Abstract
Prenatal alcohol exposure can cause a wide range of deficits in executive function that persist throughout life, but little is known about how changes in brain structure relate to cognition in affected individuals. In the current study, we predicted that the rate of white matter volumetric development would be atypical in children with fetal alcohol spectrum disorders (FASD) when compared to typically developing children, and that the rate of change in cognitive function would relate to differential white matter development between groups. Data were available for 103 subjects [49 with FASD, 54 controls, age range 6–17, mean age = 11.83] with 153 total observations. Groups were age-matched. Participants underwent structural magnetic resonance imaging (MRI) and an executive function (EF) battery. Using white matter volumes measured bilaterally for frontal and parietal regions and the corpus callosum, change was predicted by modeling the effects of age, intracranial volume, sex, and interactions with exposure status and EF measures. While both groups showed regional increases in white matter volumes and improvement in cognitive performance over time, there were significant effects of exposure status on age-related relationships between white matter increases and EF measures. Specifically, individuals with FASD consistently showed a positive relationship between improved cognitive function and increased white matter volume over time, while no such relationships were seen in controls. These novel results relating improved cognitive function with increased white matter volume in FASD suggest that better cognitive outcomes could be possible for FASD subjects through interventions that enhance white matter plasticity.
Keywords: Fetal Alcohol Syndrome, MRI, working memory, children, adolescent, executive functions, longitudinal
Highlights
-
•
Children with prenatal alcohol exposure compared with controls longitudinally
-
•
Similar increases of white matter and cognition seen in both groups
-
•
But, brain–behavior relationships differed between groups.
-
•
Different relationships seen with neurobehavior and structure in exposed children
-
•
Results suggest interventions for white matter plasticity would be useful for FASD.
1. Introduction
Prenatal alcohol exposure may lead to a range of physical and cognitive abnormalities in children that persist into adulthood (Mattson et al., 2011; Riley et al., 2011). The range of deficiencies observed in affected individuals is collectively described as fetal alcohol spectrum disorders (FASD) (Sampson et al., 1997), with fetal alcohol syndrome (FAS) representing the most severe form. The diagnosis for FASD is based on three major domains of deficiencies: brain growth, cognitive dysmorphology, and facial dysmorphology (Jones and Smith, 1973; Hoyme et al., 2005).
Numerous structural brain abnormalities have been observed in individuals with FASD. Smaller parietal and frontal white matter volumes and smaller corpus callosum size and/or area have been specifically reported in this population (Archibald et al., 2001; Wozniak and Muetzel, 2011) and may occur in tandem with abnormal gray matter thickness observed in the parietal and frontal cortices (Sowell et al., 2002; Sowell et al., 2008). Change in white matter development over time, however, remains under-investigated in the FASD literature. In typically developing children, post-natal development of white matter is more protracted than gray matter, and continues during childhood and adolescence and into midlife (Riddle et al., 2010; Welker and Patton, 2012). Specifically, during childhood and adolescence, there are significant age-related increases in white matter volume, with peak volumes occurring between 12 and 14 years in the frontal and temporal lobes to around 20–24 years for the parietal lobes (Giedd et al., 1999; Ge et al., 2002; Gogtay et al., 2004; Tamnes et al., 2010; Raznahan et al., 2011; Tanaka et al., 2012). Increases in white matter volume are attributed to increases in myelination and potentially to changes in the size, density, and number of white matter fibers over time (Paus et al., 1999; Welker and Patton, 2012).
Cognitive deficits in FASD children also persist throughout life. Past studies have shown that those with FASD demonstrate very little improvement of cognitive function over time, and differences in cognitive ability between exposed and typically developing subjects are even more apparent during adolescence and adulthood (Streissguth et al., 1991). Cross-sectional relationships between gray and white matter and cognitive function have been assessed previously in this population. For instance, earlier studies from our group have shown significant positive correlations between verbal recall and frontal gray matter thickness only in those with FASD (Sowell et al., 2008a). Similarly, white matter integrity estimated with diffusion tensor imaging (DTI) and measures of fractional anisotropy (FA) in the external capsule (significant differences near the fronto-temporal region) have been found to be correlated with performance of visuomotor tasks in individuals with FASD but not in controls (Sowell et al., 2008a; Colby et al., 2012). Others have also found lower FA (Li et al., 2009) and moderate correlations between FA and math performance in individuals with FASD (Lebel et al., 2010). However, relationships between longitudinal change in white matter and other executive functions over time in those with FASD have not been previously assessed.
While changes in white matter volume and executive function in typically developing children remain under investigated, cross-sectional studies of typically developing cohorts using DTI show that higher FA near frontal and parietal areas is positively associated with better reading ability, lexical decision making (Nagy et al., 2004; Gold et al., 2007) and IQ (Schmithorst et al., 2005). Positive relationships have also been reported between lower reaction times and greater white matter volumes in a lifespan sample of healthy volunteers (Walhovd and Fjell, 2007). However, in typically developing children, such relationships are not consistently found, and null results have also been reported when compared with those with FASD (Lebel et al., 2010; Treit et al., 2013).
In the current study, we investigated age-related changes in white matter volume over time within individuals with FASD, and how these changes relate to executive function change over time. The frontal and parietal regions were chosen specifically to investigate brain–behavior relationships with an executive function battery because of the following: i) these functions are known to be subserved by these cortical association regions (Smith and Jonides, 1997; Smith and Jonides, 1999; Cabeza, 2008); ii) due to the prominent attention and working memory deficits reported in FASD, these structures were hypothesized to be the most likely to be related to the deficits in function. The corpus callosum was additionally chosen as a ROI as numerous studies have previously documented abnormalities in its structure in FASD (Riley et al., 1995; Sowell et al., 2001; Astley et al., 2009), and we hypothesized that this structure would show differential volume changes in children with FASD compared to controls. Hence, we first investigated whether the rate of change in white matter volume in frontal and parietal regions differed between children and adolescents who were typically developing from those with FASD. Secondly, we investigated whether regional white matter volume changes predict the rate of change in executive functions over time and differ in children with FASD and typically developing children. We expected children with FASD to show smaller white matter volumes even after adjusting for overall brain size. Specifically, we hypothesized that volumes of frontal, parietal and total white matter would be smaller in participants with FASD than in controls independent of brain size and age. Given that previous studies have consistently found positive relationships between measures of white matter macro- and microstructure and cognitive function in FASD, we expected positive brain–behavior relationships in children with FASD, but not in controls.
