Abstract
Proteasome is central to proteostasis maintenance, as it degrades both normal and damaged proteins. Herein, we undertook a detailed analysis of proteasome regulation in the in vivo setting of Drosophila melanogaster. We report that a major hallmark of somatic tissues of aging flies is the gradual accumulation of ubiquitinated and carbonylated proteins; these effects correlated with a ∼50% reduction of proteasome expression and catalytic activities. In contrast, gonads of aging flies were relatively free of proteome oxidative damage and maintained substantial proteasome expression levels and highly active proteasomes. Moreover, gonads of young flies were found to possess more abundant and more active proteasomes than somatic tissues. Exposure of flies to oxidants induced higher proteasome activities specifically in the gonads, which were, independently of age, more resistant than soma to oxidative challenge and, as analyses in reporter transgenic flies showed, retained functional antioxidant responses. Finally, inducible Nrf2 activation in transgenic flies promoted youthful proteasome expression levels in the aged soma, suggesting that age-dependent Nrf2 dysfunction is causative of decreasing somatic proteasome expression during aging. The higher investment in proteostasis maintenance in the gonads plausibly facilitates proteome stability across generations; it also provides evidence in support of the trade-off theories of aging.—Tsakiri, E. N., Sykiotis, G. P., Papassideri, I. S., Gorgoulis, V. G., Bohmann, D., Trougakos, I. P. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress.
Keywords: antioxidant response elements, gonads, Keap1, Nrf2
Organismal aging has been defined as a progressive shrinkage of the homeodynamic space that promotes time-dependent decline of functional capacity and stress resistance, associated with increased risk of morbidity and mortality (1, 2, 3). Therefore, and in sharp contrast to the precisely regulated molecular events that take place during development, aging is envisioned as a more stochastic and multifactorial process being modulated by both genetic and environmental factors (4, 5). According to the free radical theory of aging, because of their high reactivity, oxygen free radicals are causally related to the onset and promotion of aging; recently, the more general molecular damage theory of aging has been proposed, based on the fact that damage to biomolecules can also originate from mechanisms in which free radicals are not always the main cause (5, 6). At the tissue and organ level, damage accumulation is a hallmark of aging and affects both postmitotic and mitotic cells, as well as the extracellular matrix (6, 7). Nevertheless, for a relatively long time (at least up to maturation), organisms retain low levels of dysfunctional biomolecules and, apparently, maintain their reproductive cell lineages (germ line) at a high-quality level that preserves viability across generations. This is achieved via the occurrence of a complex and highly regulated network of effective surveillance, maintenance, and clearance systems that ensure biomolecules' functionality, thus preventing disruption of organism homeostasis. Key players in preventing the breakdown of cellular proteostasis (homeostasis of the proteome) are the molecular chaperones, the oxidized protein repair enzymes MsrA and MsrB, the mitochondrial Lon protease, and the two main proteolytic systems (i.e., the lysosome and the proteasome), along with transcription factors that contribute to their transcriptional activation [e.g., NFE2-related factor 2 (Nrf2)] (8, 9, 10).
Intracellular protein degradation is the most efficient mechanism to prevent toxicity associated with the accumulation of altered proteins without affecting the cellular reserves of amino acids. Central to protein turnover is the ubiquitin-proteasome system (UPS), which functions at the cytosolic and nuclear compartments of eukaryotic cells to degrade both normal short-lived ubiquitinated proteins, as well as mutated or damaged proteins (11, 12). The UPS is thus involved in the regulation of numerous cellular functions, including protein quality control, transcription, cell-cycle regulation, DNA repair, signal transduction and antigen presentation (8, 13). Structural studies have established that the 26S proteasome consists of a 20S core particle that is bound to one or two 19S regulatory particles, which are involved in substrate recognition, deubiquitination, unfolding, and translocation into the core particle (11). The 20S eukaryotic barrel-like proteasome structure comprises 4 stacked heptameric rings (2 α-type rings surrounding 2 β-type rings). The α subunits form a gate at the center of the ring that restricts substrate entry, while the β subunits contribute to the proteolytic active sites, namely the caspase-like (C-L), trypsin-like (T-L), and chymotrypsin-like (CT-L) peptidase activities, which are located at the β1, β2, and β5 proteasome subunits, respectively (11). The 26S proteasome is mostly involved in the ATP-dependent degradation of normal short-lived ubiquitinated proteins, whereas the 20S proteasome exhibits a high degree of selectivity in degrading oxidized or otherwise damaged proteins (11, 12, 14).
