Abstract
To systematically identify novel gene functions essential for osteogenesis and skeletal mineralization, we performed a forward genetic mutagenesis screen in zebrafish and isolated a mutant that showed delayed skeletal mineralization. Analysis of the mutant phenotype in an osterix:nuclear-GFP transgenic background demonstrated that mutants contain osterix-expressing osteoblasts comparable to wild-type embryos. Positional cloning revealed a premature stop mutation in the macrophage-stimulating protein (msp) gene, predicted to result in a biologically inactive protein. Analysis of the embryonic expression pattern for the receptor for Msp, Ron, shows specific expression in the corpuscles of Stannius, a teleost-specific organ that produces stanniocalcin, a pivotal hormone in fish calcium homeostasis. Knockdown of Ron resulted in identical phenotypes as observed in msp mutants. Msp mutant embryos could be rescued by excess calcium. Consistent with a role for Msp/Ron in calcium homeostasis, calcium-regulating factors, such as pth1, pth2, stc1l, and trpv5/6 were significantly affected in msp mutant larvae. While Msp and Ron have previously been shown to play a critical role in a wide variety of biological processes, we introduce here the Msp/Ron signaling axis as a previously unappreciated player in calcium homeostasis and embryonic skeletal mineralization.—Huitema, L. F. A., Renn, J., Logister, I., Gray, J. K., Waltz, S. E., Flik, G., Schulte-Merker, S. Macrophage stimulating protein and calcium homeostasis in zebrafish.
Keywords: msp, ron, mineralization, bone, osteogenesis, stanniocalcin
The vertebrate skeleton is composed of bone and cartilage. Mineralization of cartilage and bone occurs by a series of physicochemical and biochemical processes that together facilitate the deposition of calcium phosphates (mostly hydroxyapatite) in specific areas of the extracellular matrix (1).
The dynamic process of bone formation in vertebrates is tightly regulated by bone-forming cells, osteoblasts, and bone-resorbing cells, osteoclasts. Osteoblasts are of mesenchymal origin, and runt-related transcription factor 2 (Runx2) and Osterix have been identified as the major transcription factors controlling osteoblast commitment and differentiation (2). Osteoclasts, on the other hand, are of hematopoietic origin and derive from the monocyte lineage (3).
Calcium and phosphate levels must be stringently controlled to guarantee healthy bone physiology (4). When extracellular fluid (or plasma) calcium levels increase, excess calcium can be incorporated into bone by the activity of osteoblasts. Hormones that influence the expression of calcium transporters, calcium channels, and calcium-sensing receptors maintain the extracellular fluid calcium content within a narrow range, and although there is a continuous turnover of total bone mass, there is no net gain or loss of calcium from bone in an adult/full-grown healthy organism (4). However, when calcium homeostasis is disturbed, pathological mineralization or demineralization can occur, and skeletal diseases, such as osteoporosis and osteopetrosis, are the consequence (5).
Terrestrial animals acquire calcium solely from food, and tight regulation of plasma calcium concentrations depends on intestinal calcium absorption, renal calcium reabsorption, and exchange of calcium from bone. In fish, calcium is readily available from the surrounding aquatic environment. Fish have the ability to extract calcium both from the ambient water via their gills (equipped with mitochondria-rich cells, so-called ionocytes, which transport calcium from water to blood; ref. 6) and from food via their intestine (7). Except for the additional uptake route via the gills, calcium physiology in fish is not very different from how terrestrial vertebrates accomplish tight regulation of calcium levels (7).
To investigate molecular mechanisms underlying skeletal mineralization in fish, we performed a large-scale forward genetic screen in zebrafish and here describe a mutant that is significantly delayed in skeletal mineralization. Positional cloning revealed a premature stop mutation in exon 8 of the macrophage-stimulating protein (Msp) (8) to be causative for the phenotype. Msp has thus far not been linked to embryonic skeletal mineralization. Although several functions have been attributed to the Msp/Ron signaling axis, the various effects of Msp appear to be dependent on cell type and/or Ron expression levels, with no single cell type showing all mentioned effects (at least in vitro). In our in vivo study, we have identified a novel role for the Msp/Ron signaling pathway, and our data show that this pathway regulates calcium homeostasis.
MATERIALS AND METHODS
Animal procedures
All zebrafish strains were maintained at the Hubrecht Institute using standard husbandry conditions. Animal experiments were approved by the Animal Experimentation Committee of the Royal Netherlands Academy of Arts and Sciences.
Media
Embryos were kept in embryonic medium 3 (E3; 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4) at 28°C. For anesthesia, a 0.2% solution of 3-aminobenzoic acid ethyl ester (MS-222; Sigma, St. Louis, MO, USA)containing Tris buffer (pH 7) was used (9).
Screening procedure
Screening was essentially done as described previously (10, 11). Embryos and larvae were grown in E3 medium until 8 days postfertilization (dpf) and subsequently fixed for skeletal staining as described below.
Skeletal staining
Skeletal staining was performed as described previously (12, 13), and specimens were stored at 4°C.
In vivo skeletal staining was performed with 0.05% Alizarin Red in E3 medium for 5 min and subsequent extensive washes with E3 medium.
Meiotic mapping and sequencing
A line for mapping was generated by crossing the Tübingen strain with individuals from a WIK strain. The F1 progeny were grown to sexual maturity, and F2 mutant embryos were used for linkage analysis. After isolation of genomic DNA and amplification of polymorphic CA-repeats, the mutation was mapped using standard simple sequence length polymorphism (SSLP) mapping.
The primers CA152 and Z80 (Fig. 3A and Supplemental Table S1) were used for fine mapping. Coding exons of the msp gene of mutant and wild-type embryos were PCR-amplified and sequenced on both strands. A mutation in the coding exon eight was confirmed with the primers msp-exon8 (Supplemental Table S1). PCR conditions are available on request.
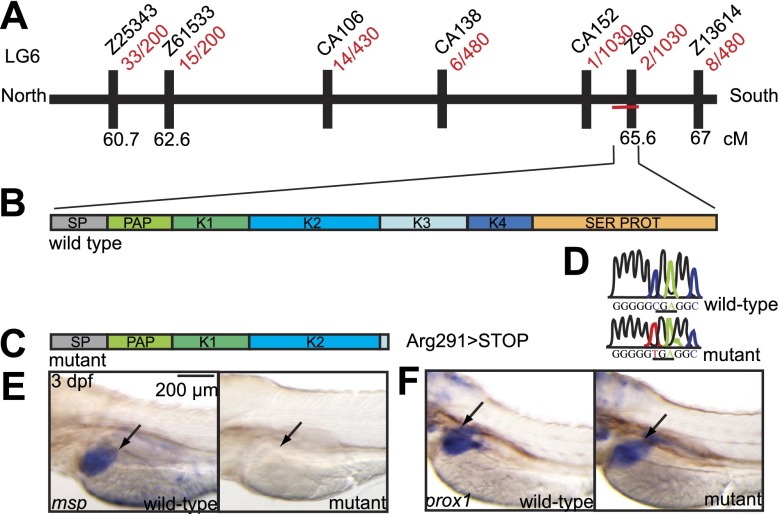
Figure 3.
