Abstract
In this study, we developed anionic polymer-coated liposome/siRNA complexes (lipoplexes) with chondroitin sulfate C (CS), poly-l-glutamic acid (PGA) and poly-aspartic acid (PAA) for siRNA delivery by intravenous injection, and evaluated the biodistribution and gene silencing effect in mice. The sizes of CS-, PGA- and PAA-coated lipoplexes were about 200?nm and their ?-potentials were negative. CS-, PGA- and PAA-coated lipoplexes did not induce agglutination after mixing with erythrocytes. In terms of biodistribution, siRNAs after intravenous administration of cationic lipoplexes were largely observed in the lungs, but those of CS-, PGA- and PAA-coated lipoplexes were in both the liver and the kidneys, indicating that siRNA might be partially released from the anionic polymer-coated lipoplexes in the blood circulation and accumulate in the kidney, although the lipoplexes can prevent the agglutination with blood components. To increase the association between siRNA and cationic liposome, we used cholesterol-modified siRNA (siRNA-Chol) for preparation of the lipoplexes. When CS-, PGA- and PAA-coated lipoplexes of siRNA-Chol were injected into mice, siRNA-Chol was mainly observed in the liver, not in the kidneys. In terms of the suppression of gene expression in vivo, apolipoprotein B (ApoB) mRNA in the liver was significantly reduced 48?h after single intravenous injection of PGA-coated lipoplex of ApoB siRNA-Chol (2.5?mg?siRNA/kg), but not cationic, CS- and PAA-coated lipoplexes. In terms of toxicity after intravenous injection, CS-, PGA- and PAA-coated lipoplexes did not increase GOT and GPT concentrations in blood. From these findings, PGA coatings for cationic lipoplex of siRNA-Chol might produce a systemic vector of siRNA to the liver.
Keywords: Liposome, Anionic polymer, siRNA delivery, Chondroitin sulfate, Poly-l-glutamic acid, Poly-aspartic acid
Graphical abstract
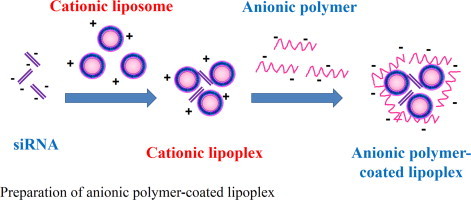
1. Introduction
RNA interference (RNAi) is a powerful gene-silencing process that holds great promise in the field of gene therapy. Synthetic small interfering RNAs (siRNAs), which are small double-stranded RNAs, are substrates for the RNA-induced silencing complex. However, there are challenges associated with the in vivo delivery of siRNA, such as enzymatic instability and low cellular uptake. In siRNA delivery, non-viral vectors such as cationic liposomes and cationic polymers have been more commonly used than viral vectors. Of all the carriers, lipid-based formulations such as cationic liposomes are currently the most widely validated means for systemic delivery of siRNA to the liver. The liver is an important organ with a number of potential therapeutic siRNA targets including cholesterol biosynthesis, fibrosis, hepatitis and hepatocellular carcinoma. For efficient siRNA delivery to liver by cationic liposome, the cationic liposome/siRNA complex (lipoplex) must be stabilized in the blood by avoiding its agglutination with blood components, and the pharmacokinetics of lipoplex after intravenous injection must be controlled. This is because electrostatic interactions between positively charged lipoplex and negatively charged erythrocytes cause agglutination [1], and the agglutinates contribute to high entrapment of lipoplex in the highly extended lung capillaries [2]. PEGylation on the surface of cationic lipoplex (PEG-modified lipoplex) can decrease accumulation in the lungs by preventing association with blood components; however, the PEGylation abolishes the effect of gene suppression by siRNA owing to high stability of the lipoplex.
One promising approach for overcoming this problem is electrostatic encapsulation of cationic lipoplex with anionic biodegradable polymers such as chondroitin sulfate (CS) and poly-l-glutamic acid (PGA). These anionic polymer coatings for lipoplex of plasmid DNA (pDNA) can prevent the agglutination with blood components [3,4]. Recently, we developed anionic polymer-coated lipoplex of pDNA and found that CS and PGA coatings for cationic lipoplex produced safe systemic vectors [5]. Anionic polymer-coated lipoplexes have already been developed for pDNA delivery; however, there is little information about the use of the anionic polymer-coated lipoplexes for siRNA delivery. Therefore, in this study, we prepared anionic polymer-coated lipoplexes with CS, PGA and poly-aspartic acid (PAA) and examined the biodistribution and gene silencing effect in the liver after intravenous injection into mice.
2. Materials and methods
2.1. Materials
1,2-Dioleoyl-3-trimethylammonium-propane methyl sulfate salt (DOTAP) was obtained from Avanti Polar Lipids Inc. (Alabaster, AL, USA). Poly-l-glutamic acid sodium salt (PGA, 10.5?kDa) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Poly-(a,ß)-dl-aspartic acid (PAA, 21?kDa) was obtained from the PolySciTech division of Akina, Inc. (West Lafayette, IN, USA). Cholesterol (Chol) and chondroitin sulfate C sodium salt (CS) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals were of the finest grade available.
2.2. Cell culture
Human breast cancer MCF-7-Luc (TamR-Luc#1) cells stably expressing firefly luciferase (pGL3) were donated by Dr. Kazuhiro Ikeda (Division of Gene Regulation and Signal Transduction, Research Center for Genomic Medicine, Saitama Medical University, Saitama, Japan) [6]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100?µg/ml kanamycin and 0.5?mg/ml G418 at 37?°C in a 5% CO2 humidified atmosphere.
