Abstract
Affect recognition deficits found in individuals with attention-deficit/hyperactivity disorder (ADHD) across the lifespan may bias the development of cognitive control processes implicated in the pathophysiology of the disorder. This study aimed to determine the mechanism through which facial expressions influence cognitive control in young adults diagnosed with ADHD in childhood. Fourteen probands with childhood ADHD and 14 comparison subjects with no history of ADHD were scanned with functional magnetic resonance imaging while performing a face emotion go/no-go task. Event-related analyses contrasted activation and functional connectivity for cognitive control collapsed over face valence and tested for variations in activation for response execution and inhibition as a function of face valence. Probands with childhood ADHD made fewer correct responses and inhibitions overall than comparison subjects, but demonstrated comparable effects of face emotion on response execution and inhibition. The two groups showed similar frontotemporal activation for cognitive control collapsed across face valence, but differed in the functional connectivity of the right dorsolateral prefrontal cortex, with fewer interactions with the subgenual cingulate cortex, inferior frontal gyrus, and putamen in probands than in comparison subjects. Further, valence-dependent activation for response execution was seen in the amygdala, ventral striatum, subgenual cingulate cortex, and orbitofrontal cortex in comparison subjects but not in probands. The findings point to functional anomalies in limbic networks for both the valence-dependent biasing of cognitive control and the valence-independent cognitive control of face emotion processing in probands with childhood ADHD. This limbic dysfunction could impact cognitive control in emotional contexts and may contribute to the social and emotional problems associated with ADHD.
Keywords: ADHD, fMRI, Emotional bias, Prefrontal cortex, Go, No-go task, Adults
Highlights
-
•
We tested adult probands diagnosed with ADHD in childhood and comparison subjects.
-
•
Emotional bias of cognitive control was modeled with a face emotion go/no-go task.
-
•
Probands made fewer correct responses and correct inhibitions on the go/no-go task.
-
•
Probands showed reduced prefrontal connectivity with limbic and paralimbic regions.
-
•
Probands showed no valence-dependent limbic activation for cognitive control.
1. Introduction
Impairments in affect recognition have been found in individuals with ADHD across the lifespan (Corbett and Glidden, 2000; Kats-Gold et al., 2007; Rapport et al., 2002) and shown to impact cognitive control in children with ADHD (Kochel et al., 2014). These basic emotion deficits have been linked to a pattern of limbic dysfunction in youth with ADHD, including amygdala hyperreactivity (Brotman et al., 2010; Posner et al., 2011b), enhanced amygdala-prefrontal connectivity (Posner et al., 2011b), and valence-dependent activation in the prefrontal cortex that may reflect the impact of affect on cognitive control (Passarotti et al., 2010; Posner et al., 2011a). However, it is not known if this limbic dysfunction persists over development or biases cognitive control in adulthood, although anomalous intrinsic connectivity in fronto-limbic networks has been reported in adults with ADHD (Cocchi et al., 2012; McCarthy et al., 2013). Establishing the developmental influence of basic emotion deficits on cognitive control in individuals with ADHD and identifying the neural mechanisms that support this emotional bias have implications for addressing the impulsivity and affective instability that are the source of much of the impairment associated with the disorder in adults (Retz et al., 2012).
Facial expressions convey emotional cues that influence cognitive control processes, including response execution and inhibition in healthy adults (Hare et al., 2005; Schulz et al., 2007). Facial expressions of happiness promote approach tendencies (Otta et al., 1994), resulting in faster responses that are more difficult to inhibit (Hare et al., 2005; Schulz et al., 2007), while expressionless (neutral) faces are often mistakenly evaluated as positive or negative (Lee et al., 2008) and interfere with responses to happy and sad faces (Schulz et al., 2009, 2013). The emotional biasing of these cognitive control processes depends on functional interactions between limbic regions specialized for the affective valuation of visual stimuli (Dolan, 2007; Haber and Knutson, 2010), orbital aspects of the inferior frontal gyrus that integrate limbic input to assign behavioral significance to stimuli (Sakagami and Pan, 2007), and the dorsolateral prefrontal cortex (DLPFC), which converts these behavioral codes into top-down control over sensorimotor effectors that directly support task performance (Dosenbach et al., 2007; Gazzaley and Nobre, 2012). The inferior frontal gyrus and DLPFC have been implicated in the cognitive control deficits in ADHD (Hart et al., 2013) and are some of the last brain regions to mature functionally, with development continuing into early adulthood (Gogtay et al., 2004; Shaw et al., 2012) and reportedly delayed in individuals with ADHD (Shaw et al., 2012). The late and protracted development of the DLPFC and inferior frontal gyrus suggests that the impact of aberrant limbic processing on cognitive control in individuals with ADHD may not manifest fully until these regions reach functional maturation in early adulthood (Goldman, 1971).
