Abstract
We report on an 8 year old boy with primary cardiac anaplastic large cell lymphoma (ALCL), in whom the diagnosis was challenging and who was treated with modified chemotherapy without radiation therapy according to the ALCL 99 study protocol [1]. Two years and 4 months after completion of therapy the boy is in complete remission with normal cardiac function.
Keywords: Childhood, ALCL, Lymphoma, Heart, Cardiac tumour, ALCL99
Highlights
-
•
We report about an 8-year old boy with intracardiac ALCL.
-
•
Multiple catheter assisted myocardial biopsies did not proof the ALCL.
-
•
Progressive disease and critical ventricular tachycardia during early treatment.
-
•
Continuous complete remission after modified ALCL99 treatment without radiation.
-
•
Complete recovery of myocardial function.
1. Report
We report on an 8 year old boy with primary cardiac anaplastic large cell lymphoma (ALCL), in whom the diagnosis was challenging and who was treated with modified chemotherapy without radiation therapy according to the ALCL 99 study protocol [1]. Two years and 5 months after completion of therapy the boy is in complete remission with normal cardiac function.
The boy was admitted with history of recurrent colds, weight loss, joint pain and fever up to 40 °C. Laboratory tests showed normal complete blood count and differentiation, normal electrolytes and renal function tests. ALAT (199 U/L), ASAT (152 U/L), LDH (528 U/L), NT-proBNP (735 ng/L) and Troponine-T (5.35 ng/L) were elevated. Chest X-ray revealed moderate cardiomegaly. ECG showed negative T-waves in V1-V6 and echocardiography (M-Mode) revealed normal right and left ventricular function, mild pericardial effusion and prominent masses in the left ventricular apex (largest: 1.3 cm). Clinically, the boy felt weak, he had palpable precordial heaving, peripheral oedema and hepatosplenomegaly were not noticed. Tissue Doppler echocardiography measuring myocardial velocity confirmed reduced left ventricular function.
Due to positive mycoplasma serum-IgG and -IgM antibodies antibiotic therapy was initiated. Cardiac magnetic resonance imaging (MRI) was performed. It confirmed a tumour in the apex of the left ventricle and showed infiltration of the interventricular septum and the right ventricle. On both sides tumours protruded into the cavities (Fig. 1a). Left heart catheter showed good left ventricular ejection fraction (EF, 56%) with muscular thickening and irregular contrast enhancement of soft tissue within the left cavity. Multiple catheter assisted biventricular myocardial biopsies showed mild fibrosis. They raised no suspicion of malignancy.
Fig. 1.
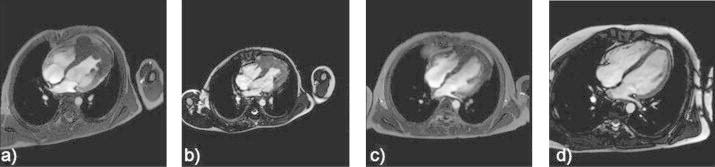
Cardiac MRI before and during ALCL treatment, Cardiac tumour shown by MR imaging before treatment (a), after prednisone prephase (b), after the first AM block (c), and two years after completion of therapy (d).
Within the following 2 weeks, moderate leucopenia (minimum 2.9/nL, neutrophils 39%, lymphocytes 49%) prompted bone marrow aspiration, which did not reveal a haematologic malignancy. Serologic tests for Echo-, Coxsackie-, Polio- and Cytomegaly-virus and HIV remained negative.
MRI of the abdomen and CNS as well as skeletal scintigraphy showed no abnormalities apart from moderate hepatosplenomegaly. Since the patient׳s clinical status deteriorated within another 2 weeks, pericardial puncture and second bone marrow aspiration were performed with no pathologic findings. Ultimately, open heart biopsy led to the diagnosis of an anaplastic lymphoma kinase-1 (ALK-1) positive ALCL of lymphohistiocytic subtype (positivity for CD30, CD2, CD3, granzyme-B, perforin, EMA and ALK1) with monoclonal T-cell receptor rearrangement.
Prednisone prephase was initiated and led to immediate improvement of clinical status. However, tumour size did not decrease within the prolonged prephase of 11 days (Fig. 1b). Despite additional cyclophosphamide (2×200 mg/m2), the patient showed deteriorating clinical condition and fever reappeared 1 week later. Pleural effusion and paravertebral cutaneous infiltration of the lymphoma indicated progressive disease. Consequently, the first AM block according to ALCL99 was started (no methotrexate due to pericardial effusion). During AM block, cardiac arrhythmia appeared and on day 6, ventricular tachycardia with hemodynamic instability resistant to chemical cardioversion occurred. Electric cardioversion and initiation of amiodarone terminated ventricular arrhythmias and normalised clinical status. The following cardiac MRI documented satisfying reduction of tumour size (Fig. 1c). Further ALCL99 treatment was given without modification. After 6 AM/BM blocks, 2-deoxy-2-(18F)fluoro-d-glucose positron emission tomography/computed tomography (FDG-PET/CT) suggested residual active tumour in the apex of the heart. Additionally, cardiac MRI revealed a large thrombus within a hypokinetic area in the apex of the left ventricle mandating warfarin therapy. We were aware that residual tumour may not necessarily predict active disease. Nevertheless, we decided to add 2 AM/BM blocks since our patient had tolerated therapy well and cumulative doses of chemotherapeutics with potential late effects (etoposide, anthracyclines) permitted intensification. Chemotherapy was completed 6 months after initiation of treatment (cumulative doses: prednisone 1000 mg/m2, dexamethasone 400 mg/m2, ifosfamide 3200 mg/m2, cytosine arabinoside 2400 mg/m2, etoposide 800 mg/m2, cyclophosphamide 4400 mg/m2, doxorubicin 200 mg/m2, methotrexate 21 g/m2).
