Abstract
Muscle force is typically proportional to muscle size, resulting in constant force normalized to muscle fiber cross-sectional area (specific force). Mice overexpressing insulin-like growth factor-1 (IGF-1) exhibit a proportional gain in muscle force and size, but not the myostatin-deficient mice. In an attempt to explore the role of the cytoplasmic volume supported by individual myonuclei [myonuclear domain (MND) size] on functional capacity of skeletal muscle, we have investigated specific force in relation to MND and the content of the molecular motor protein, myosin, at the single muscle fiber level from myostatin-knockout (Mstn−/−) and IGF-1-overexpressing (mIgf1+/+) mice. We hypothesize that the addition of extra myonuclei is a prerequisite for maintenance of specific force during muscle hypertrophy. A novel algorithm was used to measure individual MNDs in 3 dimensions along the length of single muscle fibers from the fast-twitch extensor digitorum longus and the slow-twitch soleus muscle. A significant effect of the size of individual MNDs in hypertrophic muscle fibers on both specific force and myosin content was observed. This effect was muscle cell type specific and suggested there is a critical volume individual myonuclei can support efficiently. The large MNDs found in fast muscles of Mstn−/− mice were correlated with the decrement in specific force and myosin content in Mstn−/− muscles. Thus, myostatin inhibition may not be able to maintain the appropriate MND for optimal function.—Qaisar, R., Renaud, G., Morine, K., Barton, E. R., Sweeney, H. L., Larsson, L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level?
Keywords: IGF-1, myosin, myostatin
Skeletal muscle plasticity is well documented, and muscle size is large in response to, e.g., functional overload, exercise, and hormonal influences. In general, muscle force is proportional to the cross-sectional area (CSA) of the contractile material (specific force), but a gain in muscle size need not result in maintained specific force. Muscle cells are postmitotically fixed multinuclear cells with hundreds of nuclei dispersed along the length of the cell. Each nucleus controls gene transcription in a finite volume of cytoplasm, the myonuclear domain (MND). The hypothesis that an increase in DNA content is a prerequisite for maintenance of metabolic and work demands and transport distances in hypertrophied fibers is supported in some (1–4), but not all, studies (5, 6).
In this context, two important signaling molecules that regulate muscle growth are insulin-like growth factor 1 (IGF-1) and myostatin (7–9), but they have strikingly different effects on specific force. IGF-1, a protein with anabolic effects on muscle and muscle hypertrophy, has been closely linked with an increased IGF-1 expression. A mouse model has been developed in which IGF-1 is expressed in skeletal muscle via a tissue-restricted transgene (10). These mice show a larger muscle mass than wild-type mice, which are characterized by maintenance of specific force (11–13), and satellite cell activation has been proposed to be the source of new myonuclei in these fibers (4).
Myostatin, a member of the TGF-β superfamily, is a negative regulator of skeletal muscle mass (14). Inhibition of myostatin or its gene results in larger muscle mass than in wild-type mice (14–16). However, this hypertrophy is dissimilar from IGF-1-mediated hypertrophy in that the specific force has been reported to be less in myostatin-null mice (9, 17). However, contractile measurements have typically been measured at the whole-muscle level, and it cannot be excluded that changes in specific force are secondary to changes in muscle fiber orientation rather than changes in the contractile properties at the single muscle fiber level.
Both IGF-1 and myostatin signaling pathways have been considered for the pharmacological treatment of different muscle-wasting conditions, and clinical trials have been initiated with the aim of improving muscle mass and function in patients with severe muscle wasting. Ultimately, the success of these trials will be evaluated with regard to muscle function and patient quality of life, and not solely on the changes in muscle mass. Thus, there is, accordingly, a significant need for an improved mechanistic understanding of the effects of these interventions on skeletal muscle mass and function.
We hypothesize that the myonuclear organization differs between hypertrophic muscle fibers from myostatin-knockout (Mstn−/−) and IGF-1-overexpressing (mIgf1+/+) muscle fibers. This, in turn, will have a significant effect on myofibrillar protein synthesis and turnover, affecting contractile proteins and regulation of muscle contraction at the cross-bridge level. Using a novel algorithm recently developed in our group to analyze the 3-dimensional (3D) organization of MNDs from confocal images (18), we compared the organization of myonuclei in single muscle fibers in parallel with function and myosin content from the fast-twitch extensor digitorum longus (EDL) and the slow-twitch soleus from control, Mstn−/−, and mIgf1+/+ mice.
MATERIALS AND METHODS
All animal experiments were conducted in accordance with the University of Pennsylvania Animal Care and Use Committee. C57 BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA), Mstn−/− were obtained from Dr. Se Jin Lee (Johns Hopkins University, Baltimore, MD, USA), and MyLC/IGF-I (mIgf1+/+) mice have been described in detail elsewhere (10). Four mice were included in each group (control, Mstn−/−, and mIgf1+/+). The age of the mice was 6 mo at the time of sacrifice.
Mice were sedated with a ketamine/xylazine mixture; the soleus and EDL muscles were dissected, weighed, and placed in relaxing solution at ∼110% of resting length. The muscles were chemically skinned for 24 h in relaxing solution with 50% (v/v) glycerol at 4°C, cryoprotected (19), and subsequently stored at −180°C before use. The relaxing solution contained 4 mM MgATP, 1 mM free Mg2+, 20 mM imidazole, 7 mM EGTA, 14.5 mM creatine phosphate, and sufficient KCl to adjust the ionic strength to 180. The pH was adjusted to 7.0. The free Ca2+ concentration, expressed as pCa (−log[Ca2+]), was 10−9 M. Apparent stability constant for Ca2+-EGTA was corrected for temperature and ionic strength (20). All mice were euthanized after removal of muscle tissues.
Single-fiber contractile measurements
The experimental procedure has been described in detail elsewhere (21). Briefly, skinned muscle fibers with an average segment length of 1.60 ± 0.20 mm (mean ± sd, range 1.00–2.00 mm) were exposed to the solution between the connectors of the force transducer. The sarcomere length (SL) of the single-fiber segment was set to 2.65 ± 0.05 μm (range 2.60–2.70 μm) by adjusting the overall segment length. Fiber CSA was calculated from the width and depth, assuming an elliptical circumference. Specific tension was calculated as maximum tension (Po) normalized to CSA and was corrected for the 20% swelling that is known to occur during skinning (22).