The current study used functional magnetic resonance imaging (fMRI) together with a face emotion go/no-go task to compare the emotional bias of cognitive control in young adults diagnosed with ADHD in childhood and well-matched comparison subjects with no history of ADHD. Defining the probands based on a childhood diagnosis of ADHD, rather than a current diagnosis, made it possible to test the relationship of the emotional bias of cognitive control to the persistence of ADHD in adulthood. Initial analyses disregarded face valence to focus on whole-brain activation and functional connectivity of DLPFC for cognitive control irrespective of emotion. The available literature suggested that probands would show cognitive control deficits relative to comparison subjects, as reflected in fewer correct responses and inhibitions overall on the task (Hervey et al., 2004; Willcutt et al., 2005), diminished DLPFC and inferior frontal activation for response execution and inhibition (Hart et al., 2013), and reduced DLPFC–limbic interactions that may reflect less cognitive control of emotion processing (Cocchi et al., 2012; McCarthy et al., 2013). Moreover, we predicted that DLPFC–limbic connectivity would be related to the persistence of ADHD in probands and differentially related to emotional lability in probands and comparison subjects. Further analyses used the happy, sad, and neutral facial expressions that served as cues for go and no-go trials in the task to test the influence of face valence on activation for response execution and response inhibition. We predicted that emotional biases would exacerbate the response execution and inhibition deficits in probands (e.g., fewer correct inhibitions for happy faces than sad or neutral faces) and result in greater valence-dependent variations in limbic and prefrontal activation for response execution and inhibition relative to comparison subjects.
2. Methods and materials
2.1. Participants
Participants were 14 adult males who were diagnosed with ADHD when they were 7–11 years old and 14 adult males with no history of ADHD. All participants were right-handed. The probands were recruited from a study of ADHD conducted between 1990 and 1997 (Halperin et al., 2003). Childhood diagnosis of ADHD was based on parental responses to the Diagnostic Interview Schedule for Children — Parent Version (Shaffer et al., 1989). Diagnoses of major affective disorder, schizophrenia, pervasive developmental disorder, or Tourette's syndrome were exclusionary for the initial study, as was a full-scale IQ below 70. Four probands had a comorbid diagnosis of conduct disorder in childhood, and two of these children also met diagnostic criteria for separation anxiety disorder. The comparison group was recruited from the same communities where the probands resided during an adolescent follow-up study (Miller et al., 2008). Comparison subjects had no history of childhood ADHD and no more than three inattentive or hyperactive–impulsive symptoms reported by parents on the Diagnostic Interview Schedule for Children. Other psychiatric disorders that were allowed in the childhood ADHD sample were not exclusionary for the comparison group.
The adult assessment was conducted a mean ± SD of 13.2 ± 2.3 years following the probands' childhood assessments, when probands were 19–27 years old. Comparison subjects ranged in age from 18 to 26 years. All participants were interviewed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 2002), supplemented by a semi-structured interview for ADHD that was adapted from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) and the Conners' Adult ADHD Diagnostic Interview for DSM-IV (Epstein et al., 2006). The adapted interview was previously shown to demonstrate strong internal consistency (a = 0.92) (Clerkin et al., 2013). The psychiatric status of the probands reflected the diverse adult outcomes characteristic of ADHD (Faraone et al., 2006). Seven (50%) probands met full DSM-5 diagnostic criteria for ADHD in adulthood, including six (43%) with combined presentation and one (7%) with predominantly hyperactive/impulsive presentation. Seven (50%) probands continued to report symptoms that resulted in impairment in at least one domain of functioning, but no longer met full criteria for DSM-5 ADHD as adults, and were thus considered in partial remission. None of the comparison subjects met the diagnostic criteria for ADHD in adulthood or reported more than three inattentive or hyperactive–impulsive symptoms in the past 6 months. Participants also completed the Conners' Adult ADHD Rating Scale (CAARS) (Conners et al., 1999); probands had higher ratings than comparison subjects on the Hyperactive–Impulsive Symptoms (t = 3.42, p = 0.002), Inattentive Symptoms (t= 3.94, p= 0.001), and ADHD Symptoms Total (t = 4.16, p< 0.001) subscales (Table 1).
Table 1.
Demographic and clinical characteristics.
| Probands with childhood ADHD |
Comparison subjects |
||
|---|---|---|---|
| Characteristic | (n = 14) | (n = 14) | p |
| Age, mean (SD) | 23.3 (2.3) | 22.8 (2.7) | 0.45 |
| Current mood disorder, n (%) | 2 (14) | 3 (21) | 0.62 |
| Current anxiety disorder, n (%) | 2 (14) | 1 (7) | 0.54 |
| Current substance disorder, n (%) | 5 (36) | 5 (36) | >0.99 |
| Conners' Adult ADHD Rating Scale | |||
| ADHD symptom total, mean (SD) | 66.6 (14.4) | 45.2 (12.7) | <0.001 |
| Inattentive symptoms, mean (SD) | 65.4 (11.3) | 46.1 (14.5) | 0.001 |
| Hyperactive symptoms, mean (SD) | 61.3 (16.0) | 45.1 (7.5) | 0.002 |
| Impulsivity/emotional lability, mean (SD) | 49.6 (8.1) | 41.1 (8.2) | 0.01 |
| BDI-II total score, mean (SD) | 9.1 (12.2) | 5.7 (7.5) | 0.39 |
ADHD, attention-deficit/hyperactivity disorder; BDI-II, Beck Depression Inventory — II.
