Abstract
Osteogenesis and bone remodeling are complex biological processes that are essential for the formation of new bone tissue and its correct functioning. When the balance between bone resorption and formation is disrupted, bone diseases and disorders such as Paget's disease, fibrous dysplasia, osteoporosis and fragility fractures may result. Recent advances in bone cell biology have revealed new specific targets for the treatment of bone loss that are based on the inhibition of bone resorption by osteoclasts or the stimulation of bone formation by osteoblasts. Bisphosphonates, antiresorptive agents that reduce bone resorption, are usually recommended as first-line therapy in women with postmenopausal osteoporosis. Numerous studies have shown that bisphosphonates are able to significantly reduce the risk of femoral and vertebral fractures. Other antiresorptive agents indicated for the treatment of osteoporosis include selective estrogen receptor modulators, such as raloxifene. Denosumab, a human monoclonal antibody, is another antiresorptive agent that has been approved in Europe and the USA. This agent blocks the RANK/RANKL/OPG system, which is responsible for osteoclastic activation, thus reducing bone resorption. Other approved agents include bone anabolic agents, such as teriparatide, a recombinant parathyroid hormone that improves bone microarchitecture and strength, and strontium ranelate, considered to be a dual-action drug that acts by both osteoclastic inhibition and osteoblastic stimulation. Currently, anti-catabolic drugs that act through the Wnt-β catenin signaling pathway, serving as Dickkopf-related protein 1 inhibitors and sclerostin antagonists, are also in development. This concise review provides an overview of the drugs most commonly used for the control of osteogenesis in bone diseases.
Keywords: Antiresorptive Drugs, Bone Formation, Osteoblasts, Osteogenesis, RANKL Inhibitors
INTRODUCTION
Osteoblasts play a crucial role both in the promotion of bone formation and, indirectly, in the modulation of osteoclast differentiation through the expression of the receptor activator of nuclear factor NFκB ligand (RANKL) and of osteoprotegerin (OPG), which are known, together with RANK, to regulate osteoclast formation and activity (1,2). RANKL, a transmembrane protein that is highly expressed by pre-osteoblasts and osteoblasts (3), periosteal cells (4), and osteocytes (5), binds and activates its receptor RANK, which is mainly expressed by osteoclasts and their precursors (6). After binding to RANK, RANKL stimulates the formation, activity and survival of osteoclasts (7,8), resulting in increased bone resorption (9). OPG, a member of the tumor necrosis factor (TNF) superfamily of proteins that is secreted by osteoblasts, is another key molecule in this process because it inhibits RANKL-induced osteoclastogenesis (13). In fact, OPG binds to RANKL with high affinity and competes with RANK for binding to RANKL on the surface of osteoclasts and their precursors (10,11). This RANK/RANKL/OPG system is regulated by various cytokines (interleukin (IL)-1, 4, 6, 11 and 17 and TNF-α), hormones (glucocorticoids, vitamin D and estrogen), and mesenchymal transcription factors (cbfa-1 and peroxisome proliferator-activated receptor gamma) (9) and determines osteoclast activity (Figure 1).
Figure 1.
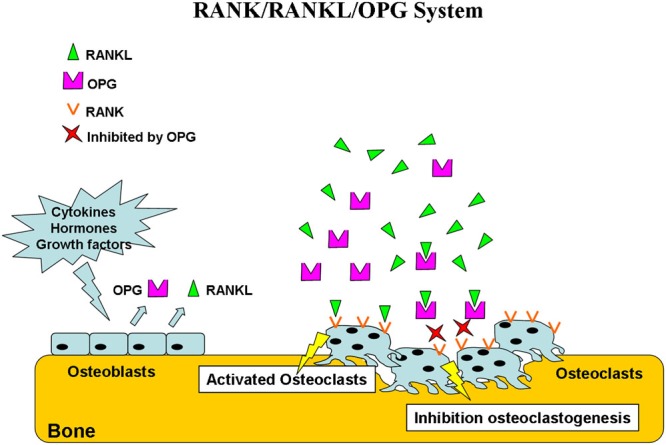
RANK/RANKL/OPG system. Osteoblasts produce RANKL and OPG under the control of various cytokines, hormones, and growth factors. OPG binds and inactivates RANKL, resulting in the inhibition of osteoclastogenesis. In the absence of OPG, RANKL activates its receptor, RANK, expressed on osteoclasts and preosteoclast precursors. The RANK-RANKL interaction leads to preosteoclast recruitment and fusion into multinucleated osteoclasts and to osteoclast activation and survival.
Bone is continually reabsorbed and formed. This process is called bone remodeling, in which bone cells have an extremely important role in ensuring a balance between the processes of bone formation and resorption. When this balance is disrupted, various diseases and conditions, such as osteogenesis imperfecta, tumors, osteoarthritis and osteoporosis may arise. In particular, osteoporosis is characterized by a progressive loss of bone mass and microarchitecture, which leads to increased fracture risk.
Currently, the available drugs used in the treatment of bone diseases can be divided into two categories: antiresorptive agents, such as bisphosphonates (BPs), estrogen, selective estrogen receptor modulators (SERMs) and RANKL inhibitors that inhibit osteoclastogenesis and bone-forming agents that increase bone strength by increasing bone mass, such as parathyroid hormone (PTH) peptides, strontium ranelate (SR) and anti-Dickkopf-related protein 1 (DKK1) and anti-sclerostin (SOST) antibodies (Figure 2 and Table 1).
Figure 2.
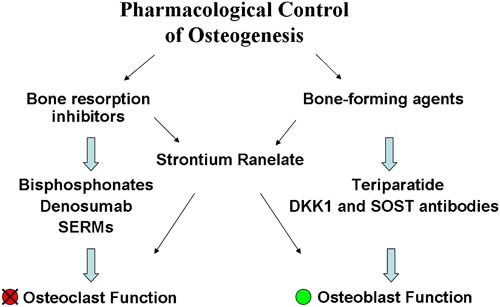
Summary of the main drugs used in the control of osteogenesis.