Relaxing and activating solutions were prepared as described previously (21). Po was calculated as the difference between the total tension in the activating solution (pCa 4.5) and the resting tension measured in the same segment while in the relaxing solution. All contractile measurements were carried out at 15°C. The contractile recordings were accepted in subsequent analyses only if Po did not change >10% from first to final activation, or if SL during isometric tension development did not change by >0.10 μm compared with SL when the fiber was relaxed (22).
Stiffness
Once steady-state isometric force was reached, small-amplitude sinusoidal changes in length (ΔL: ±0.2% of fiber length) were applied at 500 Hz at one end of the fiber (23). The resultant force response (ΔF) was measured, and the mean of 20 consecutive readings of ΔL and ΔF was used to determine stiffness. The actual elastic modulus (E) was calculated as the difference between E in activating solutions and resting E measured in the same segment in the relaxing solution. E was determined as follows (24):
Fluorescent labeling, image acquisition, and analyses of myonuclear organization
Skinned single-fiber segments were mounted at a fixed SL corresponding to optimal filament overlap for force generation. Actin and myonuclei were stained with rhodamine phalloidin and DAPI, respectively. Confocal images were analyzed by means of a novel algorithm. The volume G of a general elliptic cylinder model was developed and used to calculate the volumes of the MNDs and the CSA of the fiber. The 3D parameters of every nucleus were determined manually, and the MND size was determined by means of automatic image analyses. A detailed description of the procedures is given elsewhere (18).
Enzyme histochemistry
Frozen samples from the soleus muscles from all three groups were cut perpendicular to the longitudinal axis of the muscle fibers into 10-μm cross-sections with a cryotome (2800 Frigo-cut E; Reichert-Jung, Heidelberg, Germany) at −20°C. The sections were stained for the demonstration of myofibrillar ATPase activity after alkaline (pH 10.3) and acid (pH 4.5 and 4.3) preincubations and classified into type I, IIA, and IIB fibers. Succinate dehydrogenase (SDH) staining was performed as described elsewhere (25).
Proximity ligation assay
A proximity ligation assay was performed to quantify myosin using the Duolink in situ PLA kit (Olink Bioscience, Uppsala, Sweden), according to the manufacturer's instructions. Muscle cross-sections from control, Mstn−/−, and mIgf1+/+ mice were fixed on the same glass slide or on different glass slides using 2% paraformaldehyde. MF-20 (1:5; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) was used as the primary antibody. Slides were examined with a confocal microscope (LSM510 Meta; Zeiss, Jena, Germany) under a ×40 objective, and the analysis of the fluorescent signal was performed using Imaris 5.7.2 software (Bitplane AG, Zurich, Switzerland). Assessment of pixel counts was done after applying a constant threshold and selectivity value. Three ×40 fields were randomly chosen for analysis and averaged per condition, examining 3 different samples separately. Whether the sections were on the same slide did not yield any different results.
Single-fiber gel electrophoresis
A detailed description of procedures is given elsewhere (21). In short, the myosin heavy-chain (MyHC) composition of single fibers was determined by SDS-PAGE. The total acrylamide and bis concentrations were 4% (w/v) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Polymerization was activated by adding tetramethylethylenediamine to the stacking (0.1%) and separation gels (0.07%). Sample loads were kept small to improve the resolution of the MyHC bands, and electrophoresis was performed at 120 V for 22–24 h with a Tris-glycine electrode buffer (pH 8.3) at 10°C.
Statistical analysis
A 2-way ANOVA was used to compare fiber CSA, MND, and force measurements across all three groups. Wherever a significant difference was found, Holm-Sidak post hoc test was used for comparisons between groups. Data normality was tested according to Kolmogorov-Smirnov test. Linear regression analysis was done across individual fibers pooled according to MND size and CSA. All values are expressed as means ± se. Values of P < 0.05 were considered statistically significant.
RESULTS
Muscle weights
Higher EDL and soleus muscle weights were observed in both Mstn−/− and mIgf1+/+ mice compared with age- and strain-matched controls (Table 1). In the EDL, both Mstn−/− (59%; P<0.05) and mIgf1+/+ (21%; P<0.05) muscles were heavier than in controls, and Mstn−/− was heavier (P<0.05) than mIgf1+/+. In the soleus, the hypertrophy was 33 and 17% in Mstn−/− and mIgf1+/+ groups compared with controls, respectively, but the difference was statistically significant only in the Mstn−/− group. There was no significant difference between the two experimental groups.
Table 1.
Muscle weights in 6-mo-old control, Mstn−/−, and mIgf1+/+ mice
| Muscle | Control | Mstn−/− | mIgf1+/+ |
|---|---|---|---|
| EDL | 11.25 ± 0.47 | 18.15 ± 0.35*# | 13.7 ± 1.14* |
| Soleus | 10.17 ± 0.25 | 13.57 ± 0.88* | 11.87 ± 0.99 |
Values are expressed as means ± se.
P < 0.05 vs. control;).
P < 0.05 vs. mIgf1+/+.
Fiber CSA and MyHC isoform expression
Muscle fiber CSA was measured in a total of 553 fiber segments from control, Mstn−/−, and mIgf1+/+ EDL (n=296) and soleus (n=257) muscles fibers. All single-fiber CSA measurements were performed at a fixed SL. Significantly larger (P<0.05) CSAs in EDL and type IIa fibers from soleus were observed in both experimental groups compared with controls (Table 2). The hypertrophy was more marked in type IIa fibers from soleus (65 and 45% in Mstn−/− and mIgf1+/+ mice, respectively) than the muscle fibers expressing type IIb MyHC in the EDL (34 and 39% in Mstn−/− and mIgf1+/+ mice, respectively) when compared with controls. Type I fibers from soleus, on the other hand, were only 11 and 14% larger in the Mstn−/− and mIgf1+/+ groups, respectively, but this difference failed to reach statistical significance. There was no statistically significant difference between the two experimental groups in either muscle. No such comparison was made in type IIb fibers from soleus or slow fibers in EDL fibers due to the low number of fibers expressing these isoforms. Thus, the gain in soleus weight was primarily due to bigger fibers expressing the IIa MyHC isoform. The relative distribution of muscle fibers expressing different MyHC isoforms is summarized in Table 3. In short, both experimental groups showed a trend toward a fast phenotype, albeit not statistically significant when compared with controls.