A premature STOP mutation in the macrophage-stimulating protein (msp) gene is causative for the mutant phenotype. A) Meiotic map of the locus. The mutant phenotype was mapped to a narrow region on linkage group 6. Recombinant mutants per total amount of isolated mutants for each polymorphic marker are depicted in red. Flanking SSLP markers (CA152 and Z80) are both located on contig CU207276.12. msp is the only gene present between the flanking markers, as depicted by the horizontal red line. B) Schematic drawing of Msp. Msp consists of a heavy chain with an N-terminal domain corresponding to a signal peptide (SP), plasminogen-activating peptide (PAP), 4 kringle domains (K1-K4), and a light chain with a serine protease domain. C) Because of a premature STOP mutation in exon 8 of the msp, gene mutants are predicted to be devoid of the biologically active light chain. D) Sequencing of msp shows a premature STOP mutation (Arg291>stop) in exon 8. E, F) Whole-mount in situ hybridization of msp (E) and prox1 (F) in 3-dpf wild-type vs. mutant embryos. Note the complete absence of msp transcripts in the mutant liver.
Whole-mount in situ hybridization
In situ hybridizations were performed at least twice, as described previously (13, 14). Previously described probes used were recombination activating gene 1 (rag1), msp, prospero homeobox protein 1 (prox1), and type X collagen α1 (col10a1) (13, 15–17). Newly designed probes were transcribed from the 5′ part of the respective cDNA using the primers as described in Supplemental Table S1.
For all in situ hybridizations, mutants were derived from heterozygous parental crosses and genotyped individually as follows: first a high-quality image of a randomly chosen embryo was captured. The image was saved as A1, A2, or A3. Thereafter, the embryo was collected in a 96-well PCR plate according to the labeling of the captured images. For each gene, ≥12 embryos were imaged, after which they were genotyped.
Immunohistochemistry
Embryos were fixed for 1 h in 4% paraformaldyhyde (PFA) and stored in methanol. Embryos were rehydrated, blocked in phosphate-buffered saline (PBS) with 5% lamb serum, and incubated with 1:500 anti-phospho-histone H3 (pH3; Millipore, Bedford, MA, USA) and anti-collagen II (1:500; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) overnight at 4°C. Embryos were washed extensively and then incubated in Alexa-Fluor secondary antibodies (Molecular Probes, Eugene, OR, USA) diluted 1:500 in blocking solution for 3 h at room temperature. Embryos were washed extensively in the dark and mounted for analysis.
Morpholino (MO) antisense oligomer injection
MOs were obtained from Gene Tools (http://www.gene-tools.com) and diluted in water containing 0.2% phenol red. One-cell-stage embryos were injected with 16 to 32 ng of MO in a maximum volume of 2 nl (18). Embryos were injected with Ron-specific MOs. MO sequences were MOron-1: 5′-ATGAGTGATGCTATAATAACCTGCA-3′, MOron-2-ATG: 5′-CTAAATGTGTGGCCCAATGGACCAT-3′, and MOron-2-UTR: 5′-TCCACTCCAGGGTGAAGCAAATGGC-3′.
MO specificity controls
Only morphant embryos with a normal size, trunk circulation, and blood flow and without tissue malformations and general edema or toxic defect were included for analysis. Furthermore, MO injection was performed into Fli1:EGFP embryos, which allowed us to score, at 5 dpf, only embryos that developed a complete thoracic duct (19), an anatomical structure that is sensitive to developmental delay.
Splice MO specificity was analyzed by RT-PCR. In brief, cDNA was synthesized from RNA from 16 ng MOron-1 and uninjected control embryos [24 hours postfertilization (hpf)] using the SuperScript II RT Kit (Invitrogen, Carlsbad, CA, USA). Primers used for reverse transcriptase PCR were Ron-1.MO.F and Ron-1.MO.R (Supplemental Table S1).
ATG and UTR MO specificity controls were analyzed by cloning MOron2-ATG and MOron2-UTR binding sites upstream of the translation initiation site of a green fluorescent protein (GFP) cassette in a modified pCS2+ vector. One-cell-stage embryos were injected with 10 pg RNA or 10 pg RNA + 16-32 ng MO in a maximum volume of 2 nl. Viable embryos were anesthetized using MS-222 and mounted in a glass-bottom dish containing 0.4% agarose. Images were taken with a Leica CLSM SP2 AOBS confocal microscope.
Chloride cell staining
Mitochondria-rich chloride cells were stained as described previously (20). Life embryos were incubated for 30 min in a 5-micromolar solution of 2-(4-dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI) in E3 medium, with subsequent extensive washes with E3 medium.
Quantitative real-time PCR
Sibling adult msp mutant and wild-type fish were separated by genotyping, and the two clutches were mated simultaneously. Embryos were immediately collected and grown in E3 medium at 28°C until 6 dpf. For total RNA isolation, a maximum of n = 40 msp mutant and wild-type embryos per clutch were homogenized by shredding in 600 μl of RTL lysis buffer (Qiagen RNeasy kit; Qiagen, Valencia, CA, USA) containing 10% β-mercaptoethanol. One volume of 70% ethanol was added, and the homogenate was loaded onto a column for total RNA isolation, according to the manufacturer's protocol, followed by DNaseI (Promega, Madison, WI, USA) treatment. The RNA quality and concentration were determined using a NanoDrop and were verified by gel electrophoresis. cDNA was synthesized from total RNA (1–5 μg) with random hexamers (Integrated DNA Technologies, Coralville, IA, USA) using reverse transcriptase M-MLV (Promega). Primer sets were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) with an optimal product size of 150–200 bp that, where possible, spanned two exons to avoid genomic contamination. PCR efficiency and optimal melting temperatures were determined per primer set, and the specificity was verified by gel electrophoresis using standard real-time PCR on zebrafish cDNA (see Supplemental Table S1 for primer sequences). Quantitative PCR was performed using the MyIQ single color real-time PCR detection system and software (Bio-Rad, Hercules, CA, USA). Each reaction contained: 12.5 μl SYBR Green fluorescent label (Bio-Rad), 3 μl of 1.5 μM primer mix, 4.5 μl MQ, and 5 μl cDNA (10 ng/μl). Cycling conditions were 95°C for 3 min, 40 cycles of 95°C for 10 s and the optimal primer temperature for 45 s, followed by 95°C for 1 min, and finally 65°C for 1 min. All reactions were performed in triplicate on cDNA isolated from ≥3 different clutches of pooled 6-dpf msp mutant and wild-type embryos. Ct values were corrected for the 18S gene. Msp mutant cDNA concentrations were calculated in arbitrary units and compared with the wild-type average and are represented as fold change, with the sibling value set to 1.