Probands and comparison subjects did not differ significantly in age, ratings on the Beck Depression Inventory — II (Steer et al., 1999), or in their prevalence of mood, anxiety, and substance use disorders (Table 1). However, probands had higher ratings on the CAARS Impulsivity/Emotional Lability subscale than comparison subjects (t = 2.76, p= 0.01). All participants were screened for substance use on the day of the scan and positive urine toxicology results for amphetamines, cocaine, and opiates were exclusionary. Participants refrained from cannabis use for at least 24 h before the scan. Ten (71%) probands had a previous history of stimulant treatment for ADHD, but no patient received any psychotropic medication in the 6 months preceding this study. None of the comparison subjects reported a history of psychotropic medication use.
The study was approved by the institutional review boards of Queens College of CUNY and the Icahn School of Medicine at Mount Sinai. All probands and comparison subjects provided written informed consent for participation. Participants were compensated for their time and expenses.
2.2. Face emotion go/no-go task
The face emotion go/no-go task has been previously described (Schulz et al., 2009, 2013). The task consisted of six 252-s runs that each began and ended with a 30-s central fixation-cross. Each run contained 72 (75%) go cues and 24 (25%) no-go cues, yielding a total of 432 go cues and 144 no-go cues. Participants had to respond rapidly with the right index finger to “go” cues and withhold responses to “no-go” cues. Stimuli were presented in the center of the screen for 500 ms with an interstimulus interval that was pseudorandomized from 1250 to 1750 ms (mean per block = 1500 ms). Face stimuli consisted of gray-scaled happy, sad, and neutral facial expressions from 18 individuals (9 females, 9 males) from the MacBrain Face Stimulus Set [(Tottenham et al., 2009); available at http://www.macbrain.org]. Alternating the valence of the face stimuli used as trial cues resulted in six runs, as follows: 1) happy go/sad no-go; 2) sad go/neutral no-go; 3) neutral go/happy no-go; 4) happy go/neutral no-go; 5) sad go/happy no-go; and 6) neutral go/sad no-go. Trial order was counterbalanced across all conditions (e.g., trial type, facial expression, face ethnicity, face gender, face) to ensure that each trial type followed every other trial type equally often.
2.3. Image acquisition
All participants were scanned on a 3.0 Tesla Siemens Allegra (Siemens Medical Systems) head-dedicated MRI scanner. Six series of 84 functional T2*-weighted images were acquired with echo-planar imaging sensitive to the blood oxygenation level-dependent (BOLD) signal (repetition time = 3000 ms; echo time = 27 ms; flip angle = 85°; slice thickness = 2.5 mm; skip = 0.825 mm; 42 axial slices). The repetition time represented a trade-off for thinner slices that minimized distortions and increased sensitivity. A high-resolution T2-weighted anatomical volume was acquired at the same 42 slice locations with a turbo spin-echo pulse (slice thickness = 3.325 mm; no skip; in-plane resolution = 0.41 mm2). All images were acquired with slices positioned parallel to the intercommissural line.
2.4. Behavioral data analysis
The percentage of correct responses on go trials served as the measure of response execution, while the percentage of correct inhibitions on no-go trials was the measure of response inhibition. Reaction time (RT) was also calculated for correct go trials. Behavioral performance was tested with repeated measures analyses of variance (ANOVA) with face emotion (happy vs. sad vs. neutral) as the within-subjects factor and group (probands vs. comparison subjects) as the between-subjects factor.
2.5. fMRI data analysis
2.5.1. Preprocessing and individual-level analysis
Event-related analyses were performed with SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). The six functional series for each participant were slice-time corrected, motion corrected, co-registered to the T2 anatomical volume, spatially normalized to the Montreal Neurological Institute template, and smoothed with an 8-mm Gaussian kernel. The proband and comparison groups did not differ in mean translational movement (0.97 ± 0.64 mm vs. 0.99 ± 0.40 mm; t = 0.91, p> 0.10) or rotational displacement (0.01 ± 0.01° vs. 0.01 ± 0.01°; t= 0.74, p> 0.10) during the scan.
Single-subject general linear models (GLM) were conducted to fit beta weights to regressors for the four trial events (correct no-go, correct go, incorrect no-go, incorrect go) in each run, as well as six motion parameters of no interest (Johnstone et al., 2006), convolved with the default SPM hemodynamic response function (Friston et al., 1998). The neural effect of cognitive control was tested by applying appropriate contrasts to the beta weights for correct no-go events minus correct go events collapsed over face valence. Further analyses tested for variations in activation for response execution and inhibition as a function of face valence using linear and quadratic contrasts based on the behavioral results. The neural effects of happy, sad, and neutral faces were modeled with linear and quadratic contrasts applied separately to the beta weights for correct no-go and correct go events.
Psychophysiological interaction analyses were conducted to determine the whole-brain connectivity of the right DLPFC for cognitive control (Friston et al., 1997). The seed region was extracted from a 6-mm radius sphere at subject-specific maxima that were within 2 mm of the peak of the right DLPFC activation for the correct no-go minus correct go contrast common to all probands and comparison subjects (x= 54, y= 22, z= 30). The time series of the first eigenvariate of the BOLD signal in the seed region was calculated from the time-series of voxels within the sphere and was then deconvolved to estimate the time series of the neuronal signal (Gitelman et al., 2003). Regressors representing the baseline DLPFC neuronal time series (Y), the correct no-go minus correct go contrast (P), and the interaction between the physiological and psychological factors (PPI) were forward-convolved with the hemodynamic response function and then entered into single-subject GLM, along with six motion parameters of no interest. The effect of cognitive control on right DLPFC connectivity was tested by applying appropriate contrasts to the beta weights for the PPI regressor.