Table 2.
Muscle fiber CSA, myonuclei per muscle fiber length, and MND size in muscles from control, Mstn−/−, and mIgf1+/+ mice
| Parameter and muscle | Control | Mstn−/− | mIgf1+/+ |
|---|---|---|---|
| CSA (μm2) | |||
| EDL | 910 ± 48 (96) | 1225 ± 35 (92)* | 1267 ± 44 (108)* |
| Soleus type I | 1210 ± 90 (36) | 1277 ± 31 (25) | 1467 ± 69 (29) |
| Soleus type IIa | 1023 ± 81 (45) | 1696 ± 61 (41)* | 1487 ± 96 (53)* |
| Nuclei/L (n/mm) | |||
| EDL | 29.4 ± 1 (71) | 27.3 ± 0.8 (68) | 416 ± 1.3 (68)* |
| Soleus type I | 70.2 ± 3.8 (23) | 69.1 ± 4.7 (13) | 65.6 ± 5.3 (14) |
| Soleus type IIa | 63.1 ± 2.7 (27) | 66.5 ± 2.8 (26) | 69.1 ± 5.3 (21) |
| EDL | 28.7 ± 1.3 (71) | 41 ± 1.8 (68)* | 31.7 ± 1.3 (68) |
| Soleus type I | 18.2 ± 1.6 (23) | 19.3 ± 1.1 (13) | 23.8 ± 2.5 (14) |
| Soleus type IIa | 16.3 ± 1 (27) | 26.6 ± 1.4 (26)*,# | 23.5 ± 2 (21)* |
Values are expressed as means ± se.
P < 0.05 vs. control,
P < 0.05 vs. corresponding soleus type I; 1-way ANOVA.
Table 3.
Proportion of MyHC isoforms from single muscle fibers and cross-sections from EDL and soleus muscles of different groups, as determined by 6% SDS-PAGE
| Muscle and group | Total fibers (n) | Type I | Type IIa | Type I, IIa | Type IIb | Type IIx | Type IIb, IIx |
|---|---|---|---|---|---|---|---|
| EDL | |||||||
| Control | 105 | 1 | 1 | 53 (66 ± 3) | 22 (34 ± 3) | 23 | |
| Mstn−/− | 88 | 87 (80 ± 1) | 4 (20 ± 1) | 9 | |||
| mIgf1+/+ | 86 | 1 | 79 (76 ± 1) | 4 (24 ± 1) | 16 | ||
| Soleus | |||||||
| Controls | 98 | 40 (30 ± 1) | 45 (62 ± 1) | 8 | (5) | 3 (3) | 4 |
| Mstn−/− | 77 | 19 (29 ± 1) | 45 (44 ± 1) | 1 | 9 (14) | 11 (13) | 15 |
| mIgf1+/+ | 85 | 28 (34 ± 1) | 37 (53 ± 1) | 6 | 3 | 13 (13) | 13 |
Values for cross sections (in parentheses) are expressed as means ± se of mean. No statistically significant difference was found across both experimental groups compared to controls.
Myonuclear phenotype and spatial organization
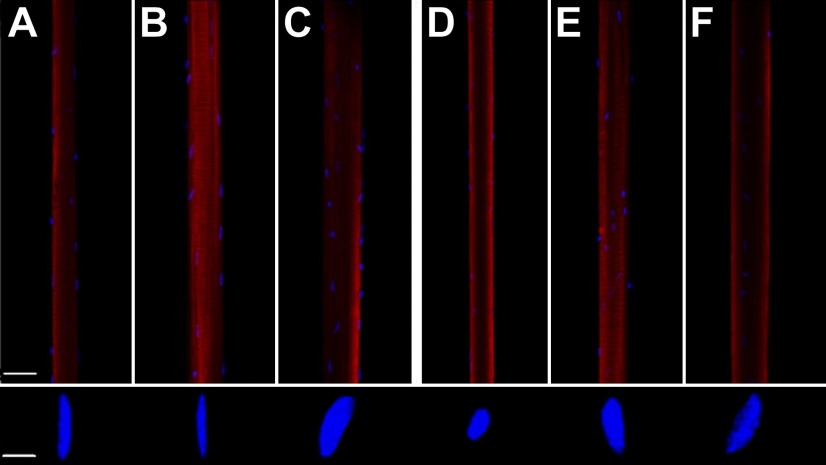
In muscle fibers expressing fast MyHC isoforms from both EDL and soleus muscles, myonuclei with an elliptical shape dominated, and the longitudinal axes of the nuclei were typically aligned with the longitudinal axis of the fiber (Fig. 1). In muscle fibers expressing the slow MyHC isoform, myonuclei with a rounded shape were predominant, but both shapes of myonuclei were frequently observed in the same fiber segments, irrespective of MyHC isoform expression. Similar patterns of myonuclei shape and arrangement were observed in control and experimental groups, with the exception of EDL muscle fibers from the mIgf1+/+ group, where a deviation from the alignment of the elliptical myonuclei along the longitudinal axis was observed. Further, EDL muscle fibers from mIgf1+/+ mice had significantly more internal nuclei than any other fiber population. Central nuclei constituted ∼10% of all nuclei in EDL fibers and ∼40% of the nuclei in muscle fibers expressing the type IIb MyHC isoform. Internal nuclei were, on the other hand, rare in soleus muscle fibers from all groups and in EDL muscle fibers from control and Mstn−/− mice and constituted only 1–2% of all nuclei in these fibers.