Calcium measurements
Zebrafish were quickly washed twice with deionized water and killed on ice. Thereafter, they were dried overnight in a speedvac. Ions were released by overnight digestion with concentrated nitric acid (21). Dried samples were diluted using deionized water and measured by inductively coupled plasma atomic emission spectrophotometry (ICP-AES; Plasma IL200; Thermo Electron, Waltham, MA, USA).
Statistical analyses
Each gene-specific MO was compared to the vehicle in every single experiment. To determine the penetrance of the phenotype, we counted the number of embryos that exhibited the different severities of the mutant or morphant phenotype. χ2 analysis was used to determine whether the severity distribution differed between treatment groups. For quantitative PCR (qPCR), data analysis and calcium measurement data analysis groups at the same developmental stage were compared by paired Student's t test.
RESULTS
Mutants are delayed in skeletal mineralization
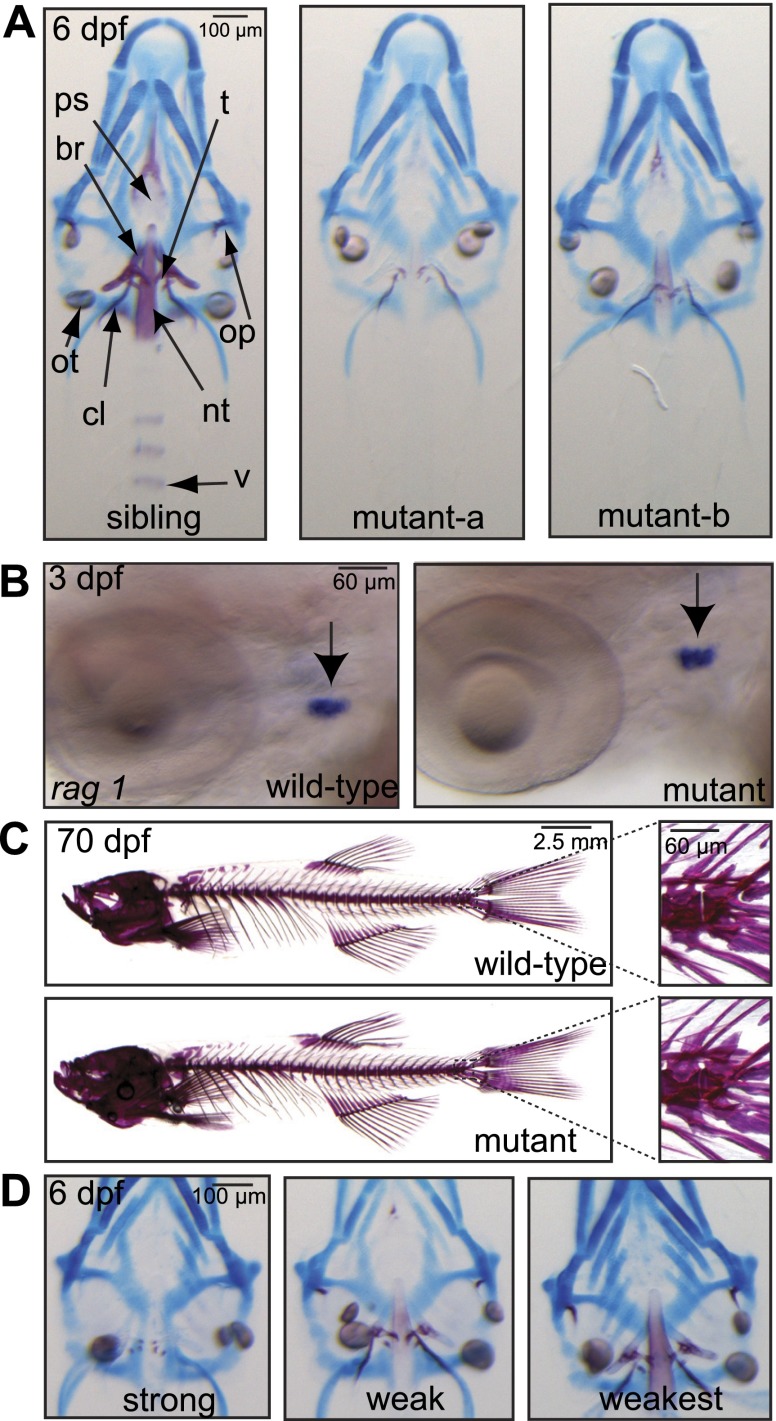
In a large-scale forward mutagenesis screen for genes that affect skeletal mineralization in zebrafish, we identified a mutant that showed a delay in skeletal mineralization at 8 dpf. Skeletal staining of mutant and sibling embryos showed that the mutant phenotype is apparent already at 6 dpf, and at this stage of development, it is characterized by the absence of mineralization of several bone elements (Fig. 1A, mutant-a). Some mutants showed trace amounts of mineralization in the operculum, parasphenoid, and the tip of the notochord (Fig. 1A, mutant-b).
Figure 1.
Phenotypic description of a zebrafish mutant delayed in embryonic skeletal mineralization. A) Alizarin red and Alcian blue staining of a sibling and 2 mutant embryos at 6 dpf. B) Whole-mount in situ hybridization of rag1 expression (arrow) in 4-dpf wild-type vs. mutant embryos, demonstrating that mutants are not delayed in development. C) Alizarin red skeletal staining of adult wild-type and mutant individuals. Insets: higher magnification of the most caudal vertebrae. D) Alizarin red and Alcian blue staining of mutant offspring derived from homozygous mutant parents. Variation of the mutant phenotype is observed at 6 dpf and can be classified into a strong phenotype, a weak phenotype, and a weakest phenotype. br, 5th branchial arch; cl, cleithrum; nt, notochord tip; op, operculum; ot, otolith; ps, parasphenoid; t, teeth; v, vertebrae.
We excluded the possibility that a general delay in development was responsible for the observed phenotype by monitoring development in life specimens and by performing in situ hybridization for thymus-specific rag1, as thymus size correlates with the developmental stage of the zebrafish embryos (17). As shown in Fig. 1B, the size of the thymus did not differ between 4-dpf mutant and wild-type embryos. Furthermore, histological Alcian blue staining and immunohistochemistry for anti-type 2 collagen on 3-, 4-, and 5-dpf embryos (Supplemental Fig. S1) demonstrate that cartilage extracellular matrix formation was not affected or delayed in msp mutants, and no difference in the expression of chondrogenic markers, such as sox9, aggrecan, and type X collagen were observed between siblings and mutants (Supplemental Fig. S2). In addition, no difference in proliferating cells in the craniofacial cartilage elements of 3-, 4-, and 5-dpf embryos between siblings and mutants was found (Supplemental Fig. S1). Together, these data demonstrate that not chondrogenesis but specifically skeletal mineralization was delayed in the mutants.