2.5.2. Group-level analysis
Subject-specific contrast images for activation and connectivity were entered into second-level group analyses conducted with random-effects GLM. One- and two-sample t-tests were conducted to analyze within-group and between-group effects in the contrasts of interest, respectively. The effect of emotional lability on right DLPFC connectivity was tested using a multiple linear regression analysis that included regressors centered on the mean for the group variable and the CAARS Impulsivity/Emotional Lability subscale T-score, and an interaction predictor, calculated as the product of the centered regressors. A second analysis tested the effect of ADHD persistence by regressing DLPFC connectivity on the CAARS ADHD Symptoms Total subscale T-score in probands. The regression analyses were restricted to regions that differed in connectivity with the right DLPFC in probands and comparison subjects.
The resultant voxel-wise statistical maps were thresholded for significance using a cluster-size algorithm that protects against false-positive results (Hayasaka et al., 2004). The height (intensity) threshold of each activated voxel was set at a p-value of 0.005 and the extent (cluster) threshold was fixed at ? > 100 contiguous voxels. A prior Monte Carlo simulation confirms the current voxel contiguity threshold (Schulz et al., 2013).
3. Results
3.1. Behavioral data
Separate ANOVAs revealed that probands with childhood ADHD made both significantly fewer correct inhibitions on no-go trials (F(1, 26) = 4.54, p= 0.04) and fewer correct responses on go trials (F(1, 26) = 8.49, p= 0.007) than comparison subjects (Fig. 1). There were also significant main effects of emotion on the percentage of correct inhibitions (F(2, 26) = 6.03, p= 0.004) and the percentage of correct responses (F(2, 26) = 7.99, p< 0.001). Post-hoc Bonferroni tests revealed: 1) a linear trend in the percentage of correct inhibitions that was due to fewer correct inhibitions for happy faces than sad faces, p < 0.05, which in turn had fewer correct inhibitions than for neutral faces, p < 0.05; and 2) a quadratic trend in the percentage of correct responses that reflected fewer correct responses for sad faces than either happy or neutral faces, both p< 0.01, which did not differ from each other, p> 0.05. However, there were no significant group × emotion interaction effects for either the percentage of correct inhibitions or the percentage of correct responses (both p> 0.05). There were no main effects or interactions for RT (all p> 0.05).
Fig. 1.
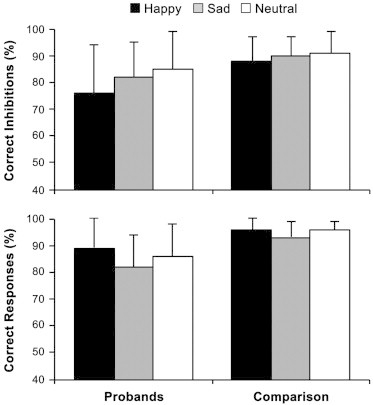
Performance on the face emotion go/no-go task as a function of face valence in probands with childhood ADHD and comparison subjects. Error bars = SD.
3.2. Activation and connectivity for cognitive control
Probands with childhood ADHD and comparison subjects demonstrated similar patterns of frontotemporal activation for cognitive control collapsed over face valence (Supplementary Table 1). As shown in Fig. 2, the two groups exhibited greater activation for correct no-go events than correct go events in overlapping areas of the right inferior frontal gyrus and right DLPFC, as well as in right middle temporal gyrus and right fusiform face area. Comparison subjects showed additional frontal and left amygdala activation that was not evident in probands. However, direct comparison of the two groups found no significant differences in activation for cognitive control.
Fig. 2.
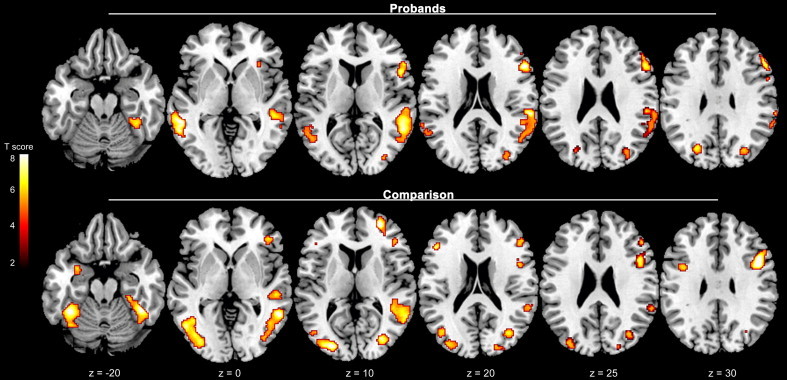
Neural activation for cognitive control (correct no-go events minus correct go events) collapsed over face emotion valence in probands with childhood ADHD and comparison subjects. Figures are thresholded at p< 0.005 (corrected for multiple comparisons with a cluster threshold > 100 voxels). Numbers at the bottom indicate z coordinates in the Montreal Neurological Institute brain template space.