2.3. siRNA
siRNAs targeting nucleotides of firefly pGL3 luciferase (Luc siRNA), Cy5.5-labeled Luc siRNA (Cy5.5-siRNA), Luc siRNA conjugated with cholesterol (Luc siRNA-Chol), Cy5.5-labeled Luc siRNA conjugated with cholesterol (Cy5.5-siRNA-Chol), nonsilencing siRNA (Cont siRNA) as a negative control for Luc siRNA, Cont siRNA conjugated with cholesterol (Cont siRNA-Chol) as a negative control for Luc siRNA-Chol, cholesterol-modified apolipoprotein B siRNA (ApoB siRNA-Chol) and Cont siRNA-Chol as a negative control for ApoB siRNA-Chol were synthesized by Sigma Genosys (Tokyo, Japan). The siRNA sequences of the Luc siRNA were as follows: sense strand: 5'-GUGGAUUUCGAGUCGUCUUAA-3', and antisense strand: 5'-AAGACGACUCGAAAUCCACAU-3. In Cy5.5-siRNA and Cy5.5-siRNA-Chol, Cy5.5 dye was conjugated at the 5'-end of the sense strand, and cholesterol was at the 3'-end of the sense strand. The siRNA sequences of the Cont siRNA were as follows: sense strand: 5'-GUACCGCACGUCAUUCGUAUC-3', and antisense strand: 5'-UACGAAUGACGUGCGGUACGU-3' [7]. In Luc siRNA-Chol and Cont siRNA-Chol, cholesterol was conjugated at the 3'-end of the sense strand. The siRNA sequences of the apolipoprotein B (ApoB) siRNA-Chol were as follows [8]: sense strand: 5'-GUCAUCACACUGAAUACCAAU*Chol-3', and antisense strand: 5'-AUUGGUAUUCAGUGUGAUGAc*a*C-3'. The siRNA sequences of the Cont siRNA-Chol were as follows : sense strand: 5'-GAACUGUGUGUGAGAGGUCCU*Chol-3', and antisense strand: 5'-AGGACCUCUCACACACAGUUc*g*C-3'. The lower-case letters represent 2'-O-methyl-modified nucleotides; asterisks represent phosphorothioate linkages.
2.4. Preparation of liposome and lipoplex
Cationic liposome was prepared from DOTAP/Chol at a molar ratio of 1/1 by a thin-film hydration method, as previously reported [9,10]. For preparation of rhodamine-labeled cationic liposome, Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (rhodamine-DHPE, Invitrogen, Carlsbad, CA, USA) was incorporated at 1?mol% into the total lipids. The particle size and ?-potential of cationic liposomes were measured by dynamic light-scattering and electrophoresis light-scattering methods, respectively (ELS-Z2, Otsuka Electronics Co., Ltd., Osaka, Japan). The size of the cationic liposomes was adjusted to approximately 80?nm.
To prepare cationic liposome/siRNA complex (cationic lipoplex), cationic liposome suspension was mixed with siRNA by vortex-mixing for 10?s at a charge ratio (-/+) of 1/4, and left for 15?min at room temperature. The theoretical charge ratio (-/+) of siRNA to cationic liposome was calculated as the molar ratio of siRNA phosphate to DOTAP nitrogen. To prepare ternary complexes with anionic polymers, cationic lipoplex was mixed with CS, PGA and PAA solutions (CS-, PGA- and PAA-coated lipoplexes, respectively) at the indicated charge ratios. The theoretical charge ratios (-/+) of CS, PGA and PAA to DOTAP were calculated as the molar ratios of sulfate and carboxylic acid of CS (two negative charges per disaccharide unit), carboxylic acid of PGA (one negative charge per glutamic acid) and carboxylic acid of PAA (one negative charge per aspartic acid) to nitrogen of DOTAP, respectively.
2.5. Gel retardation assay
After preparation of the cationic lipoplexes, CS-, PGA- and PAA-coated lipoplexes of 1?µg of siRNA or siRNA-Chol at the indicated charge ratios (-/+) of anionic polymer and siRNA to DOTAP, they were analyzed on an 18% acrylamide gel for siRNA in Tris–borate–EDTA (pH 8.0) buffer and were visualized by ethidium bromide staining, as previously reported [11].
2.6. Accessibility of siRNA in lipoplexes
siRNA condensation by anionic polymer-coated lipoplexes was analyzed by exclusion assay using an SYBR® Green I Nucleic Acid Gel Stain (Takara Bio Inc., Shiga, Japan), as previously reported [11]. The anionic polymer-coated lipoplexes of 1?µg of siRNA at various charge ratios (-/+) in a volume of 100?µL of Tris–HCl buffer (pH 8.0) were mixed with 100?µL of 2500-fold diluted SYBR® Green I Nucleic Acid Gel Stain solution with Tris–HCl buffer, and then incubated for 30?min. The fluorescence was measured at an emission wavelength of 521?nm with an excitation wavelength of 494?nm using a fluorescent spectrophotometer, F-2700 (Hitachi Co., Ltd., Tokyo, Japan). As a control, the value of fluorescence obtained upon addition of 5?µg/ml free siRNA solution was set as 100%. The amount of siRNA available to interact with the SYBR® Green I is expressed as a percentage of the control.