Figure 1.
Confocal images of single muscle fibers and representative images of myonuclei shape in control (A, D), Mstn−/− (B, E) and IGF-1-overexpressing mice (C, F) in EDL (A–C) and soleus (D–F) fibers. DAPI (blue) stained myonuclei and rhodamine phalloidin (red)-labeled actin. Scale bars = 50 μm (fiber); 3 μm (nuclei).
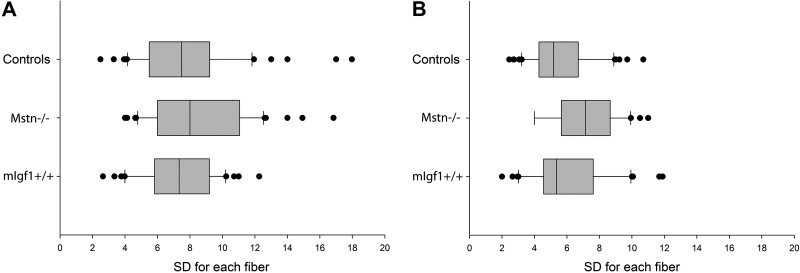
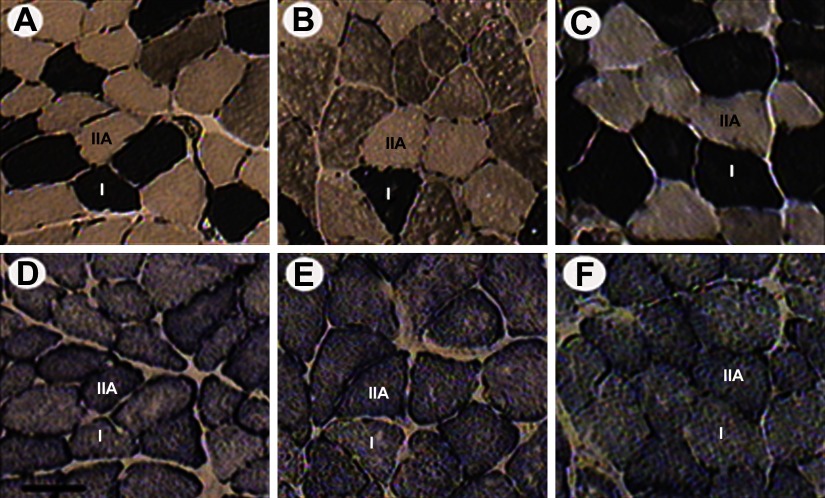
The number of nuclei per fiber length and the size of individual MNDs were measured along the length of single muscle fiber segments using a novel algorithm (18). Myonuclei close to the terminal parts of fiber segments were excluded from analysis since their MNDs may extend outside the fiber segment and give rise to erroneously small MNDs. Uniform MND sizes were observed along the length of individual muscle fibers in both control and experimental groups (Fig. 2). A significant increase (P<0.05) in myonuclear number was observed only in hypertrophic EDL muscle fibers from the mIgf1+/+ group (Table 2). This was the only fiber population showing a significant change in both size and myonuclei number per fiber length compared with control fibers, resulting in a maintained MND size in mIgf1+/+ EDL fibers. In contrast, MND sizes in hypertrophic EDL fibers from Mstn−/− and in type IIa soleus fibers from both Mstn−/− and mIgf1+/+ mice were significantly higher than in controls as a result of larger fibers without a change in nuclear content (Table 2). In the soleus, in accordance with our previous study (26), MND size was similar in fibers expressing type I and type IIa MyHC isoforms in control mice. The same finding was observed in the mIgf1+/+ group, while type IIa fibers had significantly larger domains than type I fibers in the Mstn−/− mice. We have previously suggested that protein systems besides MyHC isoforms, such as mitochondrial proteins, may have a role in defining MND size in rodents. Thus, serial cross-sections from control, Mstn−/−, and mIgf1+/+ mice were assessed for SDH activity of type I and type IIa fibers identified by mATPase staining. Type IIa fibers had deeper staining for SDH than type I fibers in all three groups, despite the fact that MND size in Mstn−/− mice was bigger in type IIa fibers than type I fibers (Fig. 3). This shows that mitochondrial content may not be the prime determinant of MND size, at least in Mstn−/− mouse fibers.
Figure 2.
Box plots show sd of MND sizes from individual muscle fiber segments from EDL (A) and soleus (B) muscles. Boxes represent 25th and 75th percentiles; median value is indicated as a band near the middle of the box. Horizontal bars denote the 10th and 90th percentiles; data outside this range are indicated as dots.
Figure 3.
Enzyme histochemical myofibrillar ATPase, pH 4.5, (A–C) and SDH (D–F) staining of soleus muscle cross-sections from control (A, D), Mstn−/− (B, E), and mIgf1+/+ (C, F) mice, respectively. Muscle fiber types I and IIa are shown. Scale bar = 50 μm.
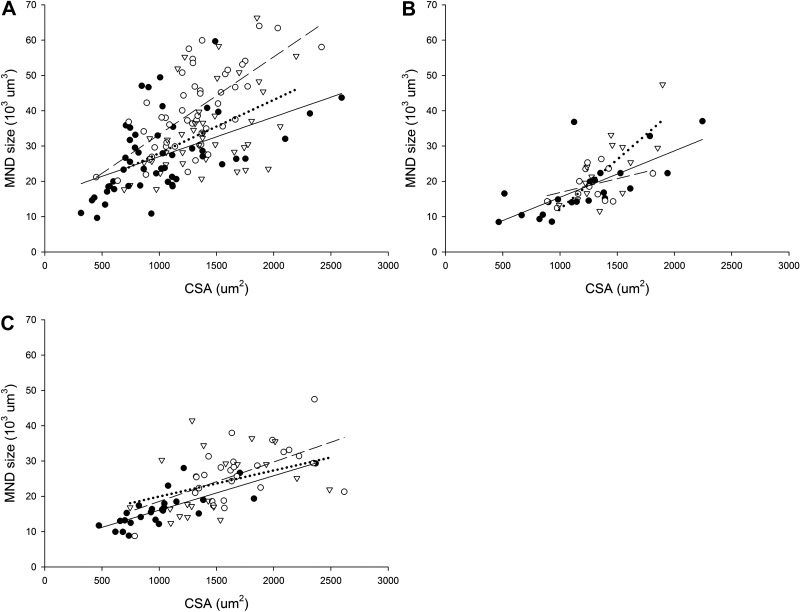
A linear relationship was observed between MND size and fiber CSA in control and experimental groups (Fig. 4). In EDL, a strong linear relationship was observed between both MND and CSA in Mstn−/− (r=0.68; P<0.001) and in mIgf1+/+ mice (r=0.64; P<0.001; Fig. 4A). In soleus, on the other hand, a significant relationship was only found in type I fibers from mIgf1+/+ mice (r=0.75; P<0.05; Fig. 4B) and type IIa fibers from Mstn−/− group (r=0.57; P<0.05; Fig. 4C).