On the basis of in vivo skeletal staining, mutants were found to be viable when separated at 6 dpf from their siblings and could be raised until adulthood. In adult mutants, we did not observe a difference in Alizarin red staining compared to wild types (Fig. 1C), indicating that mutants recover in skeletal mineralization at later stages of development. Crossing adult mutants with each other resulted in 100% offspring delayed in skeletal mineralization. As for the offspring of heterozygous breeding pairs, we observed variation in the mutant phenotype (Fig. 1D). The varying phenotypes were classified into strong, weak, and weakest, of which the phenotypic characteristics are described in Table 1. Subsequent quantification of the classified mutants from 6 to 12 dpf showed that the mutants recover in skeletal mineralization (Table 2), a process that starts around the point when fish started feeding (6 dpf).
Table 1.
Classification of the mutant phenotype of 6-dpf embryos derived from mutant parents
| Trait | Strong phenotype | Weak phenotype | Weakest phenotype |
|---|---|---|---|
| Presence | Mineralized teeth | Mineralized teeth | Mineralized teeth |
| Otoliths | Otoliths | Otoliths | |
| Parasphenoid | Parasphenoid | ||
| Tip of the notochord | Tip of the notochord | ||
| Slight mineralization in Operculum | Operculum | ||
| Slight mineralization in the Fifth branchial arch | |||
| Absence | Mineralized vertebrae | Mineralized vertebrae | Mineralized vertebrae |
| Fifth branchial arch | Fifth branchial arch | ||
| Operculum | |||
| Parasphenoid | |||
| Tip of the notochord |
Table 2.
Quantification of the mutant phenotype of 6- to 12-dpf embryos derived from mutant parents
| Age (dpf) | Strong | Weak | Weakest |
|---|---|---|---|
| 6 | 21 | 5 | 3 |
| 8 | 14 | 20 | 5 |
| 10 | 0 | 5 | 23 |
| 12 | 0 | 0 | 25 |
Mutants are delayed in osteocalcin expression
To determine whether delayed onset of mineralization is due to altered numbers or delayed maturation of mutant osteoblasts, we analyzed mutants that harbored an osterix:nuclear-GFP transgene (12, 13). As shown in Fig. 2A, no difference was observed in the number of osterix-expressing osteoblasts between sibling and mutant embryos even though mutants are delayed in skeletal mineralization. Insets show a magnification of the operculum to visualize and quantify early osteoblasts.
Figure 2.
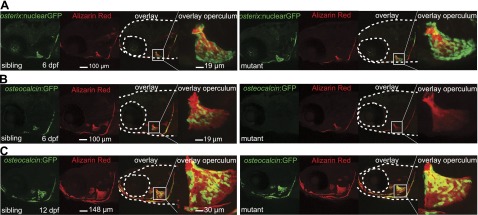
Osterix expression, marking early osteoblasts, in 6-dpf mutant embryos is comparable to siblings, but expression of osteocalcin (marking mature osteoblasts) is delayed in mutant embryos. A) Osterix:nuclear-GFP+ early osteoblasts in 6-dpf sibling vs. mutant embryos. B) Osteocalcin:GFP+ mature osteoblasts are present in 6-dpf siblings, but absent in mutant embryos. C) Osteocalcin:GFP expression is observed in 12-dpf sibling vs. mutant embryos. Insets: magnification of the operculum.
Next, we asked whether mutant osteoblasts have a delay in their maturation and later differentiation. Therefore, we analyzed the expression pattern of osteocalcin, a marker for mature osteoblasts (22, 23). We used a zebrafish osteocalcin:GFP transgenic fish reporter line (24) and, as shown in Fig. 2B, mutants did not express osteocalcin at 6 dpf, while in siblings, expression is observed in the cleithrum and operculum. At later stages of development (12 dpf), osteocalcin expression was also observed in mutants (Fig. 2C). However, we observed no difference in the expression of other mineralization markers, such as alkaline phosphatase (alp) and type I collagen (colI) (Supplemental Fig. S2). Together, these data show that osteoblasts are present, but that specific aspects of osteoblast maturation are delayed in msp mutants.
The mutant phenotype is caused by a mutation in the gene encoding macrophage-stimulating protein
To identify the molecular lesion responsible for the mutant phenotype, we used SSLP mapping. Single-embryo mapping on a limited number of embryos positioned the mutation between two flanking SSLP markers, CA152 and Z80 (Fig. 3A), on chromosome 6. The single gene flanked by both markers was msp (8). Sequencing of the zebrafish msp gene in mutant and sibling embryos revealed a C → T transversion in the 8th coding exon (Fig. 3D). This mutation resulted in an Arg > stop mutation at position 291 of the predicted protein (Fig. 3B, C). In situ hybridization for msp showed high levels of expression in the liver of wild-type embryos, but severely down-regulated mRNA levels in homozygous mutants (Fig. 3E). To exclude the possibility that a general delay of liver development was responsible for the down-regulated msp expression in the mutants, we performed in situ hybridizations for the liver-specific marker prox1 (16). As shown in Fig. 3F, a comparable level of prox1 expression was observed in the liver of msp mutants and wild-type embryos.