2.7. Luciferase activity
MCF-7-Luc cells were prepared by plating cells in a 6-well plate 24?h prior to each experiment. For transfection, each lipoplex of 200?pmol Luc siRNA, Luc-siRNA-Chol, Cont siRNA or Cont siRNA-Chol was diluted in 2?ml of medium supplemented with 10% FBS and then the mixture was added into the cells. Lipofectamine RNAiMax lipoplex (Invitrogen Corp.) was prepared according to the manufacturer’s protocol. Forty-eight hours after the transfection, luciferase activity was measured as counts per s (cps)/µg protein using the luciferase assay system (Pica gene luciferase assay kit; Toyo Ink Mfg. Co., Ltd.) and BCA reagent (Pierce, Rockford, IL, USA), as previously reported [11].
2.8. Agglutination assay
Agglutination assay was performed according to the method described in a previous report [5]. Briefly, erythrocytes were collected from mouse blood at 4?°C by centrifugation at 300g for 3?min and resuspended in PBS as a 2% (v/v) stock suspension of erythrocytes. The anionic polymer-coated lipoplexes of 2?µg of Cont siRNA or siRNA-Chol were added to 100?µL of erythrocyte suspension and then incubated for 15?min at 37?°C. The sample was placed on a glass plate and agglutination was observed by microscopy.
2.9. Biodistribution of anionic polymer-coated lipoplexes in mice
All animal experiments were performed with approval from the Institutional Animal Care and Use Committee of Hoshi University. Cationic and anionic polymer-coated lipoplexes of 50?µg of Cy5.5-siRNA or Cy5.5-siRNA-Chol were intravenously administered via lateral tail veins into female BALB/c mice (7 weeks of age; Sankyo Lab. Service Corp., Tokyo, Japan). One hour after injection, the mice were sacrificed, and the tissues were frozen on dry ice and sliced at 16?µm. The localization of Cy5.5-siRNA was examined using an Eclipse TS100-F microscope (Nikon, Tokyo, Japan).
2.10. Knockdown of liver-specific ApoB mRNA in vivo
Anionic polymer-coated lipoplexes of 50?µg of Cont siRNA-Chol or ApoB siRNA-Chol were intravenously administered via lateral tail veins into mice. At 24?h post-injection, mice were fasted for 24?h. At 48?h post-injection, mice were sacrificed by cervical dislocation and the liver was removed for analysis. Total RNA was isolated from the liver using the NucleoSpin RNA II (Macherey-Nagel, Germany). Mouse ApoB cDNA was amplified using the primers ApoB-FW, 5'-TTCCAGCCATGGGCAACTTTACCT-3', and ApoB-RW, 5'-TACTGCAGGGCGTCAGTGACAAAT-3', as previously reported [12]. Mouse ß-actin cDNA was amplified using the primers ß-actin-FW, 5'-TGTGATGGTGGGAATGGGTCAG-3', and ß-actin-RW, 5'-TTTGATGTCACGCACGATTTCC-3', as previously reported [13]. Quantitative RT-PCR was performed with the iCycler MyiQ detection system (Bio-Rad Laboratories, Hercules, CA, USA) and the SYBR Green I assay (iQ™ SYBER Green Supermix, Bio-Rad Laboratories), as previously described [13]. Samples were run in triplicate and the mRNA expression levels of ApoB were normalized to the amount of ß-actin mRNA in the same sample. A difference of 1 cycle was considered to represent a 2-fold change in gene expression.
2.11. Serum cholesterol level
PGA-coated lipoplexes of 50?µg of ApoB siRNA-Chol were intravenously administered via lateral tail veins into mice. At 24?h post-injection, mice were fasted for 24?h. At 48?h post-injection, blood was collected from the carotid arteries of mice under anesthesia, and allowed to stand for 1?h at 37?°C. Serum low-density-lipoprotein (LDL) cholesterol level was measured using a commercial LDL cholesterol detection kit according to the manufacturer’s instructions (HDL and LDL/VLDL Cholesterol Quantification Kit, Bio Vision Incorporated, Milpitas, CA, USA).
2.12. Determination of plasma transaminase activities
Serum was prepared by separation of the coagulated whole blood of female C57BL/6Cr mice (7 weeks of age; Sankyo Lab. Service Corp., Tokyo, Japan) 24?h after intravenous injection of cationic and anionic polymer-coated lipoplexes of 50?µg of Cont siRNA-Chol. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) activities in the plasma were determined using commercially available test reagents (GPT-UV test Wako and GPT-UV test Wako, respectively; Wako, Osaka, Japan). Normal values were determined using blood obtained from age-matched, untreated mice.
2.13. Statistical analysis
The statistical significance of differences between mean values was determined by using Student’s t-test. A p value of 0.05 or less was considered significant.
3. Results and discussion
3.1. Particle size and ?-potential of various anionic polymer-coated lipoplexes
The cationic lipid, 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), has frequently been used as a cationic lipid for a liposomal delivery system of siRNA by several research groups [14–17]. Among cationic liposomes, DOTAP/Chol liposome is commercially supplied as an in vivo transfection reagent (e.g., in vivo MegaFectin™ from Qbiogene Molecular Biology, in vivo Liposome Transfection Reagent from Sigma-Aldrich), which was demonstrated to have high transfection efficiency in the lungs by intravenous injection. Here, we selected chondroitin sulfate C (CS), poly-l-glutamic acid (PGA) and poly-aspartic acid (PAA) as materials for coating cationic DOTAP/Chol lipoplexes of siRNA and evaluated their potential for use as an siRNA delivery vector.