Proteasome functionality was found to decline in cells derived from aged donors (15) or during cellular senescence of normal human cells (16). Also, repeated mild heat stress increased proteasome activity in young (but not in late-passage senescent) human skin fibroblasts (17). In the few existing in vivo studies, 26S proteasome activity was found to decrease in middle-aged Drosophila melanogaster flies, as compared to young insects (18, 19), and in liver and brain homogenates of fertile vs. newborn rats (20). Moreover, C-L proteasome activity was found to be higher in females (compared to males) in both control and longevity-selected flies; interestingly, an age-related decline of this proteasome activity was observed in control but not in long-lived flies (21). Loss of function of the 19S Rpn11 or Rpn10 subunits in Drosophila caused a shorter life span (19) or larval-pupal polyphasic lethality (22), respectively, while overexpression of the Rpn11 19S proteasome subunit increased life span (19). Contrary to these findings, aging was not associated with proteasome impairment in UPS reporter mice (23), while proteasome gene expression was found to be up-regulated in aged somatic tissues of Drosophila (24).
The Nrf2/Kelch-like ECH-associated protein 1 (Keap1) signaling pathway is central to cellular responses to oxidative and electrophilic stress (9). In nonstressful conditions, Nrf2 is retained in the cytoplasm by the actin-binding protein Keap1, and it is targeted for degradation by the proteasome (25). Under increased oxidative stress, the Nrf2-Keap1 interaction is disrupted, allowing Nrf2 to translocate to the nucleus, where, by binding to antioxidant response elements (AREs), it stimulates the expression of phase II and antioxidant enzymes (9). The Nrf2/Keap1 pathway is conserved in Drosophila and appears to engage in the same regulatory interactions as in vertebrates (26). Interestingly, Nrf2 was recently identified at Drosophila cells as a candidate transcriptional regulator of proteasome component expression (27).
Given that proteasome regulation during in vivo aging of higher metazoans has not been systematically studied, and considering the significant biomedical interest in the UPS as a therapeutic target in various diseases (28), the main goal of this study was to provide a systematic and detailed characterization of the sex-, tissue-, stress-, and age-dependent proteasome regulation and functionality during the life span of the genetically tractable model organism Drosophila melanogaster. Drosophila is well suited to this line of investigation, because of its powerful genetics, its similarities in key metabolic and aging pathways with humans (29), and the fact that its proteasome structurally resembles that of mammals (30). Moreover, Drosophila comprises a soma-germ line demarcation with both postmitotic and mitotic cell lineages.
MATERIALS AND METHODS
Fly stocks
Drosophila strains used in this study were the wild-type Oregon R flies; the ARE–green fluorescent protein (GFP) transgenic reporter lines, glutathione S-transferase Delta (gstD)-ARE:GFP/II (ARE-containing enhancer of the gstD1 gene) and gstD-mARE:GFP/III (mutated version of gstD-ARE) (26), and a transgenic line carrying 4 synthetic multimer AREs [4XARE:GFP-16(R7)/II; ref. 31], as well as transgenic lines overexpressing inverted repeats corresponding to parts of either the cap‘n’collar isoform C (cncC)-specific gene or the keap1 gene-coding segment (for RNAi-mediated knockdown of cncC or keap1, respectively) (26). Tubulin-GeneSwitch-Gal4 (tubGSGal4) flies express RU486-regulated Gal4 under the control of the tubulin enhancer; thus, the conditional driver tubGSGal4 is ubiquitously activated on dietary administration of RU486 (320 μM); RU486 was applied in young (1- to 2-d-old) eclosed flies. Stocks were maintained at 25°C, 60% relative humidity on a 12-h light-dark cycle and were fed standard medium (see Supplemental Data). Oxidants were added in culture medium at the following final concentrations: tert-butyl hydroperoxide (t-BHP), 10 or 20 mM; paraquat was administered at 20 mM dissolved in 5% sucrose.