Exogenous calcium rescues the msp mutant phenotype
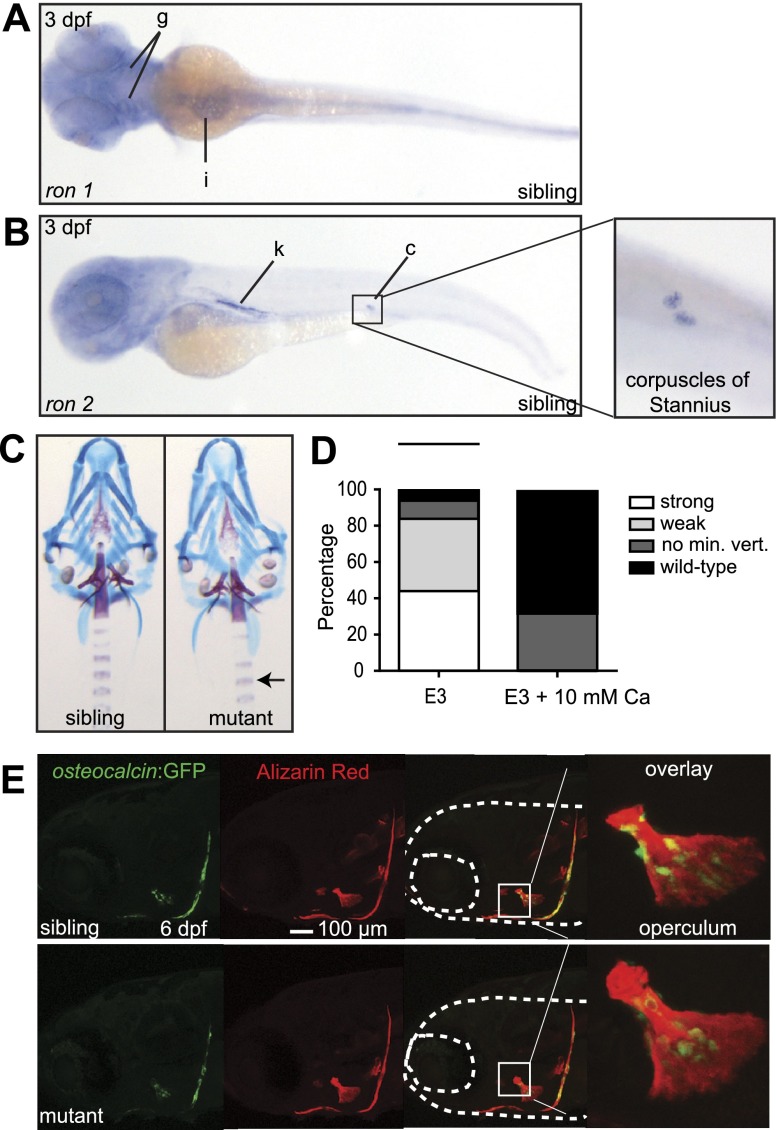
Msp is a ligand for the receptor tyrosine kinase Ron (25, 26), also known as macrophage-stimulating protein receptor 1 (MST1R). We analyzed the embryonic expression pattern of ron by whole-mount in situ hybridization. Because of a third round of genome duplication before the radiation of the teleostean lineage (27), ron is duplicated in zebrafish, resulting in the presence of two genes, ron-1 and ron-2. We observed high levels of ron-1 expression in the intestine and the gills of 3-dpf fish embryos (Fig. 4A), whereas ron-2 was highly expressed in the kidney and corpuscles of Stannius (Fig. 4B). No difference in ron-1 and ron-2 expression was observed between siblings and mutants (data not shown). Since the corpuscles of Stannius are unique organs that regulate calcium metabolism in fish (28), we hypothesized that msp mutants may have a disruption in calcium homeostasis. Therefore, we investigated whether increasing the calcium concentration in E3 medium would rescue the delayed bone formation phenotype. As shown in Fig. 4C, D, the addition of 10 mM calcium to E3 medium resulted in a significant rescue of the mutant phenotype. Rescued msp mutants even contain mineralized centra of the vertebrae (Fig. 4C, arrow) which we rarely observed in mutants grown in normal E3 medium. These experiments clearly demonstrate that the msp mutant phenotype is highly dependent on the calcium content in the medium.
Figure 4.
Excess calcium rescues the msp mutant phenotype and restores osteocalcin expression. A, B) Whole-mount in situ hybridization of 3-dpf embryos showing increased expression of ron-1 (A) in the gills (g) and intestine (i) (ventral view) and ron-2 (B) in the kidney (k) and corpuscles of Stannius (c) (lateral view). Inset: higher magnification of the corpuscles of Stannius. C) Alizarin red and Alcian blue staining of 6-dpf wild-type vs. mutant embryos treated with 10 mM calcium in E3 medium. Note the complete phenotypic rescue of mutant individuals, including Alizarin red staining in the vertebrae (arrow). D) Bar graphs represent percentages of rescued mutant embryos in E3 medium compared E3 medium with 10 mM calcium at 6 dpf (average of 3 independent experiments, n=12/experiment). E3 medium with 10 mM calcium significantly rescued the msp mutant phenotype when compared to E3 medium (P<0.001 by chi square test). E) Osteocalcin:GFP+ osteoblasts are present in 6-dpf sibling and mutant embryos treated with 10 mM calcium in E3 medium. Insets: magnification of the operculum, showing the presence of mature osteoblast cells.
To determine whether osteoblast maturation in msp mutants was also calcium concentration dependent, we analyzed osteocalcin expression in 6-dpf embryos grown in the presence of 10 mM calcium. As shown in Fig. 4E, high levels of calcium in the E3 medium resulted in osteocalcin expression levels in msp mutants comparable to those found in wild-type embryos (Fig. 2C). Interestingly, the extra calcium did not have an anabolic effect on skeletal mineralization (Fig. 4C) or osteocalcin expression in the wild-type embryos (Fig. 4E). Collectively, these data suggest that msp regulates aspects of calcium metabolism necessary for osteoblast maturation. This consequently affects normal embryonic skeletal mineralization in fish.
Knockdown of ron-2 delays embryonic bone formation
To investigate whether Msp binding to Ron affects embryonic bone formation, we silenced ron-1 and ron-2 in zebrafish embryos using MOs. Knockdown using 16 ng ron-1 (MOron-1) was confirmed at the molecular level to completely abolish ron-1 wild-type mRNA (Supplemental Fig. S3B), but absence of ron-1 mRNA did not result in a phenotype. However, knockdown of ron-2 via MO (32 ng) targeting the ATG site (MOron-2-ATG) significantly delayed embryonic bone formation (Fig. 5A, B). Another MO, targeting the UTR site of ron-2 (MOron-2-UTR; 16 ng) also delayed embryonic bone formation (Fig. 5B). As shown in Supplemental Fig. S3C, translation of capped mRNA was efficiently blocked by the respective MOs.
Figure 5.
Knockdown of ron-2 delays embryonic skeletal mineralization in zebrafish and phenocopies the msp mutant phenotype. A) Alizarin red and Alcian blue skeletal staining of zebrafish morphants. Variation of the morphant phenotype is observed at 6 dpf and can be classified into a strong phenotype, a weak phenotype, and a wild-type phenotype. B) Bar graphs represent the percentage of embryos at 6 dpf, which develop a wild-type phenotype (black), a weak phenotype (gray) or a strong phenotype (white). Injection of 32 ng MOron2-ATG (average of 3 independent experiments, n=42, n=11, n=45) significantly delayed embryonic skeletal mineralization when compared to the uninjected control (uic, n=60 for each experiment; P<0.001 by χ2 test). Injection of 16 ng MOron2-UTR (n=73) also inhibited bone formation when compared to the uic (P<0.001; χ2 test).
These results indicate that Msp binding specifically to Ron-2 is required for embryonic skeletal mineralization and that interference with the Msp/Ron-2 signaling axis affects calcium homeostasis and causes a delayed skeletal mineralization phenotype.
Calcium-regulating factors are affected in msp mutants
A group of cells known to extract calcium from water are the so-called mitochondria-rich chloride cells in the yolk sac membrane (7). By staining msp mutants and siblings at 3 dpf with DASPEI (20), we visualized these cells but observed no difference between wild-type and mutant embryos derived from heterozygous crosses (Supplemental Fig. S4) or derived from homozygous parents (data not shown). Because also the morphology of the corpuscles of Stannius appeared unaltered in mutants (Supplemental Fig. S2), we reasoned that expression levels of genes involved in calcium homeostasis might be affected in mutants. Hence, we performed microarray experiments on 3- and 6-dpf mutant and wild-type embryos (Gene Expression Omnibus: GSE33414). Analysis of the microarray data suggested that indeed calcium-regulating hormones, such as parathyroid hormone 2 (pth2) and stanniocacin 1-like (stc1l), are disturbed at 3 and 6 dpf (Table 3). On the basis of these findings, we further quantified (by qPCR) the expression levels of calcium-regulating factors, such as stc1l, pth1, pth2, and the epithelial calcium channel (trpv5/6) during the recovery of the mineralization defects in msp mutants (6 and 12 dpf). At 6 dpf, pth2 and stc1l were significantly down-regulated (Fig. 6A) in msp mutants, and at 12 dpf, pth1, pth2, stc1l, and trpv5/6 were significantly up-regulated in msp mutants (Fig. 6B). These data demonstrate that calcium regulating factors are affected in msp mutants, which substantiates that calcium homeostasis is disturbed in msp mutants.