Figure 4.
Average single muscle fiber MND vs. fiber CSA in single EDL (A) and type I soleus (B) and type IIa soleus (C) muscle fibers from control (solid circles, solid line), Mstn−/− (open circles, dashed line), and mIgf1+/+ (open triangles, dotted line) mice.
Contractile properties
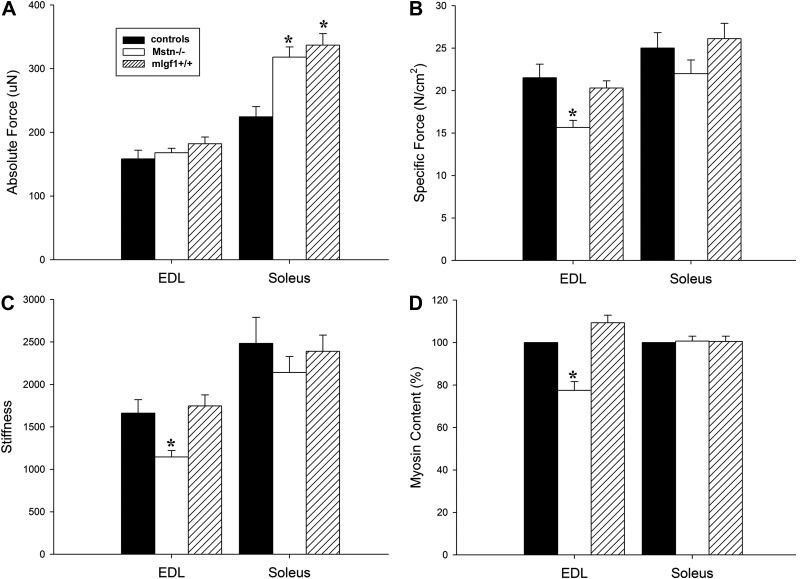
A total of 272 muscle fibers (EDL, n=131; soleus, n=141) fulfilled the criteria for acceptance and were included in the analyses of contractile properties in control, Mstn−/−, and mIgf1+/+ mice. In the EDL, absolute force (Fig. 5A) did not differ significantly between experimental and control groups. Po normalized to CSA (specific force) was lower in hypertrophied fibers from Mstn−/− mice (P<0.05), while it remained unchanged in mIgf1+/+ mice (Fig. 5B). In the soleus, Po was 41 and 50% higher in Mstn−/− (P<0.05) and mIgf1+/+ (P<0.05) mice, respectively, but specific force did not differ between experimental and control groups. Muscle fibers expressing the type I MyHC isoform in the soleus showed a similar Po and specific force in experimental groups compared with controls. Muscle fibers expressing the type IIa MyHC isoform had 55 and 48% higher Po in the Mstn−/− and mIgf1+/+ groups compared with controls, and specific force did not differ between control and experimental groups.
Figure 5.
Single muscle fiber absolute force (A), specific force (B), and stiffness (C) together with myosin content, described as percentage (D). Results from single muscle fibers expressing the type IIb MyHC isoform in the EDL and the IIa MyHC isoform in the soleus are included. Values are means ± se. *P < 0.05 vs. control; 1-way ANOVA.
In the EDL, fiber stiffness was ∼40% lower (P<0.05) in the Mstn−/− group than in the control and mIgf1+/+ groups (Fig. 5C). In the soleus, stiffness did not differ between experimental and control groups in accordance with specific force measurements. It is noted that average specific force and stiffness variables are lower, albeit not statistically significant, in Mstn−/− soleus fibers (Fig. 5B, C). Thus, the lower stiffness in parallel with the lower specific force in EDL fibers from Mstn−/− mice indicate a reduced number of functional cross-bridges underlying the reduced specific force in these mice, since fiber stiffness (Fig. 5C) is primarily dependent on the number of attached cross-bridges (27).
Myosin content
The content of the molecular motor protein myosin in the two experimental groups was measured in muscle cross-sections and related to the content in control muscles using a proximity ligation assay (Fig. 5D). Muscle sections from control and experimental groups were fixed and stained on the same glass. In the EDL, Mstn−/− mouse myosin content per CSA was 23% lower (P<0.05) than in control mice, while mIgf1+/+ mice had a similar myosin content as in controls (Fig. 5D). In the soleus, myosin content per CSA was similar in all three groups, in accordance with specific force and stiffness measurements.
DISCUSSION
Results from this study have confirmed and extended our understanding of the mechanisms underlying the altered contractile properties associated with different modes of hypertrophy, and the major findings from this study are as follows: a dramatic muscle fiber growth in response to Mstn−/− and mIgf1+/+; a commensurate gain in the cytoplasmic volume and nuclear content in EDL fibers from mIgf1+/+ mice and maintenance of MND, in contrast to larger cytoplasmic volume with no myonuclear accretion and an expansion of MND in EDL fibers from Mstn−/− mice; diminished specific force and myosin content in EDL fibers lacking myostatin; and gain in fiber size and MND, yet a maintenance of specific force in IIa soleus fibers from both Mstn−/− and mIgf1+/+ mice. These results demonstrate a divergence in the regulation of muscle mass with respect to MND by myostatin and IGF-1A. Further, there is a distinct difference in absolute MND size between fiber types, where type I and IIa have much smaller MNDs than IIb fibers. Thus, we posit that there is a critical MND above which muscle fibers cannot adequately support contractile proteins for maintenance of force production and that the cytoplasmic volume supported by individual myonuclei is a potential mechanism underlying altered functional capacity in hypertrophic muscle fibers.