Table 1.
Comparisons of the principal drugs used in bone diseases.
| Drugs | Bone resorption inhibitors | Bone-forming agents | In vitro effects | In vivo effects |
| Bisphosphonates |  |
- Inhibition of osteoclast activity and differentiation- Induction of osteoclast apoptosis | - Reduction in the risk ofnew vertebral,non-vertebral, and hipfractures | |
| Denosumab |  |
- Inhibition of osteoclast differentiation, activation and survival | - Increase in bone mass and decrease in the risk of fractures | |
| Selective Estrogen Receptor Modulators (SERMs) |  |
- Reduction in the number ofpreosteoclasts and matureosteoclasts | - Reduction in the risk ofnew vertebral fractures | |
| Intermittent PTH1-34 Therapy |  |
- Increase in the number andactivity of osteoblasts- Increase in osteogenesisand chondrogenesis duringskeletal repair | - Increase in bone mineraldensity- Enhancement of the corticalthickness and trabecularbone volume andimproved bonemicroarchitecture | |
| Strontium Ranelate |  |
 |
- Induction of the osteoblasticdifferentiation of human MSCs- Increase in osteoblastproliferation, survival anddifferentiation- Reduction in osteoblastapoptosis- Decrease in osteoclastdifferentiation- Increase in osteoclastapoptosis | - Increase in bone mineraldensity- Reduction in the risk ofnew vertebral, non-vertebral, and hipfractures |
| Anti-DKK1 and Anti-SOST Antibodies |  |
- Increase in osteoblastogenesisand proliferation | - Increased bone formation,trabecular thickness, and bonemass and strength |
The purpose of this review is to provide an overview of the drugs commonly used for the control of osteogenesis in bone diseases.
Bisphosphonates
BPs are a class of drugs generally used in the treatment of bone disorders that are characterized by excessive osteoclastic bone resorption, such as osteoporosis, Paget's disease, fibrous dysplasia, hypercalcemia of malignancy, and inflammation-related bone loss (12-15).
The clinical efficacy of BPs primarily stems from two key properties: their ability to bind strongly to bone mineral and their inhibitory effects on mature osteoclasts (16).
In fact, these drugs are able to bind with high affinity to hydroxyapatite crystals, where they remain for prolonged periods. The drugs then act selectively on osteoblasts, particularly in areas of high bone turnover, resulting in an antiresorptive effect (17,18). The drugs are subsequently released from the bone matrix upon exposure to acid and enzymes secreted by active osteoclasts (19,20).
Studies to date suggest that the mechanisms by which BPs are internalized by osteoclasts are similar for different BPs, which can be divided into two categories: nitrogen-containing BPs and non- nitrogen-containing BPs.
Nitrogen-containing BPs, such as alendronate, ibandronate, pamidronate, risedronate, and zoledronate, have a side chain that contains a nitrogen atom, in contrast to the non-nitrogen-containing BPs, such as clodronate and etidronate.
Nitrogen-containing BPs principally act by inhibiting farnesyl pyrophosphate (FPP) synthase, an enzyme in the cholesterol synthesis pathway and preventing the prenylation of small guanosine triphosphate (GTP)-binding proteins, which are indispensable for cytoskeletal organization and vesicular traffic in the osteoclast, causing osteoclast inactivation (21,22).
In contrast, in osteoclasts' cytosol, non-nitrogen-containing BPs are metabolized into adenosine triphosphate (ATP) analogs that block osteoclast function and induce osteoclast apoptosis (23).
In vitro, several BPs inhibit osteoclast differentiation in human bone marrow cultures (24) and promote the apoptosis of murine osteoclasts, which was also confirmed by in vivo studies in mice. More specifically, in vitro studies have shown that BPs are not always selective for osteoclasts and can inhibit cell growth and induce apoptosis in a wide range of cell types (16,19), and in many cancer cell types (20) at high doses.
In the 1990s, in vitro studies demonstrated that osteoblasts treated with BPs did not exhibit osteoclastogenesis (29,30). Additionally, numerous studies performed to evaluate the effects of BPs on osteoblasts have demonstrated the non-selectivity of these drugs for osteoclastic cells.
In addition, BPs are able to inhibit the apoptosis of osteocyte cell lines and primary murine osteoblasts (31), as well as human osteoblasts (32).
Nitrogen-containing BPs appear to induce collagen type I (COLIA1) gene expression (28). Moreover, alendronate and etidronate enhance IL-6 production in osteoblasts (33).
Clodronate stimulates osteoblast differentiation in ST2 and MC3T3-E1 cells, whereas etidronate promotes osteoinduction only in MC3T3-E1 cells (34). In addition, it has been shown that BPs decrease the expression of RANKL and increase the expression of OPG in human osteoblastic cells (35,36). Finally, trabecular cultures of MG-63 cells and primary human bone have shown that risedronate and alendronate each increase osteoblast and osteoblast progenitor numbers and also enhance the gene expression of bone morphogenetic protein 2 (BMP-2), COLIA1, and osteocalcin (OCN) (37,38).
It has been demonstrated that these drugs increase the proliferation and formation of mineralized nodules in murine and human bone marrow cultures in vitro (25), and promote early osteoblastogenesis in both young and aged mice in vivo (39). In contrast, other studies have demonstrated that BPs decrease proliferation and inhibit osteoblast differentiation and mineralization (27,28,43,44). In particular, an in vitro study has demonstrated that pamidronate and zoledronate decrease osteoblast proliferation in a dose-dependent manner and increase differentiation and bone-forming activities among immortalized human fetal osteoblasts (28). However, another in vitro study on mouse calvarial osteoblasts has shown that pamidronate and alendronate inhibit osteoblast growth and bone nodule formation (43).
