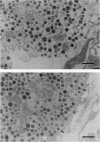
Abstract
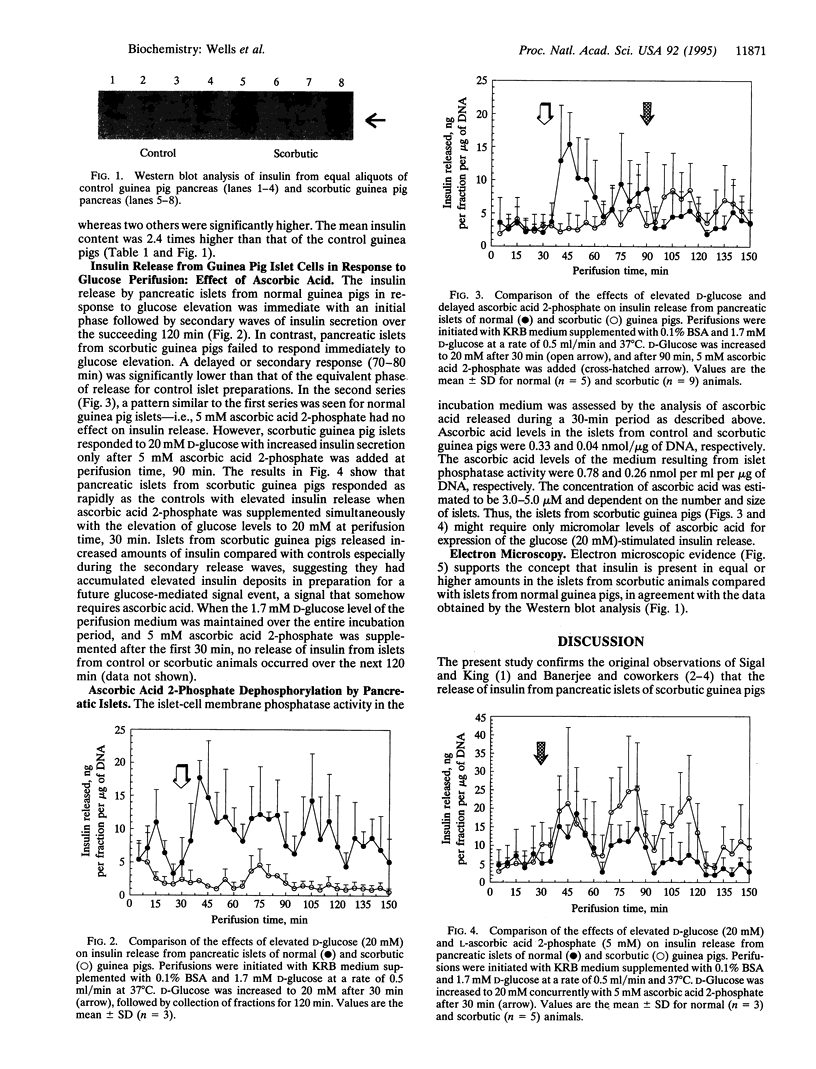
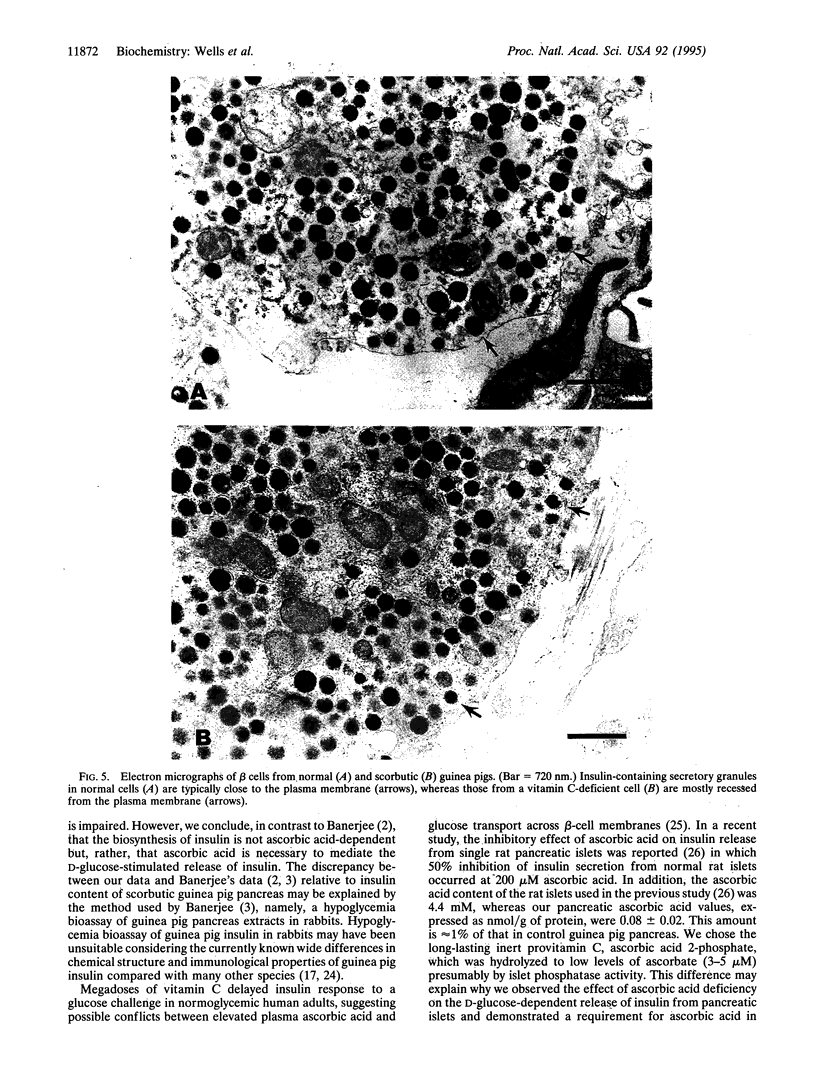
Pancreatic islets from young normal and scorbutic male guinea pigs were examined for their ability to release insulin when stimulated with elevated D-glucose. Islets from normal guinea pigs released insulin in a D-glucose-dependent manner showing a rapid initial secretion phase and three secondary secretion waves during a 120-min period. Islets from scorbutic guinea pigs failed to release insulin during the immediate period, and only delayed and decreased responses were observed over the 40-60 min after D-glucose elevation. Insulin release from scorbutic islets was greatly elevated if 5 mM L-ascorbic acid 2-phosphate was supplemented in the perifusion medium during the last 60 min of perifusion. When 5 mM L-ascorbic acid 2-phosphate was added to the perifusion medium concurrently with elevation of medium D-glucose, islets from scorbutic guinea pigs released insulin as rapidly as control guinea pig islets and to a somewhat greater extent. L-Ascorbic acid 2-phosphate without elevated D-glucose had no effect on insulin release by islets from normal or scorbutic guinea pigs. The pancreas from scorbutic guinea pigs contained 2.4 times more insulin than that from control guinea pigs, suggesting that the decreased insulin release from the scorbutic islets was not due to decreased insulin synthesis but due to abnormal insulin secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Grimm A., Lutz S., Wagner-Teschner D., Händel M., Hagenloh I. Islet glutathione and insulin release. Diabetes. 1980 Oct;29(10):830–834. doi: 10.2337/diacare.20.10.830. [DOI] [PubMed] [Google Scholar]
- BANERJEE S., DEB C., DELAVADY B. Effect of scurvy on glutathione and dehydroascorbic acid in guinea pig tissues. J Biol Chem. 1952 Mar;195(1):271–276. [PubMed] [Google Scholar]
- Bank H. L. A quantitative enzyme-linked immunosorbent assay for rat insulin. J Immunoassay. 1988;9(2):135–158. doi: 10.1080/15321818808057037. [DOI] [PubMed] [Google Scholar]
- Bergsten P., Moura A. S., Atwater I., Levine M. Ascorbic acid and insulin secretion in pancreatic islets. J Biol Chem. 1994 Jan 14;269(2):1041–1045. [PubMed] [Google Scholar]