2. Materials and methods
2.1. Recruitment
All participant data were obtained as part of the longitudinal Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) and included subjects from Los Angeles, California. Participants were recruited via advertisements and word-of-mouth, and alcohol exposed participants were also recruited through the Fetal Alcohol and Related Disorders Clinic at the University of California, Los Angeles. All participants received neuropsychological testing and structural brain imaging. Detailed developmental histories were also obtained from parental interviews during their visit(s).
2.2. Exposure and control status
Prenatally exposed participants were identified as those who were exposed to more than 4 drinks per occasion at least once per week or more than 13 drinks per week during pregnancy. Control subjects had none or less than 2 drinks (per week or on any one occasion) throughout pregnancy. Participants with incomplete exposure documentation were classified as alcohol exposed if they displayed the physical characteristics of fetal alcohol syndrome (FAS) as documented by an expert dysmorphologist. In the case of adoptive children, information about maternal alcohol use during pregnancy was gathered through sources other than the biological parent during the developmental history interview (Stratton, 1996; Hoyme et al., 2005). Out of the 49 FASD children in the study, only 7 children were living with their biological parents, and the rest were adopted. Detailed explanations of FASD diagnosis for CIFASD have been published previously (Jones et al., 2006; Mattson et al., 2010).
A total of 103 participants were included in the study, out of which 49 were classified as having FASD and 54 as controls. Of these, data were available for 41 participants (25 with FASD, 16 controls) for two time points. For the remaining 38 controls and 24 participants with FASD, data were available for one time point. Mean ages, scan intervals, and other demographic variables are presented in Table 1. There were no significant differences in age, sex, or severity of diagnosis between participants who did and did not return for a follow-up visit.
Table 1.
Demographic and cognitive variables for the control and FASD groups. Significant differences (p<0.05) are presented in bold.
| Controls | FASD group | t value | p value | |
|---|---|---|---|---|
| Two time points (n) | 16 | 25 | ||
| One time point (n) | 42 | 27 | ||
| Scan interval (range) in years | 2.36 (1.3–3.3) | 2.46 (1.6–3.5) | 0.272 | 0.788 |
| Mean age (SD) in years | 11.83 (±2.93) | 11.15 (±2.81) | 0.581 | 0.564 |
| Age range in years | 6.2–17.5 | 6.2–17.6 | ||
| Trail making Test A Response times (s) |
40.38 (±18.78) | 45.15 (±17.34) | -1.517 | 0.132 |
| Trail making Test B Response times (s) |
98.99 (±55.73) | 122.35 (±59.28) | -2.307 | 0.023 |
| Digit backward span | 7.73 (±2.53) | 6.81 (±2.14) | 2.259 | 0.025 |
| Digit forward span | 8.86 (±1.99) | 7.64 (±1.95) | 3.559 | 0.001 |
| CVLT-C Long recall | 11.40 (±2.54) | 8.86 (±3.11) | 2.259 | <0.001 |
2.3. Image acquisition
Scans were acquired on 1.5T Siemens Sonata, TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, matrix size = 256 × 256 × 160; FOV, 256 × 256 mm; total acquisition time = 8 min 8 s, voxel size = 1 × 1 × 1 mm.
2.4. Image processing
Images were processed with FreeSurfer software (v5.1, http://surfer.nmr.mgh.harvard.edu/). FreeSurfer allows for semi-automatic reconstruction of the cortical surface using T1-weighted MRI images. Major steps during image analyses include motion correction, removal of non-brain tissue, automated Talairach transformation, subcortical and cortical matter segmentation, intensity correction and delineation of gray/white/pial boundaries (Dale et al., 1999; Fischl et al., 1999). After the formation of cortical models, deformable procedures are applied including cortical inflation, registration to a spherical atlas and parcellation of the cerebral cortex into gyral and sulcal units (Desikan et al., 2006). For the participants with data at two time points, the structural scans were additionally processed through the longitudinal stream (Reuter et al., 2010).
2.5. Region selection
After initial processing by FreeSurfer, all MRI scans were visually checked slice-by-slice to ensure there was no misclassification of gray and white matter voxels; scans were reprocessed if errors were detected and rechecked visually a second time. Using the inbuilt atlas (Fischl et al., 2004), the volumes of the superior frontal, middle frontal, superior frontal, supramarginal, superior parietal, inferior parietal, and corpus callosum white matter, as well as total white matter volume was obtained from FreeSurfer and imported to SPSS 19.0 for preliminary analyses. Estimates for intracranial volumes (ICV) were also obtained through FreeSurfer. Left and right hemisphere volumes for each region in the frontal and parietal cortices were added to give bilateral estimates to reduce numbers of hypothesis tested. The ROI for frontal and parietal regions are shown in Fig. 1.
Fig. 1.