Addition of myonuclei is a prerequisite for maintained specific force in hypertrophic fast-twitch EDL muscle fibers
In accordance with previous studies, larger MNDs were observed in muscle fibers expressing fast MyHC isoforms (28, 29). The results from this study demonstrate that an additional enlargement of these large MNDs by Mstn−/− cause a loss in specific force. The addition of nuclei in hypertrophic EDL fibers from mIgf1+/+ mice, on the other hand, maintains both MND size and specific force. Measurements of stiffness suggest a decreased number of strongly attached cross-bridges being the primary source of the small specific force in muscle fibers with very large MNDs. Quantitative measurements of myosin content support this by demonstrating a proportional loss of myosin and specific force in the EDL muscle from Mstn−/− mice.
In accordance with recent studies, the fiber hypertrophy in myostatin-deficient muscles occurred without addition of myonuclei (8, 30, 31). This is probably a consequence of the lack of satellite cell activation, supported by the small number of internal nuclei in Mstn−/− mice. Mitochondrial depletion has been forwarded as a mechanism underlying the specific force in Mstn−/− mice (32). This is supported by a shift toward fast glycolytic IIb fibers and fewer mitochondria per unit volume in hypertrophic muscle from myostatin deficient mice together with a strong relationship between volume of mitochondria and MND size, particularly among fast fibers (33). Although a shift toward fast IIb fibers with a low mitochondrial content was observed in the Mstn−/− mice, this is not the cause of the loss in specific force in the membrane-permeabilized muscle fibers in this study. Use of the single-fiber preparation eliminates the influence of ATP production by mitochondria on excitation-contraction coupling. The changes observed in the force-generation capacity of these muscle fibers reside in the final functional contractile unit in the muscle cell, i.e., the sarcomere. This does not, however, exclude the possibility that mitochondrial depletion may play an important role for in vivo physiological properties of skeletal muscle in myostatin-deficient mice.
In contrast to observations in Mstn−/− mice, the muscle hypertrophy in myosin light chain mIgf1+/+ mice was associated with a proportional increase in force, and resulted in maintained specific force. This confirms with previous observations in α-actin/igf1 (13) and MyLC/migf1 transgenic models (11, 12). An increase in myonuclei number was observed in the mIgf1+/+, but the gain in muscle fiber size was proportionally larger than the increase in myonuclei number. Thus, MNDs were ∼10% larger and resulted in a domain size around 31,700 μm3. It is suggested that this volume corresponds to a MND size close to the maximum synthetic capacity a myonucleus can support to maintain myofibrillar protein content and number of functioning cross-bridges intact.
A uniform distribution of myonuclei was observed in EDL and soleus muscles from control, Mstn−/−, and mIgf1+/+ mice (Fig. 1). This is in contrast to our recent observation of an age-related increase in MND size variability in single muscle fibers from older humans (18). The aging-related loss in specific force and preferential myosin loss (34, 35) may accordingly be dependent on individual myonuclei exceeding their upper cytoplasmic volume to maintain an optimal concentration of contractile proteins.
Specific force is maintained with expanded MNDs in the slow-twitch soleus
Soleus has a higher protein turnover rate than EDL, with a protein half-life of 7 or 8 d, almost half that of EDL (36). Mouse soleus is predominantly composed of fibers expressing the type I and IIa MyHC isoform.
Despite a fiber type-specific difference in hypertrophy in Mstn−/− and mIgf1+/+ groups, both IIa and I fibers maintained their myonuclei numbers, and this resulted in a strong relationship between MND size and fiber size in both experimental groups. Despite a significantly larger MND size in type IIa fibers, the MND (23,000–26,000 μm3) was still smaller than in control EDL and suggests sufficient transport distances and nuclear transcriptional potential to maintain specific force, stiffness, and myosin quantity.
The constant myonuclear numbers in both experimental groups indicate a hypertrophic response independent of satellite cell activation in the soleus. This is supported by the few internal nuclei and conforms with previous observations in myostatin-null mice demonstrating that satellite cells did not contribute to hypertrophy (37) or to an increase in protein/DNA ratio (38).
The satellite cell-independent hypertrophy of soleus muscle fibers in mIgf1+/+ mice challenges established concepts, since high levels of the MyLC/igf-1promoter have been reported to be restricted to fast-twitch fibers (10, 39). It cannot be excluded that IGF-1-induced soleus fiber hypertrophy is mediated through other mechanisms, such as compensatory hypertrophy to an increased load by hypertrophy of fast-twitch antagonists or an increased postural load related to large body weight (40). However, because IGF-I secreted from type IIa fibers can act on type I fibers via paracrine actions (4), the effects of IGF-1 on slow fibers is likely mediated by the local IGF-I pool. Satellite cell activation is typically observed in soleus muscle compensatory hypertrophy (41–43), making this a less likely underlying mechanism. An IGF-1-induced hypertrophic response has been observed in the absence of satellite cells in differentiated muscle (4), as well as in cell culture via P13K/Akt and not calcineurin signaling (44). The Akt signaling pathway spares satellite cells and relies primarily on the promotion of protein synthesis via the mTOR pathway and inhibition of protein degradation via FOXO transcriptional factors.