Psychophysiological interaction analyses revealed differences in the whole-brain connectivity of the right DLPFC for cognitive control in probands with childhood ADHD and comparison subjects (Fig. 3; see also Supplementary Table 2). Comparison subjects showed significantly greater functional interactions for correct no-go events than correct go events between the right DLPFC and the left inferior frontal gyrus, bilateral subgenual cingulate cortex, and left putamen relative to probands. The regression analysis revealed that the CAARS Impulsivity/Emotional Lability subscale scores were positively correlated with DLPFC–subgenual cingulate cortex connectivity in probands but not comparison subjects (Fig. 4; F= 13.46, extent = 126 voxels, [10 44 0]). In contrast, the CAARS ADHD Symptoms Total score was not related to right DLPFC connectivity in probands. Probands showed significant right DLPFC connectivity with bilateral fusiform face area, but this connectivity did not differ from comparison subjects (Supplementary Table 2).
Fig. 3.
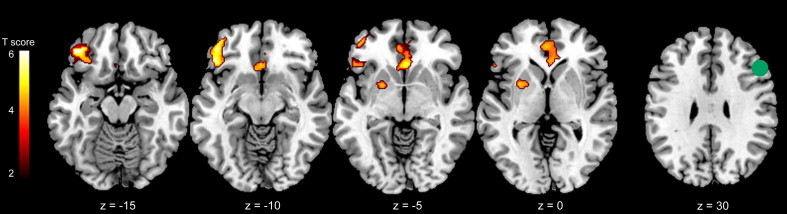
Functional connectivity of the right dorsolateral prefrontal cortex (DLPFC) for cognitive control (correct no-go events minus correct go events) collapsed over face emotion valence in probands with childhood ADHD versus comparison subjects. The seed region of interest (ROI) in the right DLPFC is displayed in green on coronal and axial sections (right column). Figures thresholded at p < 0.005 (corrected for multiple comparisons with a cluster threshold > 100 voxels). Numbers at the bottom indicate y and z coordinates in the Montreal Neurological Institute brain template space.
Fig. 4.
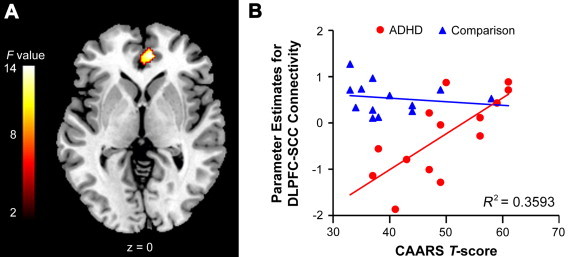
(A) The CAARS impulsivity/emotional lability score was differentially associated with the functional connectivity of the right dorsolateral prefrontal cortex (DLPFC) with the right subgenual cingulate cortex (SCC) for cognitive control (correct no-go events minus correct go events) in probands with childhood ADHD and comparison subjects. The figure is thresholded at p< 0.005 (corrected for multiple comparisons with a cluster threshold > 100 voxels). The number at the bottom indicates the z coordinate in the Montreal Neurological Institute brain template space. (B) Scatterplot of the differential association between the parameter estimates (beta values) for the functional connectivity of the right DLPFC with right SCC and the CAARS impulsivity/emotional lability T-score in probands and comparison subjects. The plot demonstrated that right DLPFC–SCC connectivity was positively related to ratings of emotional lability in probands, but not in comparison subjects.
3.3. Valence-dependent activation for response execution and inhibition
Quadratic contrasts were used to model valence-dependent variations in activation for response execution (correct go events) that matched the quadratic trend in the percentage of correct responses reported above. Direct comparison of the two groups revealed quadratic trends in activation for correct go events as a function of emotional valence in the right amygdala, left ventral striatum and orbitofrontal cortex, and right subgenual cingulate cortex in comparison subjects but not in probands with childhood ADHD (Fig. 5; see also Supplementary Table 3). Fig. 5B illustrates that the quadratic trends in activation reflected lower activation for response execution cued by sad faces than activation cued by either happy or neutral faces, which did not differ from each other. Probands showed significant quadratic trends in left motor cortex activation for response execution as a function of emotional valence, but this valence-dependent activation did not differ from comparison subjects (Supplementary Table 3).
Fig. 5.
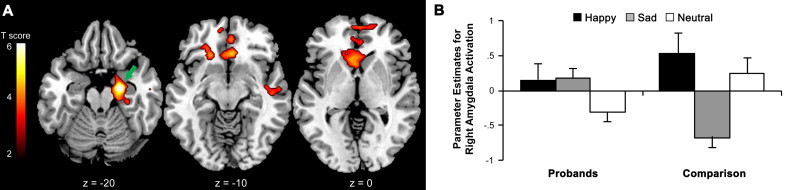
(A) Quadratic trends in neural activation for response execution (correct go events) as a function of face emotion valence in comparison subjects but not in probands with childhood ADHD. The green arrow denotes the cluster of activation in the right amygdala plotted in panel B. The figures are thresholded at p< 0.005 (corrected for multiple comparisons with a cluster threshold > 100 voxels). The numbers at the bottom of the sections indicate the z coordinates in the Montreal Neurological Institute brain template space. (B) Parameter estimates (beta values) for activation were lower for go events cued by sad faces than go events cued by either happy or neutral faces in the right amygdala in comparison subjects but not in probands.
The influence of face emotion valence on activation for response inhibition (correct no-go events) was modeled with linear contrasts based on the findings of fewer correct inhibitions for happy faces than sad faces and for sad faces than neutral faces. Group analyses revealed no significant valence-dependent variations in activation for correct no-go events in either probands or comparison subjects and no difference in such activation between the groups.