- Dionne K. E., Colton C. K., Yarmush M. L. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993 Jan;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- Feng J., Melcher A. H., Brunette D. M., Moe H. K. Determination of L-ascorbic acid levels in culture medium: concentrations in commercial media and maintenance of levels under conditions of organ culture. In Vitro. 1977 Feb;13(2):91–99. doi: 10.1007/BF02615072. [DOI] [PubMed] [Google Scholar]
- GIVOL D., GOLDBERGER R. F., ANFINSEN C. B. OXIDATION AND DISULFIDE INTERCHANGE IN THE REACTIVATION OF REDUCED RIBONUCLEASE. J Biol Chem. 1964 Sep;239:PC3114–PC3116. [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Holz G. G., Habener J. F. Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept. Trends Biochem Sci. 1992 Oct;17(10):388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Johnston C. S., Yen M. F. Megadose of vitamin C delays insulin response to a glucose challenge in normoglycemic adults. Am J Clin Nutr. 1994 Nov;60(5):735–738. doi: 10.1093/ajcn/60.5.735. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990 Jun;39(6):647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., McGarry J. D. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- Nomura H., Ishiguro T., Morimoto S. Studies on L-ascorbic acid derivatives. 3. Bis(L-ascorbic acid-3,3')phosphate and L-ascorbic acid 2-phosphate. Chem Pharm Bull (Tokyo) 1969 Feb;17(2):387–393. doi: 10.1248/cpb.17.387. [DOI] [PubMed] [Google Scholar]
- Parsey R. V., Matteson D. R. Ascorbic acid modulation of calcium channels in pancreatic beta cells. J Gen Physiol. 1993 Sep;102(3):503–523. doi: 10.1085/jgp.102.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence L. A., Mennear J. H. Inhibition effect of dehydroascorbic acid on insulin secretion from mouse pancreatic islets. Toxicol Appl Pharmacol. 1979 Aug;50(1):57–65. doi: 10.1016/0041-008x(79)90492-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. C., Francetic D. J. The importance of sample preparation and storage in glutathione analysis. Anal Biochem. 1993 Jun;211(2):183–187. doi: 10.1006/abio.1993.1254. [DOI] [PubMed] [Google Scholar]
- Smith J., Maizels M. The Plasma Phosphatase in Rickets and Scurvy. Arch Dis Child. 1932 Jun;7(39):149–158. doi: 10.1136/adc.7.39.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. F. Amino acid sequences of insulins. Diabetes. 1972;21(2 Suppl):457–460. doi: 10.2337/diab.21.2.s457. [DOI] [PubMed] [Google Scholar]