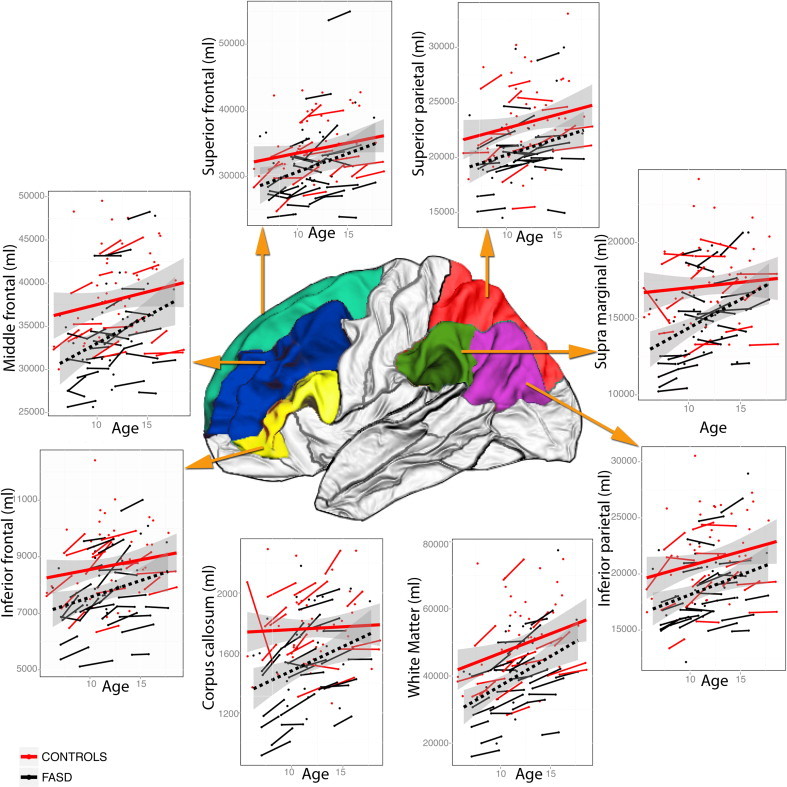
Change in total and white matter volume in the control and FASD groups. x axes: age and y axes: white matter volumes for: superior frontal; middle frontal; inferior frontal; superior parietal; inferior parietal; supramarginal; corpus callosum, and ; whole brain regions. Raw volumes have been presented. Status-by-age interactions were not significant for any region. For the supramarginal region, the FASD group had significantly smaller volumes but there were no significant age-related change in volume in either group.
2.6. Cognitive variables
Performance on verbal working memory was measured through digit-backward span (DB), while attention was measured through digit-forward span (DF), both subtests of the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (Wechsler, 2003). Attention and processing speed were measured through the Trail Making Test A (TMT-A) and mental flexibility was measured with the Trail Making Test B (TMT-B) (Reitan and Wolfson, 1985). Finally, free recall was measured through California Verbal Learning Test—C (CVLT-C) for delayed total free-recall (Delis et al., 1987). For DB, DF, and CVLT-C higher scores indicate better performance. Total completion times in seconds were utilized for TMT-A and -B, hence higher scores represent poorer performance. The cognitive tests utilized in the study are widely used in neuropsychological examinations and their test–retest reliabilities have been previously reported by other studies (for CVLT-C see Paolo et al., 1997; for DB see Iverson, 2001; and for TMT-A and -B see Cangoz et al., 2009). Raw scores were used for all cognitive variables. Descriptive statistics were analyzed using SPSS (v 19.0.) and are presented in Table 1.
2.7. Statistical analyses
Data from all participants (153 total scans) were included. This allowed us to confidently assess white matter volumes in the age ranges between 6 and 17. To minimize the effects of attrition, we adopted the linear mixed modeling (Lesaffre and Verbeke, 1998; Rabe-Hesketh et al., 2001) and maximum likelihood approaches, which are able to estimate the most likely parameters based on available time points and are robust to missing data. Statistical analyses were performed in R (R Core Team, 2012). Changes in white matter volume, as well as change in cognitive function over time were modeled taking sex, age, and ICV into account. First, the effects of age and status (prenatal alcohol exposure status: controls, FASD group) on white matter volumes were investigated for total white matter and each region of interest (ROI) separately. In the model, volumes were dependent variables while age, status, sex, ICV, and the interaction term of age-by-status were fixed variables. Subject intercept was included as the random variable. In these analyses, a significant effect for group status indicated significantly different volumes between the groups, while a significant effect for age indicated a significantly different volume with age. A significant age-by-status interaction would indicate a significant volume difference between groups in relation to age. For the second set of analyses, the effects of change in cognitive variables were modeled for each white matter ROI, and additionally included two-way interactions for ROI-by-age, ROI-by-status, age-by-status, and finally the three-way interaction of ROI-by-age-by-status. Age, sex, ICV, and status were again included as fixed variables, and subject intercept was included as a random variable. For significant three-way interactions, ROI-by-age interactions were also separately investigated for each group (controls/FASD group) to determine which group was driving the relationship among the variables. Similar to the first set of analyses, these analyses allowed us to determine if status (i.e., being alcohol exposed or not), age, and age-related change in white matter volume were differentially related to change in cognitive function.
3. Results
3.1. Demographics
Mean age for controls was 11.83 (±2.93) years and for the FASD group was 11.15 (±2.81) years. There was no statistically significant difference in age between groups (p= 0.54). Mean interval between scans was 2.36 (±3.05) years for controls and 2.46 (±2.98) years for the FASD group. There were no significant group differences in scan interval (p= 0.34). Independent sample t-tests revealed that cognitive function was significantly poorer in FASD participants compared to typically developing controls in TMT-B, DF, DB, and CVLT-C. Mean times for TMT-A were higher in those with FASD but were not significantly different from controls (Fig. 2, Table 1).
Fig. 2.