Maintenance of specific force in hypertrophic soleus muscle fibers is in accordance with previous observations in myostatin deficient (17) and IGF-1 transgenic mice (12). That is, a gain in MND size to ∼24,000 μm3 does not compromise force-generating capacity of hypertrophied fibers because MND has not reached maximum capacity. In contrast to the soleus, the upper limit of MND is exceeded in EDL fibers from the myostatin-deficient mice, resulting in depressed strength. However, postnatal inhibition of myostatin where the hypertrophic response is less robust may enable maintenance of specific force. A recent study supports this concept, since it was observed that the specific force of the EDL muscle was maintained with myostatin inhibition that resulted in 16% hypertrophy, but not when hypertrophy was nearly 40% of the control values (45). Other factors may contribute to optimal force production, for it may be that the limit of cytoplasmic expansion that can be accommodated without loss of specific force is dependent on transcriptional, translational, and protein degradation rates, as well as nuclear domain.
CONCLUSIONS
There is increasing interest in both the myostatin and IGF-1 signaling pathways in the pharmacological treatment of many different muscle-wasting conditions. The muscle hypertrophy in myostatin-null and IGF-1 overexpressing mice may be beneficial in terms of absolute muscle force secondary to fiber hypertrophy and hyperplasia. However, results from the present study show that these interventions have different and muscle-specific effects related to myonuclear organization. The fast-twitch EDL has a higher MND than either fiber type in the soleus, and this appears to be optimally tuned for generating force. Therefore, hypertrophy must be accompanied by new myonuclear incorporation to maintain MND, and this is only achieved by increased IGF-1 and not by myostatin depletion. However, because the soleus fibers have a much lower MND to start with, there is a larger dynamic range for hypertrophy without the need for new myonuclei. Thus, soleus fibers can undergo greater proportional hypertrophy and not suffer functional consequences. Because human muscle fibers span a similar varied range of MND with respect to age, gender, and fiber type (18, 19) the response differences in MND should be considered when developing human therapeutic strategies for boosting muscle mass.
Acknowledgments
The authors are grateful to Yvette Hedström, Rebeca Corpeno, and Ann-Marie Gustafson for excellent technical assistance and to Dr. Julien Ochala for invaluable help and support during experiments and constructive criticism of the manuscript.
This study was supported by grants from the Swedish Research Council (8651), the European Commission (MyoAge, EC Fp7 CT-223756 and COST CM1001), King Gustaf V and Queen Victoria's Foundation, and Association Française contre les Myopathies to L.L., and the Higher Education Commission (HEC) of Pakistan to R.Q.
Footnotes
- CSA
- cross-sectional area
- IGF-1
- insulin-like growth factor-1
- mIgf1+/+
- IGF-1-overexpressing
- MND
- myonuclear domain
- Mstn−/−
- myostatin-knockout
- MyHC
- myosin heavy chain
- SDH
- succinate dehydrogenase
- SL
- sarcomere length.
REFERENCES
- 1. Allen D. L., Monke S. R., Talmadge R. J., Roy R. R., Edgerton V. R. (1995) Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. 78, 1969–1976 [DOI] [PubMed] [Google Scholar]
- 2. Allen R. E., Boxhorn L. K. (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 138, 311–315 [DOI] [PubMed] [Google Scholar]
- 3. Dodson M. V., Allen R. E., Hossner K. L. (1985) Ovine somatomedin, multiplication-stimulating activity, and insulin promote skeletal muscle satellite cell proliferation in vitro. Endocrinology 117, 2357–2363 [DOI] [PubMed] [Google Scholar]
- 4. Barton-Davis E. R., Shoturma D. I., Sweeney H. L. (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 167, 301–305 [DOI] [PubMed] [Google Scholar]
- 5. McCarthy J. J., Esser K. A. (2007) Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl. Physiol. 103, 1100–1102; discussion 1102–1103 [DOI] [PubMed] [Google Scholar]
- 6. Wada K. I., Katsuta S., Soya H. (2003) Natural occurrence of myofiber cytoplasmic enlargement accompanied by decrease in myonuclear number. Jpn. J. Physiol. 53, 145–150 [DOI] [PubMed] [Google Scholar]
- 7. Welle S., Bhatt K., Pinkert C. A., Tawil R., Thornton C. A. (2007) Muscle growth after postdevelopmental myostatin gene knockout. Am. J. Physiol. 292, E985–E991 [DOI] [PubMed] [Google Scholar]
- 8. Amthor H., Otto A., Vulin A., Rochat A., Dumonceaux J., Garcia L., Mouisel E., Hourde C., Macharia R., Friedrichs M., Relaix F., Zammit P. S., Matsakas A., Patel K., Partridge T. (2009) Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc. Natl. Acad. Sci. U. S. A. 106, 7479–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watt K. I., Jaspers R. T., Atherton P., Smith K., Rennie M. J., Ratkevicius A., Wackerhage H. SB431542 treatment promotes the hypertrophy of skeletal muscle fibers but decreases specific force. Muscle Nerve 41, 624–629 [DOI] [PubMed] [Google Scholar]
- 10. Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 11. Colombini B., Benelli G., Nocella M., Musaro A., Cecchi G., Bagni M. A. (2009) Mechanical properties of intact single fibres from wild-type and MLC/mIgf-1 transgenic mouse muscle. J. Muscle Res. Cell Motil. 30, 199–207 [DOI] [PubMed] [Google Scholar]
- 12. Del Prete Z., Musaro A., Rizzuto E. (2008) Measuring mechanical properties, including isotonic fatigue, of fast and slow MLC/mIgf-1 transgenic skeletal muscle. Ann. Biomed. Eng. 36, 1281–1290 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez E., Messi M. L., Zheng Z., Delbono O. (2003) Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J. Physiol. 552, 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McPherron A. C., Lawler A. M., Lee S. J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90 [DOI] [PubMed] [Google Scholar]
- 15. Lee S. J. (2004) Regulation of muscle mass by myostatin. Annu. Rev. Cell. Dev. Biol. 20, 61–86 [DOI] [PubMed] [Google Scholar]