These conflicting results are explained by the fact that low concentrations of BPs, from 10−9 M to 10−6 M, were shown to increase growth and have induction effects, whereas concentrations higher than 10−5 M had inhibitory effects (45). Finally, BPs such as alendronate, risedronate, and zoledronate have been shown to reduce the risk of new vertebral, non-vertebral, and hip fractures (46-49). Interestingly, the long-term use (up to 10 years) of BPs in the treatment of osteoporosis has been associated with a good safety profile (50), although several studies have associated BP therapy with a potential risk of osteonecrosis of the jaw and atypical subtrochanteric femoral fractures (51-53).
Denosumab
The RANK/RANKL/OPG pathway is key to maintaining the balance between the activities of osteoblasts and osteoclasts to prevent bone loss and ensure normal bone turnover. Thus, manipulation of the RANKL system has been a target of pharmaceutical development. In particular, human OPG constructs, such as OPG fusion proteins (OPG-Fc) (54), have been valuable research tools because they strongly inhibit bone resorption in a variety of species, including rats (55,56), pigs (57), monkeys (58), and humans (54,59). However, the clinical development of OPG-Fc was abandoned in favor of denosumab due to several limitations concerning half-life and specificity. Denosumab (AMG 162) is currently the only RANKL-targeted therapy available, offering a new approach in the treatment of osteoporosis (60,61). This human monoclonal IgG2 antibody was developed using transgenic mouse technology. Denosumab binds RANKL with high affinity and specificity, thereby inhibiting osteoclastogenesis, as demonstrated by numerous studies (61-65) and also increasing bone mass and reducing the risk of fractures (66).
Finally, several studies have demonstrated that denosumab is able to reduce the expression of specific markers of bone resorption in postmenopausal women (67) and in subjects with bone metastases or multiple myeloma (68).
Selective Estrogen Receptor Modulators
SERMs, such as estrogen, are potent inhibitors of bone resorption and are currently Food and Drug Administration (FDA) approved for the prevention and treatment of osteoporosis in postmenopausal women (69). In particular, estrogen is a systemic hormone with direct effects on bone that plays an important role in osteoporosis. In postmenopausal women, the deficiency of estrogen leads to an upregulation of RANKL on bone marrow cells, resulting in an increase in bone resorption (70).
In contrast, estrogen itself stimulates OPG production in osteoblasts and thus exerts antiresorptive effects on bone (71). The extraskeletal effects of estrogen deficiency are mainly based on increased renal calcium excretion and decreased intestinal calcium absorption (72,73). Tamoxifen was the first SERM to be widely used in clinical practice, based on its now well-recognized estrogen antagonist activity in the breast.
The prolonged use of tamoxifen was associated with an increase in uterine cancer (74), leading to the search for other SERMs with different pharmacological profiles. Thus, raloxifene, a new SERM, was developed for the treatment and prevention of postmenopausal osteoporosis, with the goal of improving the drug safety profile. Raloxifene has a spectrum of tissue-specific agonist-antagonist effects on estrogen target tissues but acts on bone as an estrogen agonist (75). This drug has been extensively studied and data support its estrogen agonist profile in the skeletal system. The drug specifically acts on estrogenic receptor-α and estrogenic receptor-β, binding to the receptors in the same ligand-binding pocket as does estradiol, and causes the C-terminal α-helix of the receptor to change its conformation to block access to the activation function-2 region of the receptor. This event in turn likely blocks access to the transcriptional coactivators necessary to facilitate the activation of estrogen-responsive genes (76). In the ovariectomized (OVX) rat model, raloxifene acts as an antiresorptive, with preservation of both bone mineral density (BMD) and bone strength (76). It has been demonstrated that raloxifene modulates the homeostasis of bone cells in vitro by inhibiting osteoclastogenesis and bone resorption, reducing the number of preosteoclasts and mature osteoclasts in OVX rats (77) by suppressing osteoblast apoptosis and increasing osteoblast proliferation and differentiation in MC3T3-E1 cultures (78-80). Other studies in OVX rats have shown that raloxifene was able to decrease RANKL and increase OPG expression (77,81,82). Finally, an in vitro study on human fetal osteoblast cell lines treated with raloxifene, which expressed a G-protein-coupled receptor (GPR30) but lacked estrogen receptor, has shown that this drug was able to induce cell proliferation, although the function of GPR30 in bone remains unclear (83).
Parathyroid Hormone Therapy
The first molecule to be approved by the FDA as the only anabolic therapy for osteoporosis was a PTH analog (84). This analog is available in the form of human recombinant PTH peptide 1-34 (teriparatide, or PTH1-34), a fragment of PTH that has similar affinity for PTH receptor-1.
PTH is released from the parathyroid gland, and its secretion is chiefly controlled by serum [Ca2+] through negative feedback (85).
Pharmacologically, when PTH is administered intermittently (once daily) at low doses, it has an anabolic effect on osteoblasts (85), stimulating bone formation both in vitro and in vivo and increasing in BMD (84).
Many studies have demonstrated the efficacy of PTH1-34 therapy in a variety of skeletal repair models, suggesting that PTH1-34 enhanced and accelerated not only bone remodeling but also osteogenesis and chondrogenesis during skeletal repair (87). In 1999, Andreassen et al. were the first to report the efficacy of intermittent PTH1-34 therapy on rat tibial fracture healing (88). In particular, it has been shown that intermittent PTH administration promotes bone formation by increasing the number and activity of osteoblasts, enhances the mean cortical thickness and trabecular bone volume and improves bone microarchitecture (89). At the molecular level, PTH enhances Wnt signaling through inhibition of the Wnt antagonist SOST and induces the local production of bone anabolic growth factors such as insulin-like growth factor 1 (IGF1) (86). Furthermore, PTH1-34 enhances the differentiation of mesenchymal stem cells (MSCs) into osteoblasts via the induction of osterix (OSX) and Runt-related transcription factor 2 (RUNX-2) expression in vitro, increasing both OSX expression at the fracture site in vivo and the expression of osteoblastic marker genes, including COLIA1 and OCN (90). Several studies have shown that PTH1-34 can promote the proliferation and differentiation of MSCs in the early phase of bone healing (91) and to induce the proliferation of chondroprogenitors at a fracture site, contributing to increased bone formation during fracture healing (92) and accelerating articular cartilage repair (87,93,94), respectively. These data were supported by clinical studies that have demonstrated positive effects of intermittent PTH therapy, including increasing bone mass and reducing the bone fragility associated with osteoporosis due to age, sex hormone deficiency and glucocorticoid therapy (95).