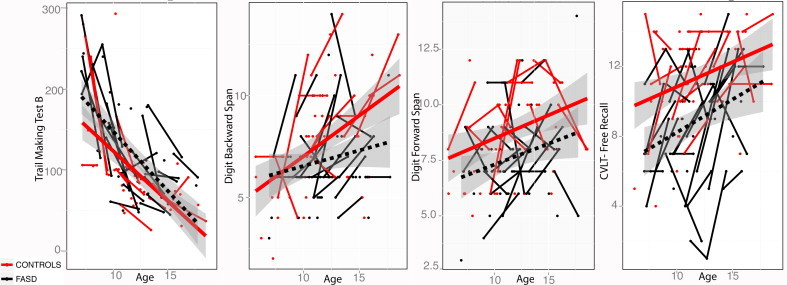
Distribution of cognitive score change in the control and FASD groups. x axes: age; y axes: cognitive scores. i) TMT-B, ii) DB, iii) DF, iv) CVLT-C. For TMT-A and -B, completion times are presented and faster times indicate better performance. For DB, DF and CVLT, total items recalled are presented, and higher scores indicate better performance. Exposure status-by-age interactions were not significant for any of the neuropsychological scores.
3.2. Age related changes and group differences
As predicted, for both groups, age was significantly related to white matter volume change over time in each of the frontal and parietal regions, including total white matter and corpus callosum, except in the supramarginal region (Table 2). Also as predicted, participants with FASD had significantly smaller regional volumes than controls, and this difference remained significant even after adjusting for intracranial volumes, for the corpus callosum (t = 2.18, p= 0.031), middle frontal (t= 2.24, p= 0.027), supramarginal (t= 2.58, p= 0.037), and inferior parietal regions (t= 2.11, p = 0.011). Both groups showed similar rates of increase in regional white matter volumes and there were no significant age-by-status interactions for any of the regions investigated. See Fig. 1 for scatterplots of volume changes for both groups. Both groups improved with age significantly on all cognitive tests (Table 3). After controlling for age, controls were also significantly better than those with FASD over time on all cognitive tests except TMT-A (Table 4).
Table 2.
Change in white matter volumes. Main effects of age and exposure status.
| Volumes | Age—tvalue | Age—tvalue | Status—tvalue | Status—p value |
|---|---|---|---|---|
| Total white matter | 8.293 | <0.001 | -1.832 | 0.069 |
| Corpus callosum | 4.830 | <0.001 | -2.184 | 0.031 |
| Inferior frontal | 5.009 | <0.001 | -1.383 | 0.169 |
| Middle frontal | 3.875 | <0.001 | -2.239 | 0.027 |
| Superior frontal | 3.074 | 0.003 | 0.012 | 0.989 |
| Inferior parietal | 3.972 | <0.001 | -2.110 | 0.037 |
| Superior parietal | 3.877 | <0.001 | -1.743 | 0.084 |
| Supramarginal | 1.379 | 0.175 | -2.584 | 0.011 |
Significant p values at <0.05 have been presented in bold font
Age, status, sex, and ICV were fixed while subject intercept was a random variable.
Table 3.
Change in cognitive function with age and exposure status.
| Cognitive variable | Age—t value | Age—p value | Status—t value | Status—p value |
|---|---|---|---|---|
| Trails making Test A | -8.536 | <0.001 | 1.026 | 0.307 |
| Trails making Test B | -9.660 | <0.001 | 2.507 | 0.013 |
| Digit backward span | 4.691 | <0.001 | -2.031 | 0.044 |
| Digit forward span | 3.513 | 0.001 | -3.308 | 0.001 |
| California verbal learning Test-C | 4.053 | <0.001 | -4.600 | <0.001 |
Subject was the random variable.
Significant p values at <0.05 have been presented in bold font.
Table 4.
Change in cognitive function predicted through change in white matter, and its interactions with age and status. Sex and ICV were fixed variables while subject was the random variable.
| Interactions |
ROI-by-age |
ROI-by-status |
ROI-by-status -by-age |
ROI-by-age |
ROI-by-status |
ROI-by-status -by-age |
ROI-by-age |
ROI-by-status |
ROI-by-status -by-age |
ROI-by-age |
ROI-by-status |
ROI-by-status -by-age |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corpus callosum | Inferior frontal | Superior frontal | Middle frontal | |||||||||
| TMT-A | 0.036 | 0.074 | 0.082 | 0.005 | 0.017 | 0.015 | 0.023 | 0.052 | 0.046 | 0.007 | 0.083 | 0.062 |
| 2.202 | –1.802 | 2.202 | 3.070 | 2.541 | –2.593 | 2.397 | 2.027 | –2.086 | 2.902 | 2.070 | –1.944 | |
| DF | 0.033 | 0.005 | 0.002 | 0.187 | 0.027 | 0.044 | 0.253 | 0.027 | 0.032 | 0.358 | 0.030 | 0.047 |
| –2.257 | –3.087 | 3.379 | –1.354 | –2.341 | 2.111 | –1.168 | –2.334 | 2.264 | –0.936 | –2.288 | 2.085 | |
| TMT-B | 0.699 | 0.036 | 0.078 | 0.018 | 0.425 | 0.997 | 0.015 | 0.323 | 0.226 | 0.092 | 0.118 | 0.292 |
| –0.390 | –2.207 | 1.836 | 2.520 | –0.809 | –0.004 | 2.583 | –1.007 | 1.240 | 0.631 | –1.472 | 1.075 | |
| CVLT | 0.370 | 0.071 | 0.042 | 0.570 | 0.937 | 0.964 | 0.420 | 0.813 | 0.837 | 0.904 | 0.962 | 0.884 |
| –1.860 | –2.028 | 2.124 | 0.605 | 0.108 | –0.005 | 0.798 | 0.223 | –0.193 | 0.105 | –0.053 | –1.860 | |
| DB | 0.302 | 0.114 | 0.044 | 0.758 | 0.433 | 0.551 | 0.796 | 0.515 | 0.625 | 0.545 | 0.403 | 0.362 |
| –1.053 | –1.637 | 2.123 | –0.312 | –0.796 | 0.604 | 0.261 | –0.661 | 0.495 | –0.614 | –0.850 | 0.928 | |