Table 3.
Microarray results of pth2 and stcl on 3- and 6-dpf msp mutant vs. wild-type fish embryos (GSE33414)
| Gene | ID_REF | 3 dpf |
6 dpf |
||
|---|---|---|---|---|---|
| wtcy3/mutcy5, sample 1 (log2) | wtcy5/mutcy3, sample 2 (log2) | wtcy3/mutcy5, sample 3 (log2) | wtcy5/mutcy3, sample 4 (log2) | ||
| stc1l | 1461 | 0.09 | −0.75 | −0.85 | −0.91 |
| pth2 | 20885 | −0.13 | −0.66 | −1.11 | −1.17 |
| pth2 | 31725 | −0.29 | −0.62 | −1.41 | −1.34 |
Figure 6.
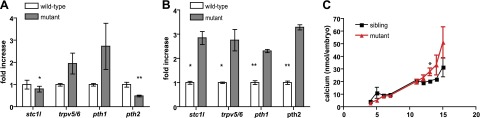
During the recovery of the msp skeletal phenotype, expression of calcium-regulating factors are significantly affected in msp mutants, and the total calcium content is mildly increased. A, B) qPCR at 6 dpf (A) and at 12 dpf (B) revealed that compared with wild types (white bars), stc1l and pth2 are significantly down-regulated in msp mutant embryos (gray bars) at 6 dpf. At 12 dpf, stc1l, trpv5/6, pth1, and pth2 are significantly up-regulated in msp mutant embryos. qPCR was performed as described in Materials and Methods. Results are expressed as means ± se of 3 independent experiments. C) Total calcium concentration in 3-15 dpf msp mutant (▲) vs. sibling (■) fish larvae. Results are expressed as means ± se of 2 independent experiments; 3 different fish embryos/larvae derived from 1 egg lay were used for each time point. *P < 0.05, **P < 0.005.
Next, we measured the total calcium content during the recovery of the mineralization defect in msp mutants and siblings. As shown in Fig. 6C, the total calcium concentration was mildly increased in phenotypically recovered msp mutant larvae at 12 dpf. This suggests that msp mutant larvae are able to overcome the decreased skeletal mineralized phenotype, possibly by adaptation in the expression of calcium-regulating factors and by extracting additional calcium from food and/or water.
DISCUSSION
In this study, we have identified Msp [also known as hepatocyte growth factor-like (HGFL) protein] as a novel factor involved in calcium homeostasis, necessary for normal embryonic bone formation in zebrafish. In mammals, MSP has been shown to be synthesized mainly by liver cells (29) and to circulate in the blood as an inactive single polypeptide protein (30). Liver expression is also found in zebrafish. Circulating MSP can be proteolytically cleaved by serum and membrane-bound proteases to form an active disulfide linked heterodimer (30–32). This active form of MSP can then bind to, and activate, the receptor tyrosine kinase Ron (also called STK), resulting in the activation of a signaling cascade (26, 33). The premature stop codon (exon 8) in the mutant is located within the third kringle domain, resulting in a mutant protein devoid of the light chain. Studies toward the functional characterization of Msp domains have shown that mutant proteins lacking the light chain are not biologically active (34). This, in conjunction with the severely reduced expression levels of msp mRNA in mutants, leads us to conclude that msp t34230 encodes a loss-of-function allele.
To date, several functions have been attributed to Msp, including the ability to stimulate mouse resident peritoneal macrophages (35), to induce cellular proliferation (26), to induce cell motility (36), to bring about apoptosis (37), and to stimulate bone resorption (38). These functions are thought to be caused by the binding of Msp to its membrane-bound receptor Ron (26, 33). The various effects of Msp appear to be dependent on cell type and/or Ron expression levels, with no single cell type showing all mentioned effects. Our current study suggests a novel function of Msp and Ron, namely, the regulation of calcium homeostasis.
Before gills become functional, teleost embryos extract calcium from the yolk and then from the ambient medium via ionocytes (chloride cells), in the yolk sac membrane and nearby skin regions (7, 39). However, ron expression was not observed in these regions nor did we observe a difference in the number or size of mitochondria-rich chloride cells. Instead, we clearly observed high levels of ron-2 transcripts in the kidney and corpuscles of Stannius. The expression of ron-2 in the corpuscles of Stannius particularly points toward a role of Msp in calcium homeostasis. The corpuscles of Stannius are small endocrine glands located in or around the kidney and are unique for bony fish (28). The corpuscles of Stannius produce stanniocalcin, a hormone that functions to prevent hypercalcemia (28) and that is released into the bloodstream in response to elevations in plasma calcium (40). In line with this, we observed that knockdown of ron-2 also delayed embryonic skeletal mineralization in zebrafish.
The normal appearance of chloride cells and of the kidney, and the presence of the corpuscles of Stannius clearly indicated that the cells and organs implicated in calcium homeostasis are present in msp mutants. Hence, we turned to analyzing the expression levels of genes correlated with calcium homeostasis by microarray and qPCR. At 6 dpf, before the onset of feeding, we observed significant down-regulation of stc1l expression, while at 12 dpf, msp mutants showed increased levels of stc1l and mildly increased levels of total calcium content. The up-regulated stc1l expression at 12 dpf is possibly a reaction to the net calcium movement, or could be a consequence of increased calcium uptake by feeding and drinking. Expression levels of the calcium channel trpv5/6 were also up-regulated, consistent with the mildly increased calcium content. Previously, a tight relation among extracellular calcium, stc1l and trpv5/6 has been demonstrated (41–43), and knockdown of stc1l has been shown to increase trpv5/6 expression in zebrafish at 2 dpf (42). Our findings demonstrate that this correlation is not present in 12 dpf msp mutants, but we carried out our analysis at a different developmental stage than Tseng et al. (42). Therefore, not all of these measurements are directly comparable.
We also studied expression levels of the calciotropic hormone PTH in msp mutants. The pth gene is duplicated in zebrafish (44), and pth1 expression has been shown to decrease when zebrafish are maintained in medium with increased calcium (45). Before onset of feeding (6 dpf), we observed a significant decrease in pth2 levels in msp mutants, while in feeding larvae (12 dpf) pth1 and pth2 are both significantly up-regulated in msp mutants, and mutants had mildly increased levels of calcium. This indicates that msp mutants are activating differential mechanisms to compensate for the disturbed calcium homeostasis under various physiologically challenging circumstances during development. Indeed, the expression of pth or pth receptors has previously been shown to be differentially regulated depending on developmental time and experimental conditions (41, 45).