Conversely, in certain studies, toxicity has been reported for the use of PTH therapy. In particular, the toxic effect of treatment with teriparatide or parathyroid hormone 1-84, which appears to be unique to animals and not applicable to human subjects, is osteosarcoma (96). In fact, it has been reported that rats treated with high doses of either teriparatide or parathyroid hormone 1-84 for prolonged periods of time developed osteosarcoma (97-99).
Treatment with teriparatide is approved by the FDA for a limited duration, from 18-24 months and in many European countries, approval is limited to 18 months. However, in several studies, the period of treatment with teriparatide was prolonged to 24-30 months (100,101).
Although it has been reported that the teriparatide-related risk of osteosarcoma development is low (102), there are still no clear scientific data. The general recommendation for this treatment is to closely follow patients who have risk factors, i.e., subjects with Paget's disease, prior skeletal irradiation, or unexplained increases in serum bone-specific alkaline phosphatase (ALP) and adolescents in whom the epiphyses have not yet closed (96).
Strontium Ranelate
SR is a drug commonly used for the treatment of osteoporosis and fragility fractures (103,104). SR consists of two cations of strontium, representing the active component, and one anion of ranelate, which acts as a carrier (105). In contrast to other drugs, SR has a dual effect on bone remodeling, both stimulating bone formation and decreasing bone resorption. In vitro experiments have shown that SR increased osteoblastic activity, enhancing preosteoblastic cell proliferation and differentiation (7,11,106,107) and stimulating osteoblastic differentiation markers, such as ALP, hydroxyapatite (HA) deposit formation, bone sialoprotein (BSP), and OCN, in primary murine osteoblasts (108).
In addition, in in vitro animal models, SR was observed to reduce osteoblast apoptosis (9,107) and to decrease osteoclast differentiation marker expression, with an enhancement of osteoclast apoptosis (109-111). Furthermore, in vitro data on primary human osteoblasts indicate that this drug promotes the ultimate differentiation of osteoblasts into osteocytes, as indicated by the increased expression of SOST, a marker of osteoblast differentiation (106). In vitro studies on rodent (112,113) and human (106) primary osteoblast cultures have shown that SR, similar to calcium, acts as an agonist of the calcium-sensing receptor (CaSR), promotes cell proliferation (106,112) via activation of the CaSR, and increases bone cell differentiation (106,113) and bone cell survival (106).
SR induces osteoblastic differentiation of human MSCs, stimulating the expression of genes of the bone extracellular matrix: COLIA1, BSP, OCN and RUNX-2. These genes are essential for osteoinduction (114). Furthermore, numerous studies using various cellular models have been performed to evaluate the effects of strontium in combination with different biomaterials on osteogenesis (115-119). In particular, it has been demonstrated that strontium released into the culture medium by a previously loaded amidated carboxymethylcellulose (CMCA) hydrogel was able to promote osteoinduction as detected based on the production of ALP and the formation of HA deposits in a clonal cell line derived from human adipose tissue-derived MSCs (120).
Wnt/β-catenin Pathway Antagonists
The Wnt/β-catenin pathway plays an important role in the main processes controlling osteogenesis (121). This pathway regulates the gene transcription of proteins important for osteoblast function (63).
In vitro and in vivo experiments have shown that activation of the canonical Wnt/β-catenin pathway induces the cellular replication and differentiation of osteoblasts, reducing adipogenic differentiation in MSCs (122,123).
The Wnt pathway is composed of Wnt proteins, frizzled transmembrane receptors and low-density lipoprotein receptor-related protein 5/6 (LRP5/6). Wnt signaling is activated by the presence of the Wnt ligand, which interacts with its receptor, thereby inhibiting the receptor. This interaction leads to cytoplasmic accumulation of β-catenin, which translocates to the nucleus, activating a fundamental transcription factor, RUNX-2, involved in osteogenic differentiation. However, in the absence of the Wnt ligand, β-catenin is phosphorylated by glycogen synthase kinase 3 beta (GSK3B), leading to its degradation, and gene transcription is halted (124). Various studies have demonstrated that modifications in Wnt signaling contributed to age-related bone loss in mouse models (125). In aged or OVX osteopenic mice, with the use of GSK3B, the Wnt signaling cascade enhanced bone formation and increased trabecular and cortical bone density and bone strength (126,127).
Studies of the Wnt/β-catenin pathway have led to the further discovery of inhibitors of Wnt signaling that are secreted by osteocytes. These inhibitors include SOST and DKK1 protein, which are Wnt antagonists specific to bone. Both block the binding of Wnt to LRP5, thereby inhibiting osteoblast stimulation (64,128). In fact, it has been observed that a loss-of-function mutation of SOST leads to an increase in bone formation and bone mass (129). Many forms of cancer are associated with such mutations within the Wnt signaling pathway (130,131).
Currently, based on promising results in animal models, monoclonal antibodies designed to block the inhibitory action of both SOST and DKK1 have been introduced for use in clinical trials (65,66,132-134).