|
Inferior parietal |
Supramarginal |
Superior parietal |
Total white matter |
|||||||||
| TMT-A | 0.012 | 0.160 | 0.117 | 0.012 | 0.160 | 0.117 | 0.011 | 0.180 | 0.094 | 0.052 | 0.209 | 0.192 |
| 2.689 | 1.444 | –1.614 | 3.335 | 2.385 | –2.432 | 2.720 | 1.375 | –1.733 | 2.030 | 1.286 | –1.335 | |
| DF | 0.117 | 0.031 | 0.047 | 0.117 | 0.031 | 0.047 | 0.60 | 0.173 | 0.279 | 0.157 | 0.026 | 0.023 |
| –1.620 | –2.275 | 2.080 | –1.092 | –1.766 | 1.687 | –0.523 | –1.400 | 1.104 | –1.456 | –2.358 | 2.407 | |
| TMT-B | 0.027 | 0.099 | 0.262 | 0.034 | 0.409 | 0.262 | 0.882 | 0.255 | 0.359 | 0.102 | 0.256 | 0.114 |
| 2.330 | –1.703 | 1.146 | 2.220 | –0.837 | 0.877 | 0.149 | –1.162 | 0.932 | 0.090 | –1.953 | 1.634 | |
| CVLT | 0.897 | 0.838 | 0.707 | 0.897 | 0.838 | 0.707 | 0.893 | 0.962 | 0.930 | 0.937 | 0.988 | 0.694 |
| 0.118 | –0.204 | 0.388 | –0.335 | –0.462 | 0.679 | –0.135 | 0.048 | 0.721 | –0.036 | 0.029 | 0.360 | |
| DB | 0.844 | 0.699 | 0.743 | 0.844 | 0.699 | 0.743 | 0.835 | 0.973 | 0.812 | 0.302 | 0.114 | 0.417 |
| –0.198 | –0.391 | 0.331 | 0.183 | 0.026 | 0.023 | 0.290 | 0.210 | –0.240 | –0.485 | –0.480 | 0.824 | |
Significant p values of <0.05 have been presented in bold font.
T-values for significance have been presented below in italics.
3.3. Cognitive change and white matter volume increase independent of age
Differential relationships between increased white matter volume and improved cognitive function were observed between the two groups (Table 4). Such ROI-by-status interactions were significant for i) DF for increases in volumes of corpus callosum (t = 3.08, p= 0.002), inferior parietal (t= 2.27, p = 0.03), middle frontal (t= 2.28, p = 0.03), superior parietal (t = 2.23, p= 0.02), inferior frontal (t= 2.34, p= 0.02), and total white matter (t= 2.35, p = 0.02); ii) TMT-B times for increases in volumes of corpus callosum (t = 2.20, p= 0.02), inferior parietal (t= 2.37, p = 0.02), supra marginal (t= 2.30, p = 0.03), middle frontal (t= 2.26, p = 0.03), and superior frontal (t= 2.03, p= 0.04) regions; and iii) DB for corpus callosum volume increase (t = 2.04, p = 0.04). These findings showed that there were significant differences in change in cognitive function and change in volumes between those with FASD and controls. Follow-up analyses confirmed the direction of the findings, where larger white matter volumes were related to better performance in those with FASD, but not in controls.
In addition, only FASD participants showed significant relationships between white matter volume increase and better cognitive function with increasing age (Table 4). Such three-way interactions (ROI-by-status-by-age) were significant for i) DF for increases in volumes of corpus callosum (t = 3.65, p < 0.001), inferior parietal (t= 2.08, p= 0.04), middle frontal (t = 2.08, p= 0.04), superior frontal (t= 2.26, p= 0.03), inferior frontal (t= 2.26, p= 0.03), and total white matter (t= 2.63, p< 0.01); ii) CVLT-C long delay free recall for corpus callosum (t = 2.11, p = 0.04). Such significant interactions show that the relationship between regional white matter volume, age, and cognition is mediated differently in the FASD and control groups. Analyses of t-values from Tables 3 and 4 reveal that individuals with FASD have consistently lower cognition and volumes compared to controls. Finally, follow-up analyses conducted for each group separately revealed significant ROI-by-age interactions for all of the regions with significant three-way interactions in the FASD group, but not in the control group. In contrast, while controls showed a significant positive effect of age on cognitive function, none of the interactions with age were significant for any of the cognitive variables. A representative plot for significant three way interactions for each group has been presented in Fig. 3. As highlighted in the plot, the relationship between white matter volume and cognitive function increases with age in the FASD group (i.e., increases in volume with age are related to better improvements in cognitive scores), but such relationships with age are not significant in controls.
Fig. 3.
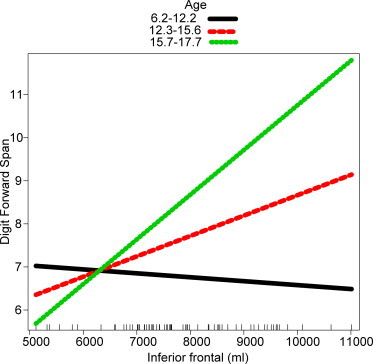
A representative plot for significant ROI-by-status-by for FASD. Color bars indicate volume–cognition relationships at increasing ages. x axes: ROI volume. y axes: cognitive score; only the FASD group showed significant ROI-by-age interactions (p< 0.05). ROI-by-age interactions were non-significant for controls.