First, we prepared DOTAP/Chol liposome and measured the particle size and ?-potential. The liposome size was about 80?nm and the ?-potential was +50?mV. When the liposomes were mixed with siRNA, the lipoplex size was about 280?nm and the ?-potential was +40?mV. Next, we coated the lipoplexes with anionic polymers, CS, PGA and PAA, at various charge ratios (-/+), and prepared CS-, PGA- and PAA-coated lipoplexes. With increasing amounts of CS, PGA and PAA being added to the lipoplex, their sizes decreased to 150–200?nm and ?-potential to a negative value (Fig. 1A–C). Although the sizes of CS-, PGA- and PAA-coated lipoplexes were smaller than that of cationic lipoplex, the anionic polymers may be able to strongly compact the cationic lipoplex by the electrostatic interaction. The ?-potentials of the lipoplexes after the addition of anionic polymers were almost consistently negative around charge ratios (-/+) of 1 in CS, 1.5 in PGA and 1.5 in PAA, indicating that nitrogen of cationic lipoplex was completely covered with a sulfate group or a carboxyl group of the anionic polymers. In a previous study, we reported that ?-potentials of the lipoplexes of pDNA after the addition of anionic polymers were almost consistently negative around charge ratios (-/+) of 5.8 in CS and 7 in PGA [5]. The amount of anionic polymer needed for covering cationic lipoplex of siRNA was sufficient at a lower level than for the lipoplex of pDNA. Therefore, in subsequent experiments, we decided to use 1 in CS, 1.5 in PGA and 1.5 in PAA as optimal charge ratios (-/+) for the preparation of anionic polymer-coated lipoplex.
Fig. 1.
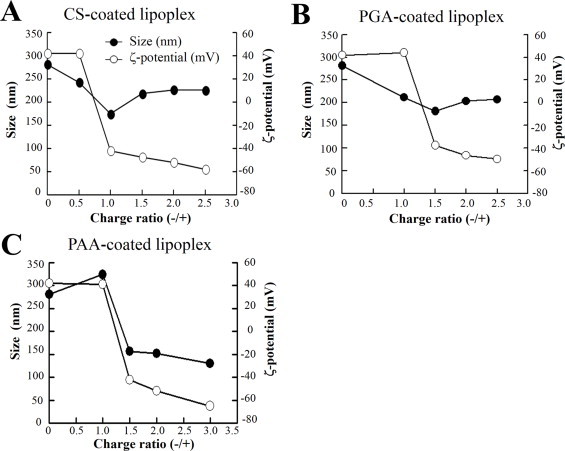
Effect of charge ratio of anionic polymer to cationic lipoplex of siRNA on particle size and ?-potential of anionic polymer-coated lipoplexes. Charge ratio (-/+) indicates the molar ratios of sulfate and/or carboxylic acid of anionic polymers/nitrogen of DOTAP.
3.2. Association of siRNA with the liposome
The association of siRNA with cationic liposome was monitored by gel retardation electrophoresis. Naked siRNA was detected as bands on acrylamide gel. Beyond a charge ratio (-/+) of 1/3, no migration of siRNA was observed for cationic lipoplex (Fig. 2A). However, migration of siRNA was observed for CS-, PGA- and PAA-coated lipoplexes at all charge ratios (-/+) of anionic polymer/DOTAP when anionic polymers were added into cationic lipoplex (Fig. 2B), indicating that anionic polymers caused dissociation of siRNA from lipoplex by competition for binding to cationic liposome. Previously, we reported that CS and PGA could coat cationic lipoplex of pDNA without releasing pDNA from the cationic lipoplex, and formed stable anionic lipoplexes [5]. In lipoplex of siRNA, the association of cationic liposome with siRNA might be weaker than that with pDNA.
Fig. 2.
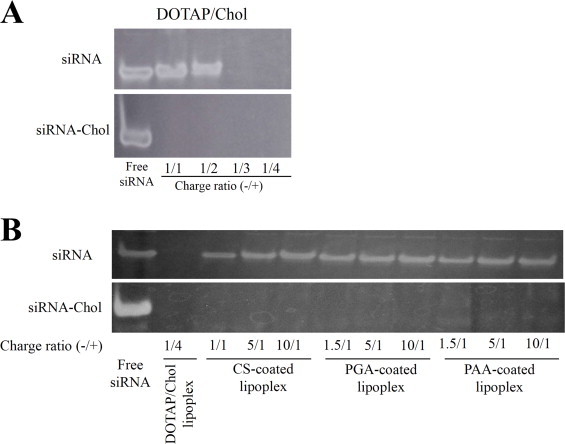
Association of siRNA with cationic liposome after coating with various anionic polymers. (A) Cationic lipoplexes of 1?µg of siRNA or siRNA-Chol at various charge ratios (+/-) were analyzed by 18% acrylamide gel electrophoresis. Charge ratio (-/+) indicates the molar ratios of siRNA phosphate to DOTAP nitrogen. (B) Anionic polymer-coated lipoplexes of 1?µg of siRNA or siRNA-Chol at various charge ratios (-/+) were analyzed by 18% acrylamide gel electrophoresis. Charge ratio (-/+) indicates the molar ratios of sulfate and/or carboxylic acid of anionic polymers/DOTAP nitrogen.
Furthermore, we examined the association of siRNA with cationic liposome using SYBR® Green I. SYBR® Green I is a DNA/RNA-intercalating agent whose fluorescence is dramatically enhanced upon binding to siRNA and quenched when displaced by condensation of the siRNA structure. Unlike gel retardation electrophoresis, fluorescence of SYBR® Green I was markedly decreased by the formation of anionic polymer-coated lipoplex, compared with that in siRNA solution (Supplemental Fig. S1). These findings suggested that the CS-, PGA- and PAA-coated lipoplexes were completely formed even at charge ratios (-/+) of 1, 1.5 and 1.5, respectively. Although a discrepancy between the results from the accessibility of SYBR® Green I and gel retardation electrophoresis was observed, siRNA might be released from the anionic polymer-coated lipoplex under electrophoresis by weak association between siRNA and cationic liposomes.