The development of pharmacological SOST and DKK1 antagonists that increase bone formation and bone mass is a new strategy in the treatment of bone disorders. In vivo studies on monkeys and OVX rats have shown that systemic administration of an anti-SOST MAB increased bone formation, bone mass, and strength (135). Furthermore, the anti-SOST antibody was able to enhance bone formation markers in postmenopausal women (136). Finally, an increase in bone formation on trabecular, periosteal, endocortical, and intracortical surfaces, without increased bone resorption and with enhanced trabecular thickness, BMD and bone strength, was shown in preclinical studies with the administration of SOST-neutralizing monoclonal antibodies (137,138).
Today, the prevention and treatment of several bone disorders are possible. This progress is due to the development of a variety of drugs that act to halt excessive bone resorption by inhibiting osteoclasts or by promoting bone formation.
BPs such as alendronate and zoledronic acid have been demonstrated to significantly reduce the risk of vertebral, non-vertebral and femoral fractures by decreasing bone remodeling via the inhibition of osteoclasts with increased bone mass, although their long-term use has been correlated with the occurrence of atypical femoral fractures (53). However, a new approach targeting the inhibition of osteoclast activity inhibits RANKL, which is involved in the survival and differentiation of mature osteoclasts. Denosumab is among the RANKL inhibitors that have been most studied and used, and numerous studies have demonstrated that denosumab exerts an inhibitory action on osteoclastogenesis (61-65).
In animal models, it has been demonstrated that teriparatide accelerates bone fracture healing, thereby enhancing bone remodeling (139). In studies of bone histomorphometry, PTH1-34 was able to increase the trabecular bone mass in postmenopausal women (140). Raloxifene also modulates the homeostasis of bone cells in vitro by inhibiting osteoclastogenesis and bone resorption, with a reduction in the numbers of preosteoclasts and mature osteoclasts in OVX rats (77). Additionally, it has been shown that raloxifene suppressed osteoblast apoptosis in MC3T3-E1 cells (78) and increased osteoblast proliferation and differentiation in murine cell cultures (79,80). Finally, in vitro studies have demonstrated that SR promotes the survival, proliferation and differentiation of osteoblasts and inhibits osteoclastic activity and clinical studies have shown that SR improves bone strength, increasing BMD. Furthermore, no change in the porosity of bone was evident in patients treated with SR (141).
In conclusion, an understanding of the molecular and cellular mechanisms of bone fragility is essential for the development of successful cell therapies that support new pharmacological approaches.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 3.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95(11):1046–57. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 4.Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, et al. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Histol. 2005;36(1-2):59–67. doi: 10.1007/s10735-004-3839-1. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng QJ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 6.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96(7):3540–5. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca JE. Rebalancing bone turnover in favour of formation with strontium ranelate: Implications for bone strength. Rheumatology (Oxford) 2008;47 Suppl 4:iv17–19. doi: 10.1093/rheumatology/ken165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9 Suppl 1:S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappa B ligand and osteopr otegerin in bone cell biology. J Mol Med. 2001;79(5-6):243–53. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 10.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292(4):490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 12.Eggelmeijer F, Papapoulos SE, Van Paassen HC, Dijkmans BA, Breedveld FC. Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: a double blind placebo controlled study. J Rheumatol. 1994;21(11):2016–20. [PubMed] [Google Scholar]
- 13.Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, De Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright sindrome. Acta Paediatr. 2000;89(2):188–93. doi: 10.1080/080352500750028816. [DOI] [PubMed] [Google Scholar]
- 14.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 15.Lane JM, Khan SN, O'Connor WJ, Nydick M, Hommen JP, Schneider R, Tomin E, et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop. 2001;(382):6–12. doi: 10.1097/00003086-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D'Alessandro A, et al. Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer. 2003;88(12):1971–8. doi: 10.1038/sj.bjc.6600986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Jung A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates on hydroxyapatite crystals. Calcif Tissue Res. 1973;11(4):269–80. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- 19.Sudhoff H, Jung JY, Ebmeyer J, Faddis BT, Hildmann H, Chole RA. Zoledronic acid inhibits osteoclastogenesis in vitro and in a mouse model of inflammatory osteolysis. Ann Otol Rhinol Laryngol. 2003;112(9 Pt 1):780–6. doi: 10.1177/000348940311200907. [DOI] [PubMed] [Google Scholar]
- 20.Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM. Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone. 2003;33(2):216–28. doi: 10.1016/s8756-3282(03)00223-0. [DOI] [PubMed] [Google Scholar]
- 21.Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9 Suppl 4:3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 22.Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2(6):571–7. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- 23.D'Aoust P, McCulloch CA, Tenenbaum HC, Lekic PC. Etidronate (HEBP) promotes osteoblast differentiation and wound closure in rat calvaria. Cell Tissue Res. 2000;302(3):353–63. doi: 10.1007/s004419900165. [DOI] [PubMed] [Google Scholar]
- 24.Hughes DE, MacDonald BR, Russell RGG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989;83(6):1930–5. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26(34):6941–9. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Still K, Phipps RJ, Scutt A. Effects of risedronate, alendronate, and etidronate on the viability and activity of rat bone marrow stromal cells in vitro. Calcif Tissue Int. 2003;72(2):143–50. doi: 10.1007/s00223-001-2066-y. [DOI] [PubMed] [Google Scholar]