4. Discussion
These results suggest that emotional cues conveyed by facial expressions bias cognitive control similarly, albeit through divergent neural mechanisms in young adults diagnosed with ADHD in childhood and comparison subjects with no history of ADHD. Probands with childhood ADHD made fewer correct responses and correct inhibitions overall than comparison subjects despite showing similar patterns of frontotemporal activation for cognitive control collapsed over face valence. The response execution and inhibition deficits may have instead been related to the anomalous functional connectivity of the right DLPFC in probands. Comparison subjects showed enhanced right DLPFC connectivity with limbic structures, including the subgenual cingulate cortex, putamen, and orbital aspects of inferior frontal gyrus compared to probands, who showed connectivity with the fusiform face area. Face emotion had comparable effects on performance in probands and comparison subjects; the two groups showed similar linear trends in the percentage of correct inhibitions and quadratic trends in the percentage correct responses as a function of face valence. However, corresponding quadratic trends in activation for response execution as a function of emotional valence in the amygdala, ventral striatum, subgenual cingulate cortex, and orbitofrontal cortex were found in comparison subjects but not in probands. The findings point to functional anomalies in both the valence-dependent biasing of cognitive control and the valence-independent cognitive control of face emotion processing in probands.
The impairments in response execution and response inhibition found in probands have long been considered core neuropsychological deficits in ADHD (Hervey et al., 2004; Willcutt et al., 2005). These cognitive control deficits have been linked to hypoactivation of the inferior frontal gyrus, DLPFC, and other frontoparietal regions that were engaged by both comparison subjects and probands in the current study (Hart et al., 2013). The lack of group differences in this valence-independent activation implies that the poor response execution and inhibition performance seen in probands was not directly related to motor or inhibitory processes. Rather, differences in right DLPFC connectivity for cognitive control suggests that probands and comparison subjects engaged distinct neural mechanisms to process discrete features of the face stimuli. The DLPFC initiates and adjusts top-down control over task-essential sensorimotor effectors and thereby determines the focus of attention (Dosenbach et al., 2007; Gazzaley and Nobre, 2012). Thus, the interaction of the right DLPFC with limbic circuits and orbital aspects of the inferior frontal gyrus suggests that comparison subjects focused on the affective valuation of the facial expressions for salience cues (Dolan, 2007; Haber and Knutson, 2010) and the behavioral encoding of these cues (Sakagami and Pan, 2007). Probands showed right DLPFC connectivity with fusiform face areas specialized to process non-emotional features of face stimuli (Kanwisher and Yovel, 2006). The top-down focus on general face processing at the expense of higher-order affective processing could have impacted performance dependent on face emotion discrimination, and may have contributed to the response execution and inhibition deficits in probands, but was not related to the severity of ADHD in adulthood. Rather, the positive correlation of DLPFC–subgenual cingulate connectivity with ratings of emotional lability in probands defined by a childhood diagnosis of ADHD suggests that this pattern of connectivity may reflect trait-like dysfunction that develops from ADHD in childhood, but is related to affective problems in adulthood.
The behavioral results suggest that face emotion biased response execution and inhibition similarly in probands with childhood ADHD and comparison subjects. Both groups showed linear trends in the percentage of correct inhibitions that are consistent with prior studies in healthy adults that found that responses to happy faces were more difficult to inhibit (Hare et al., 2005; Schulz et al., 2007). Likewise, the quadratic trends in the percentage of correct responses found in the two groups corroborate previous reports of less accurate responses to sad faces than happy and neutral faces (Schulz et al., 2009, 2013). However, differential localization of corresponding valence-dependent activation for response execution suggests that the affective cues conveyed by facial expressions biased different neural systems in probands and comparison subjects despite comparable effects on task performance. The finding of valence-dependent activation for response execution in the subgenual cingulate cortex, ventral striatum, amygdala, and orbitofrontal cortex in comparison subjects suggests that facial expressions influenced task performance by biasing the limbic network specialized for the evaluation of stimuli for salience cues (Dolan, 2007; Haber and Knutson, 2010). Conversely, the pattern of valence-dependent activation in probands hints that face emotion instead biased the primary motor cortex effectors for response execution (Lacourse et al., 2005). The top-down focusing of attention on general face processing exemplified by the connectivity results may have diminished the limbic response to the emotional features of the face stimuli in probands (Pessoa and Ungerleider, 2004). These differences in the stimulus-driven affective biasing of cognitive control processes may reflect abnormalities in the implicit and automatic limbic processing of affective cues in probands.
The absence of limbic responses for the top-down cognitive control of face emotion processing and the stimulus-driven affective biasing of cognitive control in probands with childhood ADHD differs from previous reports of amygdala hyperactivity in youth with ADHD (Brotman et al., 2010; Posner et al., 2011b). Youth with ADHD have been reported to show exaggerated stimulus-driven amygdala responses to fearful faces (Posner et al., 2011b) and enhanced amygdala activation during directed fear appraisal (Brotman et al., 2010). The discrepancies across the studies may be due to differences in task demands, face emotions, or more likely developmental differences between the samples. The samples in the previous studies all comprised children and adolescents who met the diagnostic criteria for ADHD at the time of the study. In contrast, probands in the current study were defined by a childhood diagnosis of ADHD; their status at the time of the scan reflected the diverse adult outcomes characteristic of ADHD (Faraone et al., 2006). The discrepant findings regarding limbic responsiveness may therefore reflect developmental differences across the samples, particularly in relation to the maturation of prefrontal control over limbic function (Blumberg et al., 2004). It should also be noted that reduced limbic responses to fearful faces have been reported in youth with disruptive behavior disorders, although this dysfunction was specifically linked to callous-unemotional traits, not the presence of ADHD (Marsh et al., 2008).