To increase the association between siRNA and cationic liposome, we decided to use siRNA-Chol for the preparation of anionic polymer-coated lipoplex. In siRNA-Chol, beyond a charge ratio (-/+) of 1/1, no migration of siRNA was observed for cationic lipoplex (Fig. 2A). Furthermore, no migration of siRNA-Chol was observed at CS-, PGA- and PAA-coated lipoplexes, even at a charge ratio (-/+) of 10/1, when anionic polymers were added into cationic lipoplex of siRNA-Chol formed at a charge ratio (-/+) of 1/4 (Fig. 2B). From these results, we confirmed that CS, PGA and PAA could coat cationic lipoplex without releasing siRNA-Chol from the cationic lipoplex, and formed stable anionic lipoplexes.
When anionic polymer-coated lipoplexes of siRNA-Chol were prepared at charge ratios (-/+) of 1 in CS, 1.5 in PGA and 1.5 in PAA, the sizes and ?-potentials of CS-, PGA- and PAA-coated lipoplexes were 299, 233 and 235?nm, and -22.8, -36.7 and -54.3?mV, respectively (Supplemental Table S1). In subsequent experiments, we decided to use anionic polymer-coated lipoplexes of siRNA and siRNA-Chol for comparison of transfection activity and biodistribution.
3.3. In vitro transfection efficiency
Generally, in cationic lipoplexes, strong electrostatic interaction with a negatively charged cellular membrane can contribute to high siRNA transfer through endocytosis. To investigate whether anionic polymer-coated lipoplexes could be taken up well by cells and induce gene suppression by siRNA, we examined the gene knockdown effect using a luciferase assay system with MCF-7-Luc cells. Cationic lipoplex of Luc siRNA or Luc siRNA-Chol exhibited moderate suppression of luciferase activity; however, coating of anionic polymers on the cationic lipoplex caused disappearance of gene knockdown efficacy by cationic lipoplex (Fig. 3A and B), suggesting that negatively charged lipoplexes were not taken up by the cells because they repulsed the cellular membrane electrostatically.
Fig. 3.
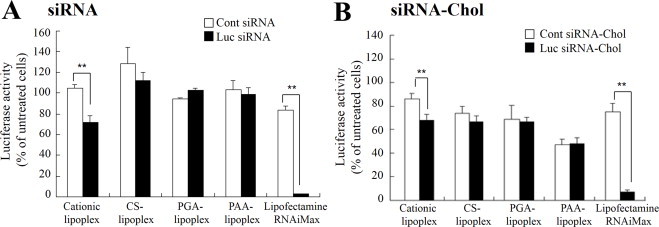
Gene suppression in MCF-7-Luc cells by anionic polymer-coated lipoplexes. Cationic, CS, PGA and PAA-coated lipoplexes of siRNA (A) and siRNA-Chol (B) were added to MCF-7-Luc cells at 100?nM siRNA, and the luciferase assay was carried out 48?h after incubation. Statistical significance was evaluated by Student’s t test. **p < 0.01, compared with Cont siRNA. Each column represents the mean ± S.D. (n = 3).
3.4. Interaction with erythrocytes
Cationic lipoplex often lead to the agglutination of erythrocytes by the strong affinity of positively charged lipoplex to the cellular membrane. To investigate whether polymer coatings for cationic lipoplex could prevent agglutination with erythrocytes, we observed the agglutination of anionic polymer-coated lipoplex with erythrocytes by microscopy (Fig. 4). CS-, PGA- and PAA-coated lipoplexes of siRNA or siRNA-Chol showed no agglutination, although cationic lipoplexes did. This result indicated that the negatively charged surface of anionic polymer-coated lipoplexes could prevent the agglutination with erythrocytes.
Fig. 4.
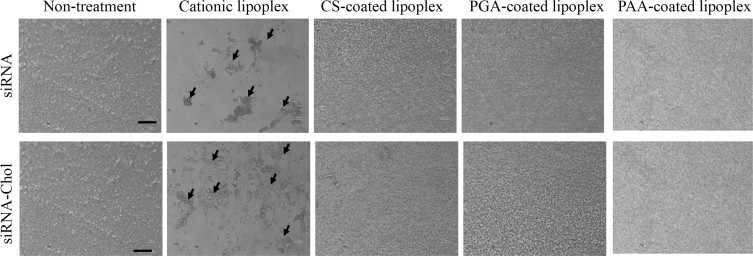
Agglutination of anionic polymer-coated lipoplexes of siRNA or siRNA-Chol with erythrocytes. Each lipoplex was added to erythrocytes, and agglutination was observed by phase contrast microscopy. Arrows indicate agglutination. Scale bar = 100?µm.