- 27.Reinholz GG, Getz B, Sanders ES, Karpeisky MY, Padyukova NS, Mikhailov SN, et al. Distinct mechanisms of bisphosphonate action between osteoblasts and breast cancer cells: identity of a potent new bisphosphonate analogue. Breast Cancer Res Treat. 2002;71(3):257–68. doi: 10.1023/a:1014418017382. [DOI] [PubMed] [Google Scholar]
- 28.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60(21):6001–7. [PubMed] [Google Scholar]
- 29.Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993;91(5):2004–11. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa M, Akatsu T, Katayama Y, Yasutomo Y, Kado S, Kugal N, et al. Bisphosphonates act on osteoblastic cells and inhibit osteoclast formation in mouse marrow cultures. Bone. 1996;18(1):9–14. doi: 10.1016/8756-3282(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104(10):1363–74. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Y Abe Y, Kawakami A, Nakashima T, Ejima E, Fujiyama K, Kiriyama T, et al. Etidronate inhibits human osteoblast apoptosis by inhibition of pro-apoptotic factor(s) produced by activated T cells. J Lab Clin Med. 2000;136(5):344–54. doi: 10.1067/mlc.2000.109757. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani N, Pedrazzoni M, Passeri G, Girasole G. Bisphosphonates inhibit IL-6 production by human osteoblast-like cells. Scand. J. Rheumatol. 1998;27(1):38–41. doi: 10.1080/030097498441155. [DOI] [PubMed] [Google Scholar]
- 34.Itoh F, Aoyagi S, Furihata-Komatsu H, Aoki M, Kusama H, Kojima M, et al. Clodronate stimulates osteoblast differentiation in ST2 and MC3T3-E1 cells and rat organ cultures. Eur J Pharmacol. 2003;477(1):9–16. doi: 10.1016/j.ejphar.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Grundker C, et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun. 2002;291(3):680–6. doi: 10.1006/bbrc.2002.6510. [DOI] [PubMed] [Google Scholar]
- 36.Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K, et al. The nitrogencontaining bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblastlike cells by activating TNF-alpha converting enzyme (TACE) J Bone Miner Res. 2004;19(1):147–54. doi: 10.1359/jbmr.2004.19.1.147. [DOI] [PubMed] [Google Scholar]
- 37.Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25(18):4105–15. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Yang HJ, Feng J, Shi ZL, Wu LD. Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res. 2009;37(2):407–16. doi: 10.1177/147323000903700216. [DOI] [PubMed] [Google Scholar]
- 39.Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22(5):455–61. doi: 10.1016/s8756-3282(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 40.Klein BY, Ben-Bassat H, Breuer E, Solomon V, Golomb G. Structurally different bisphosphonates exert opposing effects on alkaline. J Cell Biochem. 1998;68(2):186–94. [PubMed] [Google Scholar]
- 41.Kim HK, Kim JH, Abbas AA, Yoon TR. Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop Relat Res. 2009;467(12):3121–8. doi: 10.1007/s11999-008-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S, et al. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone. 2004;34(1):112–23. doi: 10.1016/j.bone.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Idris AI, Rojas J, Greig IR, Van't Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008;82(3):191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 44.Orriss IR, Key ML, Colston KW, Arnett TR. Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem. 2009;106(1):109–18. doi: 10.1002/jcb.21983. [DOI] [PubMed] [Google Scholar]
- 45.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone. 2011;49(1):50–5. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 47.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 48.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 49.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 50.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 51.Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, Felsenberg D, et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42(5):841–7. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a european society on clinical and economic aspects of osteoporosis and osteoarthritis, and international osteoporosis foundation working group report. Osteoporos Int. 2011;22(2):373–90. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–94. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 54.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97(3 Suppl):887–92. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 55.Stolina M, Adamu S, Ominsky MS, Dzamba BJ, Asuncion F, Geng Z, et al. RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. J Bone Miner Res. 2005;20(10):1756–65. doi: 10.1359/JBMR.050601. [DOI] [PubMed] [Google Scholar]
- 56.Padagas J, Colloton M, Shalhoub V, Kostenuik PJ, Morony S, Munyakazi L, et al. The receptor activator of nuclear factor-kB ligand inhibitor osteoprotegerin is a bone-protective agent in a rat model of chronic renal insufficiency and hyperparathyroidism. Calcif Tissue Int. 2006;78(1):35–44. doi: 10.1007/s00223-005-0161-1. [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Morgan-Bagley S, Kostenuik PJ. RANKL inhibition: A novel strategy to decrease femoral head deformity after ischemic necrosis. J Bone Miner Res. 2006;21(12):1946–54. doi: 10.1359/jbmr.060905. [DOI] [PubMed] [Google Scholar]
- 58.Ominsky MS, Kostenuik PJ, Cranmer P, Smith SY, Atkinson JE. The RANKL inhibitor OPG-Fc increases cortical and trabecular bone mass in intact cynomolgus monkeys. Osteoporos Int. 2007;18(8):1073–82. doi: 10.1007/s00198-007-0363-7. [DOI] [PubMed] [Google Scholar]
- 59.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001;16(2):348–60. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 60.European Public Assessment Report. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001120/human_med_001324.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124 (last accessed 2011)
- 61.Rizzoli R, Yasothan U, Kirkpatrick P. Denosumab. Nat Rev Drug Discov. 2010;9(8):591–2. doi: 10.1038/nrd3244. [DOI] [PubMed] [Google Scholar]
- 62.Belavic JM. Denosumab (Prolia): a new option in the treatment of osteoporosis. Nurse Pract. 2011;36(1):11–2. doi: 10.1097/01.NPR.0000391178.47878.73. [DOI] [PubMed] [Google Scholar]
- 63.Lipton A, Goessl C. Clinical development of anti- RANKL therapies for treatment and prevention of bone metastasis. Bone. 2011;48(1):96–9. doi: 10.1016/j.bone.2010.10.161. [DOI] [PubMed] [Google Scholar]