Since end of treatment no tumour growth has been seen in cardiac MRI (Fig. 1d), FDG-PET/CT or echocardiography and the patient is back to his former active life. The only evident sequela two years after completion of therapy is a left ventricular scar, which does not influence myocardial function.
Non-Hodgkin-Lymphomas (NHL) account for <10% of malignant tumours in childhood and 10–15% of childhood NHL are ALK-positive ALCL. More than 90% of childhood ALK-positive ALCL are characterised by NPM-ALK-fusion proteins from a reciprocal translocation t(2;5) [2]. In childhood ALCL, different chemotherapy protocols reach an EFS of 65–75% [1,3,4], the 5 year overall survival is >90% [1,4]. Treatment failure most often occurs within 6 months after the end of treatment. Relapse later than 3 years after initiation of therapy or 2 years after completion of chemotherapy is rare [1,4].
Primary cardiac tumours are most often observed in adulthood. Within the group of cardiac tumours, lymphomas represent <2% [5,6] and childhood cardiac lymphomas have only been reported in case reports [7–9]. However, primary cardiac childhood ALCL has not been described before and in the German paediatric NHL-BFM and the European ALCL99 studies, in which nearly 100% of all children with this disease are registered, no patient with cardiac ALCL was registered within the last 3 decades [1,4].
Clinically, cardiac tumours are responsible for a variety of symptoms, depending on the cardiac site of involvement. Commonly seen are chest pain, pericardial effusion, arrhythmias, coronary sinus obstruction and congestive heart failure [6,10]. In our patient recurrent colds, weight loss, joint pains and fever prompted the diagnostic work-up. Remarkably, even though diagnostic work-up had shown a FDG-PET/CT positive cardiac mass, neither pericardial effusion cytology nor myocardial biopsies via cardiac catheterisation could confirm the diagnosis.
Our clinical concern was whether myocardial function would be retained after treatment of myocardial lymphoma. Since the myocardium showed pathological contrast enhancement throughout the ventricular wall, ventricular rupture would have been the worst complication. Therefore, prednisone prephase was prolonged and intensified by cyclophosphamide. For better management, treatment was monitored on the paediatric cardiology intensive care unit with standby cardiac surgery and extracorporeal membrane oxygenation facility. However, other than severe cardiac arrhythmias including one episode of ventricular tachycardia, therapy was well tolerated. From the second block (BM) onwards, chemotherapy could be given without dose reductions.
To our best knowledge, this is the first report on a child with primary cardiac ALCL surviving after chemotherapy without additional treatment.
References
- 1.Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27:897–903. doi: 10.1200/JCO.2008.18.1487. [DOI] [PubMed] [Google Scholar]
- 2.Damm-Welk C, Klapper W, Oschlies I, Gesk S, Rottgers S, Bradtke J. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: a molecular-histological correlation. Br J Haematol. 2009;146:306–309. doi: 10.1111/j.1365-2141.2009.07754.x. [DOI] [PubMed] [Google Scholar]
- 3.Lowe EJ, Sposto R, Perkins SL, Gross TG, Finlay J, Zwick D. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of children׳s cancer group study 5941. Pediatr Blood Cancer. 2009;52:335–339. doi: 10.1002/pbc.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin–Frankfurt–Munster group trial NHL-BFM 90. Blood. 2001;97:3699–3706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- 5.Lim ZY, Grace R, Salisbury JR, Creamer D, Jayaprakasam A, Ho AY. Cardiac presentation of ALK positive anaplastic large cell lymphoma. Eur J Haematol. 2005;75:511–514. doi: 10.1111/j.1600-0609.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 6.Gowda RM, Khan IA. Clinical perspectives of primary cardiac lymphoma. Angiology. 2003;54:599–604. doi: 10.1177/000331970305400510. [DOI] [PubMed] [Google Scholar]
- 7.Meshref M, Sassolas F, Schell M, Chalabreysse L, Chassagne C, Mialou V. Primary cardiac Burkitt lymphoma in a child. PediatrBlood Cancer. 2004;42:380–383. doi: 10.1002/pbc.20005. [DOI] [PubMed] [Google Scholar]
- 8.Park HK, Choi JY, Park YH. Primary cardiac T cell lymphoma in a child. PediatrCardiol. 2006;27:177–179. doi: 10.1007/s00246-005-8022-2. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulou AL, Argiriou M, Bonoris M, Papadopoulos GS, Van Vliet-Constantinidou C, Stavrinadis C. Ki-1 lymphoma with cardiac involvement at initial presentation. Pediatr Hematol Oncol. 1998;15:265–269. doi: 10.3109/08880019809028795. [DOI] [PubMed] [Google Scholar]
- 10.Park SM, Shim CY, Choi D, Lee JH, Kim SA, Choi EY. Coronary sinus obstruction by primary cardiac lymphoma as a cause of dyspnea due to significant diastolic dysfunction and elevated filling pressures. J Am Soc Echocardiogr. 2010;23:682. doi: 10.1016/j.echo.2009.10.008. (-e5-7) [DOI] [PubMed] [Google Scholar]