3.5. Biodistribution of siRNA after injection of lipoplex
We intravenously injected anionic polymer-coated lipoplexes of Cy5.5-siRNA or Cy5.5-siRNA-Chol into mice, and observed the biodistribution of siRNA at 1?h after the injection by fluorescent microscopy. When naked siRNA and siRNA-Chol were injected, the accumulations were strongly observed only in the kidneys (Figs. 5 and 6), indicating that naked siRNA was quickly eliminated from the body by filtration in the kidneys. For siRNA lipoplex, cationic lipoplex was largely accumulated in the lungs. CS, PGA and PAA coatings of cationic lipoplex decreased the accumulation of siRNA in the lungs and increased it in the liver and the kidneys (Fig. 5). To confirm whether siRNA observed in the kidneys was siRNA or lipoplex of siRNA, we prepared cationic and PGA-coated lipoplexes using rhodamine-labeled liposome and Cy5.5-siRNA, and the localizations of siRNA and liposome after intravenous injection were observed by fluorescent microscopy (Supplemental Fig. S2). When cationic lipoplex was intravenously injected into mice, both the siRNA and the liposome were mainly detected in the lungs, and the localizations of siRNA were almost identical to those of the liposome, indicating that most of the siRNA was distributed in the tissues as a lipoplex. In contrast, when PGA-coated lipoplex was intravenously injected, siRNA was strongly detected in both the liver and the kidneys, but the liposomes were mainly in the liver. From this finding, although anionic polymer coatings prevent the accumulation of lipoplex in the lungs by inhibiting interaction with erythrocytes, siRNA dissociated from anionic polymer-coated lipoplexes in blood may accumulate in the kidneys. In contrast to siRNA lipoplex, CS, PGA and PAA coatings of cationic lipoplex of siRNA-Chol induced the high accumulation of siRNA-Chol in the liver, but diminished fluorescence of siRNA was observed in the kidneys compared with the lipoplexes of siRNA (Fig. 6). From this result, CS-, PGA- and PAA-coated lipoplexes of siRNA-Chol might have potential as a targeting vector of siRNA to the liver.
Fig. 5.
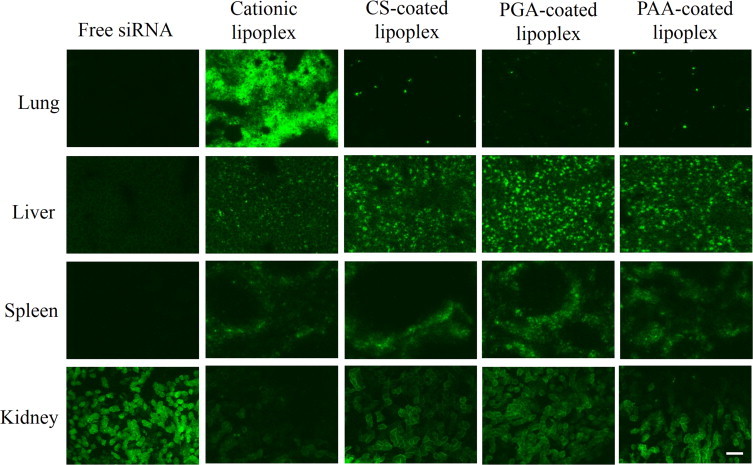
Biodistribution of Cy5.5-siRNA at 1?h after intravenous administration by anionic polymer-coated lipoplexes into mice. Green signals indicate localization of Cy5.5-siRNA. Scale bar = 100?µm.
Fig. 6.
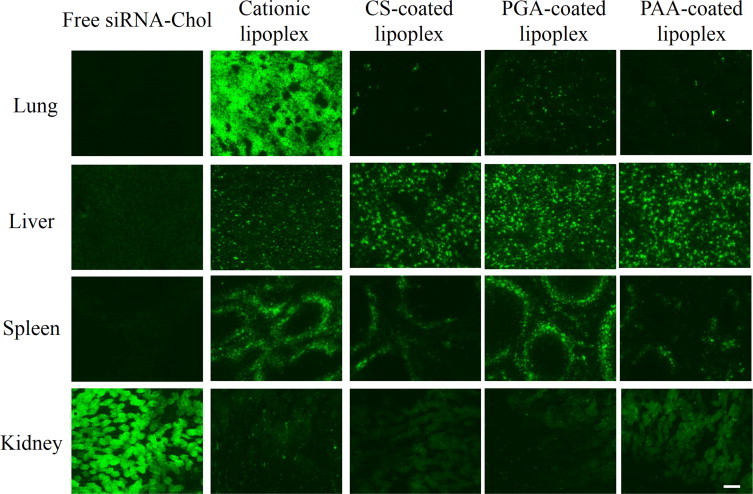
Biodistribution of Cy5.5-siRNA-Chol at 1?h after intravenous administration by anionic polymer-coated lipoplexes into mice. Green signals indicate localization of Cy5.5-siRNA-Chol. Scale bar = 100?µm.
3.6. Gene suppression in vivo
To investigate whether anionic polymer-coated lipoplex of siRNA-Chol could suppress the expression of a targeted gene in the liver, we chose to target the mouse ApoB gene, a hepatocyte-expressed gene involved in cholesterol transport, and evaluated the knockdown efficiency into mice by assaying the level of ApoB mRNA at 48?h after intravenous injection of anionic polymer-coated lipoplex of ApoB siRNA-Chol (Fig. 7). The injections of naked ApoB siRNA-Chol, cationic, CS- and PAA-coated lipoplexes of ApoB siRNA-Chol did not affect the ApoB mRNA level in the liver compared with those of Cont siRNA-Chol, respectively. In contrast, the injection of PGA-coated lipoplex of ApoB siRNA-Chol could significantly induce suppression of the ApoB mRNA level in the liver compared with that of Cont siRNA-Chol (about 40% knockdown).
Fig. 7.
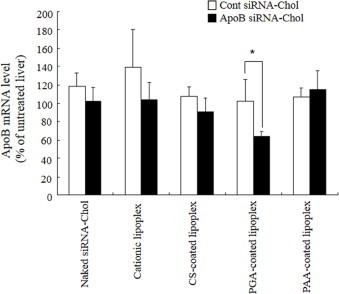
In vivo knockdown of ApoB mRNA in the liver of mice after injection of anionic polymer-coated lipoplex of Cont siRNA-Chol or ApoB siRNA-Chol. Liver ApoB mRNA levels were quantified relative to ß-actin mRNA 48?h after i.v. administration of siRNA. Each column represents the mean ± S.D. (n = 3–6). Statistical significance was evaluated by Student’s t test. *p < 0.05, compared with Cont siRNA.