- 64.Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Lewiecki EM. Treatment of osteoporosis with denosumab. Maturitas. 2010;66(2):182–6. doi: 10.1016/j.maturitas.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 67.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 68.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kB ligant inhibitor, Denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12(4):1221–8. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 69.Khajuria DK, Razdan R, Mahapatra DR. Drugs for the management of osteoporosis: a review. Rev Bras Reumatol. 2011;51(4):365–71. 379–82. [PubMed] [Google Scholar]
- 70.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32(2):136–41. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 72.McKane WR, Khosla S, Burritt MF, Kao PC, Wilson DM, Ory SJ, et al. Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause - a clinical research center study. J Clin Endocrinol Metab. 1995;80(12):3458–64. doi: 10.1210/jcem.80.12.8530583. [DOI] [PubMed] [Google Scholar]
- 73.Gennari C, Agnusdei D, Nardi P, Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71(5):1288–93. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- 74.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 75.Cosman F, Lindsay R. Selective estrogen receptor modulators: Clinical spectrum. Endocr Rev. 1999;20(3):418–34. doi: 10.1210/edrv.20.3.0371. [DOI] [PubMed] [Google Scholar]
- 76.Muchmore DB. Raloxifene: A Selective Estrogen Receptor Modulator (SERM) with Multiple Target System Effects. Oncologist. 2000;5(5):388–92. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- 77.Luvizuto ER, Queiroz TP, Dias SM, Okamoto T, Dornelles RC, Garcia IR, Jr, et al. Histomorphometric analysis and immunolocalization of RANKL and OPG during the alveolar healing process in female ovariectomized rats treated with oestrogen or raloxifene. Arch Oral Biol. 2010;55:52–9. doi: 10.1016/j.archoralbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Olivier S, Fillet M, Malaise M, Piette J, Bours V, Merville MP, et al. Sodium nitroprusside-induced osteoblast apoptosis is mediated by long chain ceramide and is decreased by raloxifene. Biochem Pharmacol. 2005;69(6):891–901. doi: 10.1016/j.bcp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 79.Taranta A, Brama M, Teti A, De luca V, Scandurra R, Spera G, Agnusdeiet al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002;30(2):368–76. doi: 10.1016/s8756-3282(01)00685-8. [DOI] [PubMed] [Google Scholar]
- 80.Viereck V, Gründker C, Blaschke S, Niederkleine B, Siggelkow H, Frosch KH, et al. Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. J Clin Endocrinol Metab. 2003;88(9):4206–13. doi: 10.1210/jc.2002-021877. [DOI] [PubMed] [Google Scholar]
- 81.Kawamoto S, Ejiri S, Nagaoka E, Ozawa H. Effects of oestrogen deficiency on osteoclastogenesis in the rat periodontium. Arch Oral Bio. 2002;47(1):67–73. doi: 10.1016/s0003-9969(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 82.Michael H1, Härkönen PL, Kangas L, Väänänen HK, Hentunen TA. Differential effects of selective oestrogen receptor modulators (SERMs) tamoxifen, ospemifene and raloxifene on human osteoclasts in vitro. Br J Pharmacol. 2007;151(3):384–95. doi: 10.1038/sj.bjp.0707232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noda-Seino H, Sawada K, Hayakawa J, Ohyagi-Hara C, Mabuchi S, Takahashi K, et al. Estradiol and Raloxifene induce the proliferation of osteoblasts through G-protein-coupled receptor GPR30. J Endocrinol Invest. 2013;36(1):21–7. doi: 10.3275/8301. [DOI] [PubMed] [Google Scholar]
- 84.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 85.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–80. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 86.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bukata SV, Puzas JE. Orthopedic uses of teriparatide. Curr Osteoporos Rep. 2010;8(1):28–33. doi: 10.1007/s11914-010-0006-3. [DOI] [PubMed] [Google Scholar]
- 88.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14(6):960–8. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 89.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40(6):1447–52. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Kaback LA, Soung do Y, Naik A, Geneau G, Schwarz EM, Rosier RN, et al. Teriparatide (1-34 human PTH) regulation of osterix during fracture repair. J Cell Biochem. 2008;105(1):219–26. doi: 10.1002/jcb.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34) J Bone Miner Res. 2002;17(11):2038–47. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 92.Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37(5):711–9. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Bukata SV, Puzas JE. Orthopedic Uses of Teriparatide. Curr Osteoporos Rep. 2010;8(1):28–33. doi: 10.1007/s11914-010-0006-3. [DOI] [PubMed] [Google Scholar]
- 94.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. Journal of bone and mineral research. J Bone Miner Res. 2007;22(12):1903–12. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 95.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 96.Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int. 2010;21(6):1041–5. doi: 10.1007/s00198-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 97.Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–39. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 98.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32(4):426–38. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 99.Wilker C, Jolette J, Smith S, Doyle N, Hardisty J, Metcalfe AJ, et al. A no observable carcinogenic effect dose level identified in Fischer 344 rats following daily treatment with PTH(1-84) for 2 years: role of the C-terminal PTH receptor? J Bone Miner Res. 2004;19 Supp 1:SA435. [Google Scholar]
- 100.Ryder KM, Tanner SB, Carbone L, Williams JE, Taylor HM, Bush A, et al. Teriparatide is safe and effectively increases bone biomarkers in institutionalized individuals with osteoporosis. J Bone Miner Metab. 2010;28(2):233–9. doi: 10.1007/s00774-009-0123-1. [DOI] [PubMed] [Google Scholar]
- 101.Losada B, Zanchetta J, Zerbini C, Molina J, de la Pena P, Liu C, et al. Active comparator trial of teriparatide vs alendronate for treating glucocorticoid-induced osteoporosis: results from the Hispanic and non-Hispanic cohorts. J Clin Densitom. 2009;12(1):63–70. doi: 10.1016/j.jocd.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Harper KD, Krege JH, Marcus R, Mitlak BH. Osteosarcoma and teriparatide. J Bone Miner Res. 2007;22(2):334. doi: 10.1359/jbmr.061111. [DOI] [PubMed] [Google Scholar]