- Tager H. S. Coupling of peptides to albumin with difluorodinitrobenzene. Anal Biochem. 1976 Apr;71(2):367–375. [PubMed] [Google Scholar]
- Treacy G. B., Shaw D. C., Griffiths M. E., Jeffrey P. D. Purification of a marsupial insulin: amino-acid sequence of insulin from the eastern grey kangaroo Macropus giganteus. Biochim Biophys Acta. 1989 Mar 24;990(3):263–268. doi: 10.1016/s0304-4165(89)80043-1. [DOI] [PubMed] [Google Scholar]
- VENETIANER P., STRAUB F. B. STUDIES ON THE MECHANISM OF ACTION OF THE RIBONUCLEASE-REACTIVATING ENZYME. Acta Physiol Acad Sci Hung. 1965;27:303–315. [PubMed] [Google Scholar]
- VENETIANER P., STRAUB F. B. THE MECHANISM OF ACTION OF THE RIBONUCLEASE-REACTIVATING ENZYME. Biochim Biophys Acta. 1964 Jul 8;89:189–190. doi: 10.1016/0926-6569(64)90123-3. [DOI] [PubMed] [Google Scholar]
- Welch R. W., Wang Y., Crossman A., Jr, Park J. B., Kirk K. L., Levine M. Accumulation of vitamin C (ascorbate) and its oxidized metabolite dehydroascorbic acid occurs by separate mechanisms. J Biol Chem. 1995 May 26;270(21):12584–12592. doi: 10.1074/jbc.270.21.12584. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Rocque P. A., Xu D. P., Meyer E. B., Charamella L. J., Dimitrov N. V. Ascorbic acid and cell survival of adriamycin resistant and sensitive MCF-7 breast tumor cells. Free Radic Biol Med. 1995 Apr;18(4):699–708. doi: 10.1016/0891-5849(94)00188-p. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P. Dehydroascorbate reduction. J Bioenerg Biomembr. 1994 Aug;26(4):369–377. doi: 10.1007/BF00762777. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]
- Wells W. W., Yang Y., Deits T. L., Gan Z. R. Thioltransferases. Adv Enzymol Relat Areas Mol Biol. 1993;66:149–201. doi: 10.1002/9780470123126.ch4. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Meda P., Halban P. A. Isolation of pancreatic islets and primary culture of the intact microorgans or of dispersed islet cells. Methods Enzymol. 1990;192:188–223. doi: 10.1016/0076-6879(90)92071-k. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S., Rasmussen H. Control of insulin secretion: a model involving Ca2+, cAMP and diacylglycerol. Mol Cell Endocrinol. 1990 Apr 17;70(2):119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. E., Yip C. C. Guinea pig insulin. I. Purification and physical properties. J Biol Chem. 1974 Jul 10;249(13):4021–4025. [PubMed] [Google Scholar]