Msp-deficient mice are viable and grow to adulthood with few visible defects (46); hence, the adult murine Msp mutant phenotype is in line with the msp zebrafish mutant phenotype. At adult stages, both teleost and mammals extract calcium from food. During embryonic stages, however, there are differences between embryos of these two vertebrate classes, as mouse embryos develop in utero and acquire calcium from the mother during embryonic stages. Therefore, in order to detect a delay in embryonic bone formation in Msp-deficient mice, one might have to keep homozygous mutant mothers on a restricted calcium diet, and even then a clear embryonic phenotype might still be hard to detect (due to resorption of calcium from maternal calcium storage). An advantage of using zebrafish for embryonic bone development is that we were able to control the surrounding calcium environment of the embryos. Next to that, we observed that as soon as we started feeding the fish larvae, the osteoblasts matured, and bone was formed. Therefore, we suggest that the recovery of the phenotype is very likely due to the fact that the fish larvae extract calcium from the food.
In summary, in this study we identified a novel function for Msp and Ron, and show that Msp plays a role in embryonic skeletal mineralization and calcium homeostasis. A null mutation was shown to delay embryonic bone formation in fish, and Ron, the receptor for Msp, was demonstrated to be specifically expressed in organs controlling calcium homeostasis. Knockdown of ron-2 also delayed embryonic bone formation. Growing msp mutant embryos in medium containing 10 mM calcium resulted in normal osteoblast maturation and bone formation. On the basis of the findings of this study, we postulate that the Msp/Ron signaling pathway regulates calcium homeostasis.
Supplementary Material
Acknowledgments
The authors thank the Tübingen 2000 screen consortium members for identifying the mspt34230 mutant phenotype; this mutant was identified while S.S.-M. was working at Exelixis Deutschland GmbH (Tübingen, Germany). Many thanks also go to C. Hammond and J. Vanoevelen for editorial review and W. Atsma for calcium measurements. The authors acknowledge the Hubrecht Institute Imaging Center for expert advice on imaging. All members of the S.S.-M. laboratory are acknowledged for comments and scientific discussions.
S.S.-M. gratefully recognizes the support of the Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture, and Science.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- alp
- alkaline phosphatase
- col10a1
- type X collagen α1
- DASPEI
- 2-(4-dimethylamino)styryl)-N-ethylpyridinium iodide
- dpf
- days postfertilization
- E3
- embryonic medium 3
- GFP
- green fluorescent protein
- HGFL
- hepatocyte growth factor like
- hpf
- hours postfertilization
- MO
- morpholino
- MS-222
- 3-aminobenzoic acid ethyl ester
- MSP
- macrophage stimulating protein
- MST1R
- macrophage stimulating protein receptor
- PBS
- phosphate buffered saline
- PFA
- paraformaldehyde
- pH3
- phospho-histone
- prox1
- prospero homeobox protein 1
- pth2
- parathyroid hormone 2
- qPCR
- quantitative PCR
- rag1
- recombination activating gene 1
- SSLP
- simple sequence length polymorphism
- stc1l
- stanniocalcin 1-like
REFERENCES
- 1. Blair H. C., Zaidi M., Schlesinger P. H. (2002) Mechanisms balancing skeletal matrix synthesis and degradation. Biochem. J. 364, 329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karsenty G. (2008) Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 [DOI] [PubMed] [Google Scholar]
- 3. Teitelbaum S. L. (2000) Bone resorption by osteoclasts. Science 289, 1504–1508 [DOI] [PubMed] [Google Scholar]
- 4. Bushinsky D. A. (2010) Contribution of intestine, bone, kidney, and dialysis to extracellular fluid calcium content. Clin. J. Am. Soc. Nephrol. 5(Suppl. 1), S12–S22 [DOI] [PubMed] [Google Scholar]
- 5. Mundy G. R., Guise T. A. (1999) Hormonal control of calcium homeostasis. Clin. Chem. 45, 1347–1352 [PubMed] [Google Scholar]
- 6. Flik G., Verbost P. M. (1995) Cellular mechanisms in calcium transport and homeostasis in fish. In: Biochemistry and Molecular Biology of Fishes (Hochachka P. W., Mommsen T. P., eds) pp. 251–265, Elsevier, Amsterdam [Google Scholar]
- 7. Flik G., Verbost P. M., Wendelaar Bonga S. E. (1995) Calcium transport processes in fishes. Cell. Mol. Approaches Fish Ionic Regul. 14, 317–342 [Google Scholar]
- 8. Shimamoto A., Kimura T., Matsumoto K., Nakamura T. (1993) Hepatocyte growth factor-like protein is identical to macrophage stimulating protein. FEBS Lett. 333, 61–66 [DOI] [PubMed] [Google Scholar]
- 9. Brand M., Granato M., Nüsslein-Volhard (2002) Keeping and raising zebrafish. In: Zebrafish, A Practical Approach (Nüsslein-Volhard C., Dahm R., eds) pp. 7–37, New York, Oxford University Press [Google Scholar]
- 10. Haffter P., Granato M., Brand M., Mullins M. C., Hammerschmidt M., Kane D. A., Odenthal J., van Eeden F. J., Jiang Y. J., Heisenberg C. P., Kelsh R. N., Furutani-Seiki M., Vogelsang E., Beuchle D., Schach U., Fabian C., Nusslein-Volhard C. (1996) The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 [DOI] [PubMed] [Google Scholar]
- 11. Spoorendonk K. M., Hammond C. L., Huitema L. F. A., Vanoevelen J., Schulte-Merker S. (2009) Zebrafish as a unique model system in bone research: the power of genetics and in vivo imaging. J. Appl. Ichthyol. 26, 219–224 [Google Scholar]
- 12. Hammond C. L., Schulte-Merker S. (2009) Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development 136, 3991–4000 [DOI] [PubMed] [Google Scholar]
- 13. Spoorendonk K. M., Peterson-Maduro J., Renn J., Trowe T., Kranenbarg S., Winkler C., Schulte-Merker S. (2008) Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 135, 3765–3774 [DOI] [PubMed] [Google Scholar]
- 14. Schulte-Merker S. (2002) Looking at embryos. In Zebrafish, A Practical Approach (Nüsslein-Volhard C., Dahm R., eds) pp. 41–43, Oxford University Press, New York [Google Scholar]
- 15. Bassett D. I. (2003) Identification and developmental expression of a macrophage stimulating 1/ hepatocyte growth factor-like 1 orthologue in the zebrafish. Dev. Genes Evol. 213, 360–362 [DOI] [PubMed] [Google Scholar]
- 16. Ober E. A., Verkade H., Field H. A., Stainier D. Y. (2006) Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688–691 [DOI] [PubMed] [Google Scholar]