4. Discussion
We show for the first time that age-related regional increase in white matter volume is differentially related to cognitive change between children with FASD and typically developing children. Specifically, age-related increases in callosal, frontal, and parietal white matter volume showed significant associations with cognitive improvements in children with FASD, but this relationship was not observed in those that were typically developing. Since both groups showed improvement of cognitive performance and increases in white matter volume during the periods examined, results support the fact that age-related plasticity in white matter structure impacts the development of cognitive function in FASD children and adolescents. These findings suggest that FASD-related disturbances or disorganization of white matter structure contribute to cognitive impairments over time in individuals with FASD and/or that variations in other structural characteristics not measured here (such as gray matter volume or thickness) play a more central role in cognitive development in typically developing controls. Abnormalities in white matter macro- and micro-structure are widely documented in FASD (Riley et al., 1995; Sowell et al., 2008b; Yang et al., 2012). Here, we have extended these findings to show that while developmental trajectories for white matter volume continues at the same rate as typical children, these trajectories have a strong positive effect on cognitive function in those with FASD. These findings may have implications for continuing effective social and behavioral support for better health, and socioeconomic status shown to relate to better brain outcomes in those with FASD that may in turn have long-lasting positive effects on executive function.
4.1. Changes with age
4.1.1. Cognitive function
Results revealed that both typically developing children and those with FASD showed significant improvement of cognitive function over time, and age and time were significantly related to cognitive function for both groups. Significant group differences still remained for all the cognitive variables after taking age into account, and FASD children performed more poorly than typically developing children for all tasks examined with the exception of the TMT-A, which showed a trend for faster completion times in controls. These results suggest that while those with FASD do not catch up to typically developing children in standardized tests, they nonetheless make significant improvements in cognitive function over time.
Our findings may have several important implications for future care of those with FASD. Since results show that those with FASD can potentially improve in executive functioning with age, it appears to be of particular importance to enhance opportunities to support the social and behavioral development of those with FASD. Environmental support is especially relevant, as previous studies have shown that the biological parents of those with FASD have poorer health behaviors and education and are of lower socioeconomic status (SES) (Streissguth et al., 2004; May et al., 2005) than those of typically developing children. Furthermore, other studies of early life adversity have been related to smaller white matter volume (Teicher et al., 2004) while adequate care early in life in typically developing children is associated with greater white matter volume later on (Als et al., 2004). Taken together, these results suggest that providing an environment with health and educational opportunities at home and in the community for those with FASD might continue to enhance overall cognitive development. Given that most of the children with FASD in the current study were not living with biological parents, but instead with adopted families, it is possible that participants in this study were exposed to improved home and school environments, which might have led to better brain and cognitive outcomes.
4.1.2. White matter volume
Similarly, although individuals with FASD had significantly smaller white matter volumes at both time points, results revealed that the rate of increase in white matter volume with age is similar in the two groups: there were no significant exposure status-by-age interactions for change in white matter volume. These findings suggest that even though those with FASD begin with smaller white matter volumes, this does not affect the subsequent increase/development of white matter over time in this population. Furthermore, results also showed that despite having smaller volumes, these differences only remained significant for the middle and superior frontal and the supramarginal regions for individuals with FASD after taking brain sizes into account.
Our results of similar rate of white matter volume increase in those with FASD are in contrast with studies of gray matter development, where rates of change of gray matter have been found to be different between FASD and control children. Lebel et al. (2012) found that while control children showed an “inverted-U” shaped development of the gray matter, those with FASD only showed linear decreases implying different timing of gray matter maturation between these two populations. Hence, while gray matter development seems to show ongoing difference in developmental trajectory between groups, our results suggest that differences in white matter are static with pronounced but stable group differences.
These results could possibly be explained through the different developmental timelines for gray and white matter. Though some fiber connections develop early, the majority of white matter development (myelination and changes in the size, density and organization of axons) continues to develop after birth (Welker and Patton, 2012) and increases in volume are reported at least until the first four decades of life (Thompson et al., 2005; Riddle et al., 2010). Hence, postnatal development of white matter occurs after the direct teratogenic effect of alcohol in utero. In contrast, gray matter development, begins in utero (Tanaka et al., 2012; Rubia, 2013), hence the initial brain neurons may be directly affected by alcohol for a longer period. Gray matter volume also has a narrower developmental trajectory, as volumes peak in early adolescence (Giedd et al., 1999; Lenroot et al., 2009) instead of adulthood. While no longitudinal studies addressing white matter volume development over time in FASD children exist, follow-up on preterm children have shown that after controlling for brain size, gray matter volumes are more affected than white matter at adolescence (Cheong et al., 2013). The hypoxic–ischemic injury to the brain during preterm birth also occurs prenatally. Hence, it is possible that, due to its relatively prolonged maturation period, white matter development could be more resistant to the long-lasting effects of prenatal alcohol exposure and/or be more amenable to plasticity relating to environmental factors. Future investigations should test our findings across a wider age range to verify the long-term effects of prenatal alcohol exposure beyond adolescence.
4.2. Inter-relationships among cognitive function, age, and exposure
Diagnostic group differences were pronounced for tests of attention. Further, as mentioned above, increase in white matter volume showed different relationships with cognitive function depending on exposure status. Specifically, performance in DF improved with age and larger white matter regional volumes in those with FASD. However, for typically developing children who also improved over time in cognitive function, this relationship was not shown as mediated by white matter volume increases. Significant interactions were also observed for DB and CVLT-C where increased callosal volume with age was related to better cognitive function in the exposed group. These results were less robust than those observed for tests of attention capacity and correlations were significant predominantly for the corpus callosum.