Several limitations should be mentioned. First, the analyses of group differences in activation for cognitive control and behavioral measures of face emotion would have benefitted from a larger sample size. The relatively small sample size may have limited the power to detect more subtle effects, but does not detract from our findings of significant group differences in activation, connectivity, and behavior. Second, the uniqueness of the probands in our study might limit the generalization of the findings to all adults with ADHD. As noted, probands were defined by a childhood diagnosis of ADHD but presented with different degrees of symptoms as adults. Conversely, this method enabled us to test the relationship of the emotional bias of cognitive control to the persistence of ADHD symptoms in adulthood. Finally, the inclusion of participants with mood and substance use disorders in the sample, while balanced between the proband and comparison groups, may have influenced the results. Depressive disorders are characterized by mood-congruent biases that would be expected to enhance responding to sad faces on the go/no-go task (Blaney, 1986). Instead, probands and comparison subjects in the current study both made fewer correct responses (i.e., more errors) to sad faces than happy and neutral faces. Likewise, the two groups had similar rates of substance use disorders, but showed divergent patterns of activation in ventral striatal regions associated with substance use (Koob and Volkow, 2010). Excluding participants with these disorders would have further limited the generalizability of our findings.
In summary, the present data suggest that emotional cues conveyed by facial expressions bias cognitive control through sensorimotor effectors rather than limbic networks in young adults diagnosed with ADHD in childhood. This limbic dysfunction could impact cognitive control in emotional contexts and may contribute to the social and emotional problems associated with ADHD.
Financial disclosures
J.H. Newcorn is a recipient of research grants from Shire, and is or has been an advisor/consultant for Alcobra, Biobehavioral Diagnostics, Enzymotec, GencoSciences, Neos, Shire, and Sunovian. No other authors have potential conflicts of interest to declare.
Acknowledgments
This research was supported by a Young Investigator's Award to KPS from the National Association for Research on Schizophrenia and Affective Disorders (NARSAD) and by grant R01 MH060698 to JMH from the National Institute of Mental Health. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at nimtottenham@ucla.edu for more information concerning the stimulus set. Thanks to our participants and to Hanna Oltarzewska and Frank Macaluso for their invaluable support in data acquisition.
Appendix A. Supplementary Material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nicl.2014.05.016.
Appendix A. Supplementary Materials
Supplementary materials for Emotional Bias of Cognitive Control in Adults with Childhood Attention-Deficit/Hyperactivity Disorder.
References
- Blaney P.H. Affect and memory: a review. Psychological Bulletin. 1986;99:229–246. 3515383 [PubMed] [Google Scholar]
- Blumberg H.P., Kaufman J., Martin A., Charney D.S., Krystal J.H., Peterson B.S. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Annals of the New York Academy of Sciences. 2004;1021:376–383. doi: 10.1196/annals.1308.048. 15251913 [DOI] [PubMed] [Google Scholar]
- Brotman M.A., Rich B.A., Guyer A.E., Lunsford J.R., Horsey S.E., Reising M.M., Thomas L.A., Fromm S.J., Towbin K., Pine D.S., Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. 19917597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin S.M., Schulz K.P., Berwid O.G., Fan J., Newcorn J.H., Tang C.Y., Halperin J.M. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. American Journal of Psychiatry. 2013;170:1011–1019. doi: 10.1176/appi.ajp.2013.12070880. 24030612 [DOI] [PubMed] [Google Scholar]
- Cocchi L., Bramati I.E., Zalesky A., Furukawa E., Fontenelle L.F., Moll J., Tripp G., Mattos P. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2012;32:17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. 23223295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Erhardt D., Sparrow M.A. Conners' Adult ADHD Rating Scales (CAARS) Multi-Health Systems; New York: 1999. [Google Scholar]
- Corbett B., Glidden H. Processing affective stimuli in children with attention-deficit hyperactivity disorder. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2000;6:144–155. doi: 10.1076/chin.6.2.144.7056. 16210210 [DOI] [PubMed] [Google Scholar]
- Dolan R.J. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. 17576922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.N., Johnson D., Conners C.K. Conners’ Adult ADHD Diagnostic Interview for DSM-IV. Multi-Health Systems; North Tonawanda, NY: 2006. [Google Scholar]
- Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. 16420712 [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Structured Clinical Interview For DSM-IV-TR Axis I Disorders. esearch Version, Patient Edition (SCID-I-NP) Biometrics Institute, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. 9344826 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Fletcher P., Josephs O., Holmes A., Rugg M.D., Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. 9500830 [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Nobre A.C. Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. 22209601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. 12781739 [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. 15148381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P.S. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Experimental Neurology. 1971;32:366–387. doi: 10.1016/0014-4886(71)90005-7. 4999861 [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. 19812543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin J.M., Schulz K.P., McKay K.E., Sharma V., Newcorn J.H. Familial correlates of central serotonin function in children with disruptive behavior disorders. Psychiatry Research. 2003;119:205–216. doi: 10.1016/s0165-1781(03)00136-7. 12914892 [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Davidson M.C., Glover G.H., Casey B.J. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. 15780849 [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. 23247506 [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Phan K.L., Liberzon I., Worsley K.J., Nichols T.E. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. 15193596 [DOI] [PubMed] [Google Scholar]