- 16. Mosher D. S., Quignon P., Bustamante C. D., Sutter N. B., Mellersh C. S., Parker H. G., Ostrander E. A. (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 3, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendias C. L., Marcin J. E., Calerdon D. R., Faulkner J. A. (2006) Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J. Appl. Physiol. 101, 898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cristea A., Qaisar R., Edlund P. K., Lindblad J., Bengtsson E., Larsson L. (2010) Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell 9, 685–697 [DOI] [PubMed] [Google Scholar]
- 19. Frontera W. R., Larsson L. (1997) A methodological study on three different techniques for membrane permeabilization of human single muscle cells obtained by percutaneous biopsy. Muscle Nerve 20, 948–952 [DOI] [PubMed] [Google Scholar]
- 20. Fabiato A. (1988) Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 157, 378–417 [DOI] [PubMed] [Google Scholar]
- 21. Larsson L., Moss R. L. (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J. Physiol. 472, 595–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moss R. L. (1979) Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J. Physiol. 292, 177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martyn D. A., Smith L., Kreutziger K. L., Xu S., Yu L. C., Regnier M. (2007) The effects of force inhibition by sodium vanadate on cross-bridge binding, force redevelopment, and Ca2+ activation in cardiac muscle. Biophys. J. 92, 4379–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonald K. S., Fitts R. H. (1995) Effect of hindlimb unloading on rat soleus fiber force, stiffness, and calcium sensitivity. J. Appl. Physiol. 79, 1796–1802 [DOI] [PubMed] [Google Scholar]
- 25. Dubowitz V. (1985) Muscle Biopsy: A Practical Approach, Balliére-Tindall, London [Google Scholar]
- 26. Liu J. X., Hoglund A. S., Karlsson P., Lindblad J., Qaisar R., Aare S., Bengtsson E., Larsson L. (2009) Myonuclear domain size and myosin isoform expression in muscle fibres from mammals representing a 100,000-fold difference in body size. Exp. Physiol. 94, 117–129 [DOI] [PubMed] [Google Scholar]
- 27. Seow C. Y., Shroff S. G., Ford L. E. (1997) Detachment of low-force bridges contributes to the rapid tension transients of skinned rabbit skeletal muscle fibres. J. Physiol. 501, 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruusgaard J. C., Liestol K., Ekmark M., Kollstad K., Gundersen K. (2003) Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 551, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruusgaard J. C., Liestol K., Gundersen K. (2006) Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 100, 2024–2030 [DOI] [PubMed] [Google Scholar]
- 30. Haidet A. M., Rizo L., Handy C., Umapathi P., Eagle A., Shilling C., Boue D., Martin P. T., Sahenk Z., Mendell J. R., Kaspar B. K. (2008) Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc. Natl. Acad. Sci. U. S. A. 105, 4318–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsakas A., Foster K., Otto A., Macharia R., Elashry M. I., Feist S., Graham I., Foster H., Yaworsky P., Walsh F., Dickson G., Patel K. (2009) Molecular, cellular and physiological investigation of myostatin propeptide-mediated muscle growth in adult mice. Neuromuscul. Disord. 19, 489–499 [DOI] [PubMed] [Google Scholar]
- 32. Amthor H., Macharia R., Navarrete R., Schuelke M., Brown S. C., Otto A., Voit T., Muntoni F., Vrbova G., Partridge T., Zammit P., Bunger L., Patel K. (2007) Lack of myostatin results in excessive muscle growth but impaired force generation. Proc. Natl. Acad. Sci. U. S. A. 104, 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tseng B. S., Kasper C. E., Edgerton V. R. (1994) Cytoplasm-to-myonucleus ratios and succinate dehydrogenase activities in adult rat slow and fast muscle fibers. Cell Tissue Res. 275, 39–49 [DOI] [PubMed] [Google Scholar]
- 34. Cristea A., Vaillancourt D. E., Larsson L. (2010) Aging-related changes in motor unit structure and function. In Sarcopenia—Age-Related Muscle Wasting and Weakness: Mechanisms and Treatments (Lynch G. S., ed) pp. 55–74, Springer, New York [Google Scholar]
- 35. Thompson L. V., Durand D., Fugere N. A., Ferrington D. A. (2006) Myosin and actin expression and oxidation in aging muscle. J. Appl. Physiol. 101, 1581–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis S. E., Kelly F. J., Goldspink D. F. (1984) Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 217, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu X., Hadhazy M., Wehling M., Tidball J. G., McNally E. M. (2000) Dominant-negative myostatin produces hypertrophy without hyperplasia in muscle. FEBS Lett. 474, 71–75 [DOI] [PubMed] [Google Scholar]
- 38. Welle S., Bhatt K., Pinkert C. A. (2006) Myofibrillar protein synthesis in myostatin-deficient mice. Am. J. Physiol. Endocrinol. Metab. Physiol. 290, E409–E415 [DOI] [PubMed] [Google Scholar]
- 39. Donoghue M. J., Alvarez J. D., Merlie J. P., Sanes J. R. (1991) Fiber type- and position-dependent expression of a myosin light chain-CAT transgene detected with a novel histochemical stain for CAT. J. Cell. Biol. 115, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paul A. C., Rosenthal N. (2002) Different modes of hypertrophy in skeletal muscle fibers. J. Cell. Biol. 156, 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanzlikova V., Mackova E. V., Hnik P. (1975) Satellite cells of the rat soleus muscle in the process of compensatory hypertrophy combined with denervation. Cell Tissue Res. 160, 411–421 [DOI] [PubMed] [Google Scholar]
- 42. Rosenblatt J. D., Yong D., Parry D. J. (1994) Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17, 608–613 [DOI] [PubMed] [Google Scholar]
- 43. Snow M. H. (1990) Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat. Rec. 227, 437–446 [DOI] [PubMed] [Google Scholar]
- 44. Quinn L. S., Anderson B. G., Plymate S. R. (2007) Muscle-specific overexpression of the type 1 IGF receptor results in myoblast-independent muscle hypertrophy via PI3K, and not calcineurin, signaling. Am. J. Physiol. Endocrinol. Metab. Physiol. 293, E1538–E1551 [DOI] [PubMed] [Google Scholar]
- 45. Morine K. J., Bish L. T., Pendrak K., Sleeper M. M., Barton E. R., Sweeney H. L. (2010) Systemic myostatin inhibition via liver-targeted gene transfer in normal and dystrophic mice. PLoS One 5, e9176. [DOI] [PMC free article] [PubMed] [Google Scholar]