Overall, our findings suggest that in typically developing children, cognitive function may rely more heavily on other neurodevelopmental changes in brain macro- or microstructure. For instance, previous research has also shown that relationships between brain measures and cognitive function are stronger in study populations with neurological disorders, whereas such brain–structure relationships during normal development are less consistently found (Van Petten, 2004; Treit et al., 2013). Thus, in a typically developing population, it is possible that the contribution of other neurodevelopmental processes might mask white matter contributions, making these relationships harder to detect. In contrast, children with FASD have documented abnormalities of gray matter structure as well as function. For instance, abnormal activation of the parietal and frontal cortices (O’Hare et al., 2009; Meintjes et al., 2010) as well as atypical morphology of cortical regions (Riley et al., 1995; Mattson et al., 1996) have been previously reported. For children with FASD, typical relationships between gray and white matter structure and their connectivity across the brain might be lacking throughout development. Since children with FASD have smaller overall white matter volumes compared to age-matched typically developing peers, increase of white matter might significantly contribute to better communication among brain regions and consequently lead to improved cognitive abilities. Since increases in white matter volume are at least partly attributable to age-related increases in myelination (Welker and Patton, 2012), improvements in conduction velocity of axonal fibers may facilitate communication among brain regions. Not surprisingly, the time course of increased myelination is related to language function in typically developing young children (Pujol et al., 2006) and may provide a possible explanation for the improvement of executive function with age in the current sample.
Several factors may contribute to our observations for more pronounced group differences in attention than in other functional domains such as mental flexibility as measured by TMT-B. First, since tasks like working memory and recall require more complex functional networks for successful task completion, it is possible that gross measures of white matter volume were not sensitive to variations associated with these specific cognitive functions. Second, only a small proportion of the participants in the FASD group had the full FAS diagnosis (n= 5). Less severe neuropsychological deficits have been previously documented for children in the spectrum compared to those with full FAS, and children with fetal alcohol effects but without the facial dysmorphology or growth abnormalities to warrant an FAS diagnosis can show normal IQ and display only subtle behavioral or cognitive deficits. Hence, the results could be more representative of those with less severe deficits who show fewer differences from control children in executive tasks.
4.3. Limitations
Although we did not find differences in the developmental trajectory of white matter volume between diagnostic groups, it is possible that there might be changes occurring at the microstructural level. For instance, a recent study has found different rates of white matter maturation using DTI measures between controls and children with FASD (Treit et al., 2013). Although this study did not examine executive functioning, they reported positive correlations between change in mean diffusivity (the overall magnitude of water diffusion in a voxel) and reading scores in the FASD group. Future longitudinal research will need to clarify relationships between diffusion imaging indices of white matter integrity and structural connectivity and executive functions.
In addition, while we included participants with data on both first and follow-up visits in order to increase power in detecting significant relationships, this is a limitation of our study and our findings need to be understood in this context. Although there were no significant age, sex, or diagnosis severity differences in participants who did and did not complete the follow-up study, there might be other unknown factors that determined attrition. Finally, additional time points of 3 or more, would also have allowed us to assess more detailed changes in developmental trajectories. Since development is a time of rapid change, it is possible that increasing the sample size and sampling at more time points would have allowed us to detect other subtler group differences.
5. Conclusions
This study showed that while the rate of development of white matter volume is similar with age in both typically developing and exposed children, there are differential relationships between cognitive function and white matter change over time. Specifically, for those with FASD, age-related increases in volumes were related to better cognitive function, while for controls, this positive relationship was not detected. Findings suggest that increasing white matter volume with age allows those with FASD to improve in their cognitive abilities over time, possibly by facilitating information processing among connected brain areas. Findings also suggest that better cognitive outcomes could be possible for FASD subjects through interventions targeting better cognitive and behavioral outcomes through education and/or other environmental factors.
Acknowledgments
This work was performed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at http://www.cifasd.org. This work was also supported by NIAAA grant U01 AA017122 to ERS, National Institute of Drug Abuse grant R01 DA017830 to ERS, National Institute of Child Health and Human Development grant R01 HD053893 to ERS, National Institute of Mental Health grant R01 MH087563 to ERS, and March of Dimes grant 6-FY08-562 to ERS.
References
- Als H., Duffy F.H. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. 15060237 [DOI] [PubMed] [Google Scholar]
- Archibald S.L., Fennema-Notestine C. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43(3):148–154. 11263683 [PubMed] [Google Scholar]
- Astley S.J., Aylward E.H. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. 19572986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. 18439631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangoz B., Karakoc E. Trail making test: normative data for Turkish elderly population by age, sex and education. Journal of the Neurological Sciences. 2009;283(1–2):73–78. doi: 10.1016/j.jns.2009.02.313. 19264326 [DOI] [PubMed] [Google Scholar]
- Cheong J.L., Anderson P.J. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS ONE. 2013;8(10):e77475. doi: 10.1371/journal.pone.0077475. 24130887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J.B., Smith L. White matter microstructural alterations in children with prenatal methamphetamine/polydrug exposure. Psychiatry Research. 2012;204(2–3):140–148. doi: 10.1016/j.pscychresns.2012.04.017. 23149028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. 9931268 [DOI] [PubMed] [Google Scholar]
- Delis D., Kramer J. California Verbal Learning Test. San Antonio Psychological Corporation, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Desikan R.S., Segonne F. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. 16530430 [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. 9931269 [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A. Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y.: 1991) 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. 14654453 [DOI] [PubMed] [Google Scholar]
- Ge Y., Grossman R.I. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR. American Journal of Neuroradiology. 2002;23(8):1327–1333. 12223373 [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. 10491603 [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. 15148381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.T., Powell D.K. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. 17509627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme H.E., May P.A. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. 15629980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G.L. Interpreting change on the WAIS-III/WMS-III in clinical samples. Archives of Clinical Neuropsychology. 2001;16(2):183–191. 14590186 [PubMed] [Google Scholar]