- 17. Willett C. E., Zapata A. G., Hopkins N., Steiner L. A. (1997) Expression of zebrafish rag genes during early development identifies the thymus. Dev. Biol. 182, 331–341 [DOI] [PubMed] [Google Scholar]
- 18. Nasevicius A., Ekker S. C. (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- 19. Hogan B. M., Bos F. L., Bussmann J., Witte M., Chi N. C., Duckers H. J., Schulte-Merker S. (2009) Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 [DOI] [PubMed] [Google Scholar]
- 20. Varsamos S., Diaz J. P., Charmantier G., Blasco C., Connes R., Flik G. (2002) Location and morphology of chloride cells during the post-embryonic development of the european sea bass, Dicentrarchus labrax. Anat. Embryol. 205, 203–213 [DOI] [PubMed] [Google Scholar]
- 21. Guerreiro P. M., Fuentes J., Flik G., Rotllant J., Power D. M., Canario A. V. (2004) Water calcium concentration modifies whole-body calcium uptake in sea bream larvae during short-term adaptation to altered salinities. J. Exp. Biol. 207, 645–653 [DOI] [PubMed] [Google Scholar]
- 22. Inohaya K., Takano Y., Kudo A. (2007) The teleost intervertebral region acts as a growth center of the centrum: in vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev. Dyn. 236, 3031–3046 [DOI] [PubMed] [Google Scholar]
- 23. Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. (1976) Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. U. S. A. 73, 1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knopf F., Hammond C., Chekuru A., Kurth T., Hans S., Weber C. W., Mahatma G., Fisher S., Brand M., Schulte-Merker S., Weidinger G. (2011) Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Cell. 20, 713–724 [DOI] [PubMed] [Google Scholar]
- 25. Wang M. H., Iwama A., Skeel A., Suda T., Leonard E. J. (1995) The murine stk gene product, a transmembrane protein tyrosine kinase, is a receptor for macrophage-stimulating protein. Proc. Natl. Acad. Sci. U. S. A. 92, 3933–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaudino G., Follenzi A., Naldini L., Collesi C., Santoro M., Gallo K. A., Godowski P. J., Comoglio P. M. (1994) RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 13, 3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Postlethwait J. H. (2007) The zebrafish genome in context: ohnologs gone missing. J. Exp. Zool. B Mol. Dev. Evol. 308, 563–577 [DOI] [PubMed] [Google Scholar]
- 28. Gerritsen M. E., Wagner G. F. (2005) Stanniocalcin: no longer just a fish tale. Vitam. Horm. 70, 105–135 [DOI] [PubMed] [Google Scholar]
- 29. Bezerra J. A., Witte D. P., Aronow B. J., Degen S. J. (1993) Hepatocyte-specific expression of the mouse hepatocyte growth factor-like protein. Hepatology 18, 394–399 [PubMed] [Google Scholar]
- 30. Wang M. H., Skeel A., Leonard E. J. (1996) Proteolytic cleavage and activation of pro-macrophage-stimulating protein by resident peritoneal macrophage membrane proteases. J. Clin. Invest. 97, 720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang M. H., Yoshimura T., Skeel A., Leonard E. J. (1994) Proteolytic conversion of single chain precursor macrophage-stimulating protein to a biologically active heterodimer by contact enzymes of the coagulation cascade. J. Biol. Chem. 269, 3436–3440 [PubMed] [Google Scholar]
- 32. Wang M. H., Gonias S. L., Skeel A., Wolf B. B., Yoshimura T., Leonard E. J. (1994) Proteolytic activation of single-chain precursor macrophage-stimulating protein by nerve growth factor-gamma and epidermal growth factor-binding protein, members of the kallikrein family. J. Biol. Chem. 269, 13806–13810 [PubMed] [Google Scholar]
- 33. Wang M. H., Ronsin C., Gesnel M. C., Coupey L., Skeel A., Leonard E. J., Breathnach R. (1994) Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science 266, 117–119 [DOI] [PubMed] [Google Scholar]
- 34. Waltz S. E., McDowell S. A., Muraoka R. S., Air E. L., Flick L. M., Chen Y. Q., Wang M. H., Degen S. J. (1997) Functional characterization of domains contained in hepatocyte growth factor-like protein. J. Biol. Chem. 272, 30526–30537 [DOI] [PubMed] [Google Scholar]
- 35. Leonard E. J., Skeel A. H. (1978) Isolation of macrophage stimulating protein (MSP) from human serum. Exp. Cell Res. 114, 117–126 [DOI] [PubMed] [Google Scholar]
- 36. Santoro M. M., Collesi C., Grisendi S., Gaudino G., Comoglio P. M. (1996) Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol. Cell. Biol. 16, 7072–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iwama A., Yamaguchi N., Suda T. (1996) STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 15, 5866–5875 [PMC free article] [PubMed] [Google Scholar]
- 38. Kurihara N., Iwama A., Tatsumi J., Ikeda K., Suda T. (1996) Macrophage-stimulating protein activates STK receptor tyrosine kinase on osteoclasts and facilitates bone resorption by osteoclast-like cells. Blood 87, 3704–3710 [PubMed] [Google Scholar]
- 39. Varsamos S., Nebel C., Charmantier G. (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141, 401–429 [DOI] [PubMed] [Google Scholar]
- 40. Wagner G. F., Milliken C., Friesen H. G., Copp D. H. (1991) Studies on the regulation and characterization of plasma stanniocalcin in rainbow trout. Mol. Cell. Endocrinol. 79, 129–138 [DOI] [PubMed] [Google Scholar]
- 41. Lafont A. G., Wang Y. F., Chen G. D., Liao B. K., Tseng Y. C., Huang C. J., Hwang P. P. (2011) Involvement of calcitonin and its receptor in the control of calcium-regulating genes and calcium homeostasis in zebrafish (Danio rerio). J. Bone Min. Res. 26, 1072–1083 [DOI] [PubMed] [Google Scholar]
- 42. Tseng D. Y., Chou M. Y., Tseng Y. C., Hsiao C. D., Huang C. J., Kaneko T., Hwang P. P. (2009) Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R549–R557 [DOI] [PubMed] [Google Scholar]
- 43. Vanoevelen J., Janssens A., Huitema L. F., Hammond C. L., Metz J. R., Flik G., Voets T., Schulte-Merker S. (2011) Trpv5/6 is vital for epithelial calcium uptake and bone formation. FASEB J. 25, 3197–3207 [DOI] [PubMed] [Google Scholar]
- 44. Hogan B. M., Danks J. A., Layton J. E., Hall N. E., Heath J. K., Lieschke G. J. (2005) Duplicate zebrafish pth genes are expressed along the lateral line and in the central nervous system during embryogenesis. Endocrinology 146, 547–551 [DOI] [PubMed] [Google Scholar]
- 45. Hoshijima K., Hirose S. (2007) Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J. Endocrinol. 193, 481–491 [DOI] [PubMed] [Google Scholar]
- 46. Bezerra J. A., Carrick T. L., Degen J. L., Witte D., Degen S. J. (1998) Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J. Clin. Invest. 101, 1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.