- 103.Cesareo R, Napolitano C, Iozzino M. Strontium ranelate in postmenopausal osteoporosis treatment: a critical appraisal. Int J Womens Health. 2010;2:1–6. [PMC free article] [PubMed] [Google Scholar]
- 104.Roux C, Fechtenbaum J, Kolta S, Isaia G, Andia JB, Devogelaer JP. Strontium ranelate reduces the risk of vertebral fracture in young postmenopausal women with severe osteoporosis. Ann Rheum Dis. 2008;67(12):1736–8. doi: 10.1136/ard.2008.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marie P. Strontium ranelate: a novel mode of action optimizing bone formation and resorption. Osteoporos Int. 2005;16:7–10. doi: 10.1007/s00198-004-1753-8. [DOI] [PubMed] [Google Scholar]
- 106.Brennan TC, Rybchyn MS, Green W, Atwa S, Conigrave AD, Mason RS. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol. 2009;157(7):1291–1300. doi: 10.1111/j.1476-5381.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marie PJ. Strontium ranelate: new insights into its dual mode of action. Bone. 2007:40-S5-8. [Google Scholar]
- 108.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42(1):129–38. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 109.Baron R, Tsouderos Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol. 2002;450(1):11–7. doi: 10.1016/s0014-2999(02)02040-x. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi N, Sasaki T, Tsouderos Y, Suda T. S 12911-2 Inhibits osteoclastic bone resorption in vitro. J Bone Miner Res. 2003;18(6):1082–7. doi: 10.1359/jbmr.2003.18.6.1082. [DOI] [PubMed] [Google Scholar]
- 111.Mentaverri R, Hurtel AS, Kamel S, Robin B, Brazier M. Extracellular concentrations of strontium directly stimulates osteoclast apoptosis. J Bone Min Res. 2003;18:M237. [Google Scholar]
- 112.Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol. 2007;74(3):438–47. doi: 10.1016/j.bcp.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 113.Zhu LL, Zaidi S, Peng Y, Zhou H, Moonga BS, Blesius A, et al. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem Biophys Res Commun. 2007;355(2):307–11. doi: 10.1016/j.bbrc.2007.01.120. [DOI] [PubMed] [Google Scholar]
- 114.Sila-Asna M, Bunyaratvej A, Maeda S, Kitaguchi H, Bunyaratavej N. Osteoblast Differentiation and Bone Formation Gene Expression in Strontium-inducing Bone Marrow Mesenchymal Stem Cell. Kobe J Med Sci. 2007;53(1-2):25–35. [PubMed] [Google Scholar]
- 115.Hao Y, Yan H, Wang X, Zhu B, Ning C, Ge S. Evaluation of osteoinduction and proliferation on nano-Sr-HAP: a novel orthopedic biomaterial for bone tissue regeneration. J Nanosci Nanotechnol. 2012;12(1):207–12. doi: 10.1166/jnn.2012.5125. [DOI] [PubMed] [Google Scholar]
- 116.Ni GX, Yao ZP, Huang GT, Liu WG, Lu WW. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med. 2011;22(4):961–7. doi: 10.1007/s10856-011-4264-0. [DOI] [PubMed] [Google Scholar]
- 117.Isaac J, Nohra J, Lao J, Jallot E, Nedelec JM, Berdal A, et al. Effects of strontium-doped bioactive glass on the differentiation of cultured osteogenic cells. Eur Cell Mater. 2011;21:130–43. doi: 10.22203/ecm.v021a11. [DOI] [PubMed] [Google Scholar]
- 118.Liu F, Zhang X, Yu X, Xu Y, Feng T, Ren D. In vitro study in stimulating the secretion of angiogenic growth factors of strontium-doped calcium polyphosphate for bone tissue engineering. J Mater Sci Mater Med. 2011;22(3):683–92. doi: 10.1007/s10856-011-4247-1. [DOI] [PubMed] [Google Scholar]
- 119.Zhang W, Shen Y, Pan H, Lin K, Liu X, Darvell BW, et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011;7(2):800–8. doi: 10.1016/j.actbio.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 120.Nardone V, Fabbri S, Marini F, Zonefrati R, Galli G, Carossino A, et al. Osteodifferentiation of human preadipocytes induced by strontium released from hydrogels. Int J Biomater. 2012;2012:865291. doi: 10.1155/2012/865291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signalling. J Clin Invest. 2006;116(5):1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7(1-2):33–9. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 123.Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22(11):1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 124.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148(6):2635–43. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 125.Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Molecular Endocrinology. 2007;21(11):2605–14. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 126.Clément-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssière B, Belleville C, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, et al. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res. 2006;21(6):910–20. doi: 10.1359/jbmr.060316. [DOI] [PubMed] [Google Scholar]
- 128.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol. 2011;165(1):1–10. doi: 10.1530/EJE-11-0132. [DOI] [PubMed] [Google Scholar]
- 129.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 130.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn. 2010;239(1):102–14. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Enders GH. Wnt therapy for bone loss: golden goose or Trojan horse? J Clin Invest. 2009;119(4):758–60. doi: 10.1172/JCI38973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 134.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–71. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–88. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 136.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 137.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010;25(5):948–59. doi: 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]
- 138.Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010;25(12):2647–56. doi: 10.1002/jbmr.182. [DOI] [PubMed] [Google Scholar]
- 139.Cipriano CA, Issack PS, Shindle L, Werner CM, Helfet DL, Lane JM. Recent advances toward the clinical application of PTH (1-34) in fracture healing. HSS J. 2009;5(2):149–53. doi: 10.1007/s11420-009-9109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. Bone Miner Res. 2005;20(7):1244–53. doi: 10.1359/JBMR.050309. [DOI] [PubMed] [Google Scholar]
- 141.Briot K, Benhamou CL, Roux C. Hip cortical thickness assessment in postmenopausal women with osteoporosis and strontium ranelate effect on hip geometry. J Clin Densitom. 2012;15(2):176–85. doi: 10.1016/j.jocd.2011.11.006. [DOI] [PubMed] [Google Scholar]