- Jones K.L., Robinson L.K. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6):e1734–e1738. doi: 10.1542/peds.2006-1037. 17088402 [DOI] [PubMed] [Google Scholar]
- Jones K.L., Smith D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. 4127281 [DOI] [PubMed] [Google Scholar]
- Lebel C., Mattson S.N. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. 23115162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Rasmussen C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcoholism, Clinical and Experimental Research. 2010;34(2):354–363. doi: 10.1111/j.1530-0277.2009.01097.x. 19930234 [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Schmitt J.E. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2009;30(1):163–174. doi: 10.1002/hbm.20494. 18041741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesaffre E., Verbeke G. Local influence in linear mixed models. Biometrics. 1998;54(2):570–582. 9629645 [PubMed] [Google Scholar]
- Li L., Coles C.D. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Human Brain Mapping. 2009;30(10):3265–3274. doi: 10.1002/hbm.20747. 19278010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Crocker N. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. 21503685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Foroud T. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol (Fayetteville, N.Y.) 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. 20036488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Riley E.P. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism, Clinical and Experimental Research. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. 8892532 [DOI] [PubMed] [Google Scholar]
- May P.A., Gossage J.P. Maternal risk factors for fetal alcohol syndrome in the Western Cape province of South Africa: a population-based study. American Journal of Public Health. 2005;95(7):1190–1199. doi: 10.2105/AJPH.2003.037093. 15933241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes E.M., Jacobson J.L. An fMRI Study of number processing in children with fetal alcohol syndrome. Alcoholism, Clinical and Experimental Research. 2010;34(8):1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. 20528824 [DOI] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. 15453975 [DOI] [PubMed] [Google Scholar]
- O’Hare E.D., Lu L.H. Altered frontal–parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Human Brain Mapping. 2009;30(10):3200–3208. doi: 10.1002/hbm.20741. 19263420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo A.M., Troster A.I. Test–retest stability of the California Verbal Learning Test in older persons. Neuropsychology. 1997;11(4):613–616. doi: 10.1037//0894-4105.11.4.613. 9345705 [DOI] [PubMed] [Google Scholar]
- Paus T., Zijdenbos A. Structural maturation of neural pathways in children and adolescents: in vivo study. Science (New York, N.Y.) 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. 10082463 [DOI] [PubMed] [Google Scholar]
- Pujol J., Soriano-Mas C. Myelination of language-related areas in the developing brain. Neurology. 2006;66(3):339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. 16476931 [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. http://www.R-project.org/ [Google Scholar]
- Rabe-Hesketh S., Yang S. Multilevel models for censored and latent responses. Statistical Methods in Medical Research. 2001;10(6):409–427. doi: 10.1177/096228020101000604. 11763550 [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P. How does your cortex grow? Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. 21562281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M., Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Neuropsychological Press; Tucson, AZ: 1985. [Google Scholar]
- Reuter M., Rosas H.D. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. 20637289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle W.R., DonLevy S.C. Modeling brain tissue volumes over the lifespan: quantitative analysis of postmortem weights and in vivo MR images. Magnetic Resonance Imaging. 2010;28(5):716–720. doi: 10.1016/j.mri.2010.01.003. 20233647 [DOI] [PubMed] [Google Scholar]
- Riley E.P., Infante M.A. Fetal alcohol spectrum disorders: an overview. Neuropsychology Review. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. 21499711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.P., Mattson S.N. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism, Clinical and Experimental Research. 1995;19(5):1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. 8561290 [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. European Child & Adolescent Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. 22729957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson P.D., Streissguth A.P. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. 9451756 [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Wilke M. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. 15858815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. 9212720 [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Storage and executive processes in the frontal lobes. Science (New York, N.Y.) 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. 10073923 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Johnson A. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2008;28(6):1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. 18256251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N. Abnormal cortical thickness and brain–behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex (New York, N.Y.: 1991) 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. 17443018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57(2):235–244. doi: 10.1212/wnl.57.2.235. 11468307 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex (New York, N.Y.: 1991) 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. 12122034 [DOI] [PubMed] [Google Scholar]
- Stratton K. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academies Press; 1996. [Google Scholar]
- Streissguth A.P., Aase J.M. Fetal alcohol syndrome in adolescents and adults. J.A.M.A.: The Journal of the American Medical Association. 1991;265(15):1961–1967. 2008025 [PubMed] [Google Scholar]
- Streissguth A.P., Bookstein F.L. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics: JDBP. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. 15308923 [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Ostby Y. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and White matter volume and microstructure. Cerebral Cortex (New York, N.Y.: 1991) 2010;20:534–548. doi: 10.1093/cercor/bhp118. 19520764 [DOI] [PubMed] [Google Scholar]
- Tanaka C., Matsui M. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Developmental Neuroscience. 2012;34:477–487. doi: 10.1159/000345152. 23257954 [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Dumont N.L. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. 15231439 [DOI] [PubMed] [Google Scholar]
- Thompson P.M., Sowell E.R. Structural MRI and brain development. International Review of Neurobiology. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. 16291026 [DOI] [PubMed] [Google Scholar]
- Treit S., Lebel C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2013;33(24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. 23761905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. 15193947 [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M. White matter volume predicts reaction time instability. Neuropsychologia. 2007;45(10):2277–2284. doi: 10.1016/j.neuropsychologia.2007.02.022. 17428508 [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-IV Technical and Interpretive Manual. The Psychological Association; San Antonio: 2003. [Google Scholar]
- Welker K.M., Patton A. Assessment of normal myelination with magnetic resonance imaging. Seminars in Neurology. 2012;32(1):15–28. doi: 10.1055/s-0032-1306382. 22422203 [DOI] [PubMed] [Google Scholar]
- Wozniak J., Muetzel R. What Does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychology Review. 2011;21(2):133–147. doi: 10.1007/s11065-011-9162-1. 21347880 [DOI] [PubMed] [Google Scholar]
- Yang Y., Phillips O.R. Callosal thickness reductions relate to facial dysmorphology in fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2012;36(5):798–806. doi: 10.1111/j.1530-0277.2011.01679.x. 22150665 [DOI] [PMC free article] [PubMed] [Google Scholar]