ApoB is an essential protein in the formation of LDL in the metabolism of dietary and endogenous cholesterol. Therefore, we measured the LDL level in serum 48?h after treatment with PGA-coated lipoplex of ApoB siRNA-Chol. This treatment of mice resulted in an approximately 34% reduction (0.073 ± 0.021?mg/ml), compared with no treatment (0.112 ± 0.027?mg/ml) (data not shown). This result indicated that the reduction of ApoB level in the liver induced a decrease of LDL cholesterol level in serum.
It was not clear why CS- and PAA-coated lipoplexes did not induce a gene silencing effect. HARE/Stab-2 is known as the primary scavenger receptor for systemic turnover of most types of CS, which is found primarily in the sinusoidal endothelial cells of the liver [18]. With regard to CS-coated lipoplex, it might be captured by the sinusoidal endothelial cells in the liver, and not be delivered to hepatocytes.
3.7. Serum GOT and GPT concentrations
Finally, for evaluation of toxicity to mice, we assessed GOT and GPT levels in serum after intravenous injection of cationic, CS-, PGA- and PAA-coated lipoplexes. Loisel et al. reported that cationic lipoplexes prepared with cationic lipids as DOTAP and cationic phospholipid compounds induced toxic effects in liver [19]. When cationic lipoplexes were intravenously injected into mice, increased concentration of GOT and GPT in blood were observed at 24?h, but not after injection of naked siRNA-Chol, CS-, PGA- and PAA-coated lipoplexes (Fig. 8A and B). These results suggested that CS-, PGA and PAA-coated lipoplexes had less side effects with regard to hepatoxicity by intravenous injection compared to cationic lipoplexes.
Fig. 8.
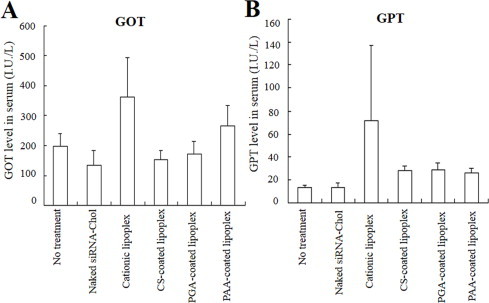
Toxicity after intravenous injection of anionic polymer-coated lipoplexes into mice. Concentrations of GOT (A) and GPT (B) in blood were measured at 24?h after intravenous administration of anionic polymer-coated lipoplexes of siRNA-Chol into mice. Each column represents the mean ± S.D. (n = 3).
Previously, naked ApoB siRNA-Chol showed a significant reduction of the level of ApoB mRNA (57% reduction) in the liver compared with that in a saline control when it was intravenously injected into mice at 50?mg?siRNA/kg (1?mg per mouse) [8]. In this study, we synthesized and used the same chemically modified ApoB siRNA-Chol as in the previous report for an experiment on ApoB mRNA suppression; however, naked ApoB siRNA-Chol did not show reduction of the level of ApoB mRNA (Fig. 7). This can be explained by the difference in injected dose of ApoB siRNA-Chol in this study (2.5?mg?siRNA/kg, 50?µg per mouse). This finding indicates that PGA-coated lipoplex of siRNA-Chol could deliver siRNA to hepatocytes and suppress ApoB expression at a 1/20-fold dose of naked siRNA-Chol without hepatoxicity. Although PGA-coated lipoplex of siRNA-Chol did not induce gene suppression in vitro (Fig. 3B), it had potential for in vivo delivery of siRNA-Chol into liver by intravenous injection.
4. Conclusion
In this study, we developed anionic polymer-coated DOTAP/Chol lipoplexes for systemic gene delivery of siRNA. Among them, PGA coating for cationic lipoplex of siRNA-Chol induced accumulation in the liver after intravenous injection, and could suppress the mRNA level of the targeted gene. From our results, PGA-coated lipoplex might be an outstanding tool for safe siRNA delivery to the liver. Further study should be performed to examine the increase of the gene silencing effect in the liver and further therapeutic applications.
Acknowledgement
We thank Mr. Ryou Okamoto, Ms. Yumiko Shingu and Ms. Eriko Hara for assistance in the experimental work. This project was supported in part by a Grant-in-Aid for Young Scientists (B), Japan Society for the Promotion of Science (KAKENHI Grant no. 23790203), the Advanced Research for Medical Products Mining Programme of the NIBIO, and the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary Material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.rinphs.2014.01.001.
Appendix. Supplementary Materials
Effect of charge ratio (-/+) on siRNA association by coating with anionic polymer on cationic lipoplex. Accessibility of siRNA in anionic polymer-coated lipoplex was analyzed with the fluorescent siRNA-binding dye SYBR Green I. Each column represents the mean ± S.D. (n = 3).
Localization of rhodamine-labeled cationic and PGA-coated lipoplexes of Cy5.5-siRNA at 1?h after intravenous administration into mice. Rhodamine-labeled cationic liposome was used for preparation of cationic and PGA-coated lipoplexes of siRNA. The localizations of Cy5.5-siRNA and rhodamine-labeled liposome are shown as green and red signals, respectively. Scale bar = 100 µm.
Formulation, size and ?-potentials of cationic lipoplexes of siRNA-Chol.