- Hervey A.S., Epstein J.N., Curry J.F. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. 15291727 [DOI] [PubMed] [Google Scholar]
- Johnstone T., Ores Walsh K.S., Greischar L.L., Alexander A.L., Fox A.S., Davidson R.J., Oakes T.R. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping. 2006;27:779–788. doi: 10.1002/hbm.20219. 16456818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. 17118927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats-Gold I., Besser A., Priel B. The role of simple emotion recognition skills among school aged boys at risk of ADHD. Journal of Abnormal Child Psychology. 2007;35:363–378. doi: 10.1007/s10802-006-9096-x. 17243015 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. 9204677 [DOI] [PubMed] [Google Scholar]
- Kochel A., Leutgeb V., Schienle A. Disrupted response inhibition toward facial anger cues in children with attention-deficit hyperactivity disorder (ADHD): an event-related potential study. Journal of Child Neurology. 2014;29:459–468. doi: 10.1177/0883073813476139. 23449686 [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. 19710631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse M.G., Orr E.L., Cramer S.C., Cohen M.J. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage. 2005;27:505–519. doi: 10.1016/j.neuroimage.2005.04.025. 16046149 [DOI] [PubMed] [Google Scholar]
- Lee E., Kang J.I., Park I.H., Kim J.J., An S.K. Is a neutral face really evaluated as being emotionally neutral? Psychiatry Research. 2008;157:77–85. doi: 10.1016/j.psychres.2007.02.005. 17804083 [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G., Reid M.E., Sims C., Kosson D.S., Towbin K.E., Leibenluft E., Pine D.S., Blair R.J. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. 18281412 [DOI] [PubMed] [Google Scholar]
- McCarthy H., Skokauskas N., Mulligan A., Donohoe G., Mullins D., Kelly J., Johnson K., Fagan A., Gill M., Meaney J., Frodl T. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. 24132732 [DOI] [PubMed] [Google Scholar]
- Miller C.J., Miller S.R., Newcorn J.H., Halperin J.M. Personality characteristics associated with persistent ADHD in late adolescence. Journal of Abnormal Child Psychology. 2008;36:165–173. doi: 10.1007/s10802-007-9167-7. 17701339 [DOI] [PubMed] [Google Scholar]
- Otta E., Lira B.B., Delevati N.M., Cesar O.P., Pires C.S. The effect of smiling and of head tilting on person perception. Journal of Psychology. 1994;128:323–331. doi: 10.1080/00223980.1994.9712736. 8046666 [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. 20855051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Ungerleider L.G. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. 14650848 [DOI] [PubMed] [Google Scholar]
- Posner J., Maia T.V., Fair D., Peterson B.S., Sonuga-Barke E.J., Nagel B.J. The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry Research. 2011;193:151–160. doi: 10.1016/j.pscychresns.2011.02.005. 21778039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Nagel B.J., Maia T.V., Mechling A., Oh M., Wang Z., Peterson B.S. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:828–837. doi: 10.1016/j.jaac.2011.05.010. 21784302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport L.J., Friedman S.R., Tzelepis A., Van Voorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. 11853351 [DOI] [PubMed] [Google Scholar]
- Retz W., Stieglitz R.D., Corbisiero S., Retz-Junginger P., Rosler M. Emotional dysregulation in adult ADHD: what is the empirical evidence? Expert Review of Neurotherapeutics. 2012;12:1241–1251. doi: 10.1586/ern.12.109. 23082740 [DOI] [PubMed] [Google Scholar]
- Sakagami M., Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Current Opinion in Neurobiology. 2007;17:228–233. doi: 10.1016/j.conb.2007.02.008. 17350248 [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Fan J., Magidina O., Marks D.J., Hahn B., Halperin J.M. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Archives of Clinical Neuropsychology: the Official Journal of the National Academy of Neuropsychologists. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. 17207962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K.P., Clerkin S.M., Halperin J.M., Newcorn J.H., Tang C.Y., Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human Brain Mapping. 2009;30:2821–2833. doi: 10.1002/hbm.20706. 19086020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K.P., Clerkin S.M., Fan J., Halperin J.M., Newcorn J.H. Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology. 2013;226:261–271. doi: 10.1007/s00213-012-2893-8. 23086020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Piacentini J., Schwab-Stone M., Wicks J. Diagnostic Interview Schedule for Children—Parent Version (DISC-2.1P) NY Psychiatric Institute; New York: 1989. [Google Scholar]
- Shaw P., Malek M., Watson B., Sharp W., Evans A., Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. 22418014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer R.A., Ball R., Ranieri W.F., Beck A.T. Dimensions of the Beck Depression Inventory—II in clinically depressed outpatients. Journal of Clinical Psychology. 1999;55:117–128. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. 10100838 [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. 19564050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. 15950006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials for Emotional Bias of Cognitive Control in Adults with Childhood Attention-Deficit/Hyperactivity Disorder.