References
- 1.Eliyahu H, Servel N, Domb AJ, Barenholz Y. Lipoplex-induced hemagglutination: potential involvement in intravenous gene delivery. Gene Ther. 2002;9:850–858. doi: 10.1038/sj.gt.3301705. 12080379 [DOI] [PubMed] [Google Scholar]
- 2.Simberg D, Weisman S, Talmon Y, Faerman A, Shoshani T, Barenholz Y. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003;278:39858–39865. doi: 10.1074/jbc.M302232200. 12869564 [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T, Kitahara T, Fumoto S, Nishida K, Yamamoto K, Nakagawa H, Kodama Y, Higuchi N, Nakamura T, Sasaki H. Chondroitin sulfate capsule system for efficient and secure gene delivery. J Pharm Pharm Sci. 2010;13:351–361. doi: 10.18433/j3gk52. 21092708 [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki T, Kitahara T, Kawakami S, Higuchi Y, Yamaguchi A, Nakagawa H, Kodama Y, Hamamoto T, Hashida M, Sasaki H. Gamma-polyglutamic acid-coated vectors for effective and safe gene therapy. J Control Release. 2010;142:404–410. doi: 10.1016/j.jconrel.2009.11.010. 19931327 [DOI] [PubMed] [Google Scholar]
- 5.Hattori Y, Yamasaku H, Maitani Y. Anionic polymer-coated lipoplex for safe gene delivery into tumor by systemic injection. J Drug Target. 2013;21:639–647. doi: 10.3109/1061186X.2013.789035. 23594095 [DOI] [PubMed] [Google Scholar]
- 6.Oyama M, Nagashima T, Suzuki T, Kozuka-Hata H, Yumoto N, Shiraishi Y, Ikeda K, Kuroki Y, Gotoh N, Ishida T, Inoue S, Kitano H, Okada-Hatakeyama M. Integrated quantitative analysis of the phosphoproteome and transcriptome in tamoxifen-resistant breast cancer. J Biol Chem. 2011;286:818–829. doi: 10.1074/jbc.M110.156877. 21044952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueyama K, Ikeda K, Sato W, Nakasato N, Horie-Inoue K, Takeda S, Inoue S. Knockdown of Efp by DNA-modified small interfering RNA inhibits breast cancer cell proliferation and in vivo tumor growth. Cancer Gene Ther. 2010;17:624–632. doi: 10.1038/cgt.2010.19. 20467453 [DOI] [PubMed] [Google Scholar]
- 8.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. 15538359 [DOI] [PubMed] [Google Scholar]
- 9.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. 5859039 [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Hattori Y, Kubo M, Maitani Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. Int J Pharm. 2012;423:428–434. doi: 10.1016/j.ijpharm.2011.12.015. 22197775 [DOI] [PubMed] [Google Scholar]
- 11.Hattori Y, Nakamura T, Ohno H, Fujii N, Maitani Y. siRNA delivery into tumor cells by lipid-based nanoparticles composed of hydroxyethylated cholesteryl triamine. Int J Pharm. 2013;443:221–229. doi: 10.1016/j.ijpharm.2012.12.017. 23279939 [DOI] [PubMed] [Google Scholar]
- 12.Wang HX, Xiong MH, Wang YC, Zhu J, Wang J. N-acetylgalactosamine functionalized mixed micellar nanoparticles for targeted delivery of siRNA to liver. J Controll Release. 2013;166:106–114. doi: 10.1016/j.jconrel.2012.12.017. 23266452 [DOI] [PubMed] [Google Scholar]
- 13.Kawano K, Hattori Y, Iwakura H, Akamizu T, Maitani Y. Combination therapy with gefitinib and doxorubicin inhibits tumor growth in transgenic mice with adrenal neuroblastoma. Cancer Med. 2013;2:286–295. doi: 10.1002/cam4.76. 23930205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geusens B, Lambert J, Smedt SC De, Buyens K, Sanders NN, Van GM. Ultradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytes. J Control Release. 2009;133:214–220. doi: 10.1016/j.jconrel.2008.10.003. 18973779 [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330:755–759. doi: 10.1016/j.bbrc.2005.03.041. 15809061 [DOI] [PubMed] [Google Scholar]
- 16.Sioud M, S?rensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. 14652004 [DOI] [PubMed] [Google Scholar]
- 17.S?rensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. 12654261 [DOI] [PubMed] [Google Scholar]
- 18.Harris EN, Weigel PH. The ligand-binding profile of HARE: Hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology. 2008;18:638–648. doi: 10.1093/glycob/cwn045. 18499864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loisel S, Le GC, Doucet L, Ferec C, Floch V. Contribution of plasmid DNA to hepatotoxicity after systemic administration of lipoplexes. Hum Gene Ther. 2001;12:685–696. doi: 10.1089/104303401300057405. 11426467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of charge ratio (-/+) on siRNA association by coating with anionic polymer on cationic lipoplex. Accessibility of siRNA in anionic polymer-coated lipoplex was analyzed with the fluorescent siRNA-binding dye SYBR Green I. Each column represents the mean ± S.D. (n = 3).
Localization of rhodamine-labeled cationic and PGA-coated lipoplexes of Cy5.5-siRNA at 1?h after intravenous administration into mice. Rhodamine-labeled cationic liposome was used for preparation of cationic and PGA-coated lipoplexes of siRNA. The localizations of Cy5.5-siRNA and rhodamine-labeled liposome are shown as green and red signals, respectively. Scale bar = 100 µm.
Formulation, size and ?-potentials of cationic lipoplexes of siRNA-Chol.