Measurement of reactive oxygen species (ROS), H2O2, and GFP with fluorometry
To assay ROS levels, somatic tissues or gonads were collected in PBS and incubated in CM-H2DCFDA (Invitrogen, Carlsbad, CA, USA) dye for 30 min at 25°C in the dark. Following centrifugation and dye removal, tissues were incubated for 10 min at 24°C in PBS for cellular esterases to hydrolyze the acetoxymethyl ester or acetate groups and render the dye responsive to oxidation. Samples were washed in PBS, lysed in Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, and 50 mM Tris, pH 8.0), and cleared by centrifugation at 19,000 g for 10 min at 4°C. The supernatant was diluted 1:4 (v/v) in ddH2O, and fluorescent dichlorodihydrofluorescein was measured using a VersaFluor Fluorometer System (Bio-Rad Laboratories, Hercules, CA, USA) at excitation, 490 nm, and emission, 520 nm. Negative controls were either unstained tissues incubated with only PBS buffer to detect autofluorescence, or cell-free mixtures of dye and buffers with or without t-BHP (in experiments involving t-BHP exposure).
H2O2 was measured using the Amplex Red hydrogen peroxide assay kit (Invitrogen) as per manufacturer's instructions. Briefly, isolated somatic tissues were homogenized in 100 μl 1× reaction buffer and were then centrifuged at 3000 g for 3 min at 4°C. Working solution (50 μl; 100 μΜ Amplex Red Reagent and 0.2 U/ml HRP diluted in 1× reaction buffer) was added to 50 μl of the collected supernatant, and samples were incubated for 30 min at room temperature. H2O2 concentration was measured in a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories) at 560 nm. Negative controls were free of tissue preparations, whereas in positive controls, 10 and 20 μΜ of H2O2 were added. The absorbance was normalized to the total protein input.
For fluorometric measurement of GFP levels in the reporter transgenic flies, somatic tissues or gonads were lysed in the dark in Nonidet P-40 lysis buffer. Lysates were cleared by centrifugation at 19,000 g (4°C), and collected supernatants were immediately used to measure fluorescence (excitation, 490 nm; emission, 510 nm); fluorescence intensity was normalized to total protein concentration.
RNA extraction and polymerase chain reaction (PCR) analysis
Reverse transcription-PCR (RT-PCR) was used to determine mRNA expression. Total RNA was extracted from Drosophila somatic tissues or gonads using RNAzol (Molecular Research Center, Cincinnati, OH, USA) and converted to cDNA with the iScript cDNA synthesis kit (Bio-Rad Laboratories). Aliquots of 1 μl cDNA from each RT reaction were then subjected to PCR using the Go Taq Green master mix (Promega, Madison, WI, USA). Semiquantitative PCR analyses were run for 25–28 cycles and were performed at least in duplicate on an MJ Research Minicycler thermocycler (Bio-Rad Laboratories)by using the following specific primers. rpn11-forward (F): 5′-ACTTAAAGACTATGGTGTCCA-3′, rpn11-reverse (R): 5′-TGGATCGTCTGCTACGTCTT-3′; rpn6-F: 5′-TCTACTGTCCGCCAAAGGTG-3′, rpn6-R: 5′-TTCCACTGACGAGCTGGTTG-3′; α4-F: 5′-GCGCCAACTGCGTTGTGCTT-3′, α4-R: 5′-GACTGCGCCACCTCGAGCAG-3′; α7-F: 5′-TTTTCGCCTGATGGCCGCGT-3′, α7-R: 5′-ACCGGTTACCCTGCCCACCAA-3′; β1-F: 5′-CGAGTCCTGCACCATCGGCG-3′, β1-R 5′-TGCCAATGCGCACCACACCA-3′; β2-F: 5′-AGCCACCGACCACCACCAAGA-3′, β2-R: 5′-CCACAACGCGCACCTCACGA-3′, β5-F: 5′-TGGCTGCTCCGCCATTCGAG-3′, β5-R: 5′-CCGGCCAGCATCATGCCCAT-3′; cncC-F: 5′-TGGAATTGGGCACCCATGGCG-3′, cncC-R: 5′-ATCATTGAGGGCGGCGGTGC-3′; keap1-F: 5′-TCCGCCGGCATGGAGTACCA-3′, keap1-R: 5′-CGCTCCACTGCGACCCGTTT-3′. Primers were designed using the primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). PCR products were analyzed by agarose gel electrophoresis, and quantification of gels was performed by scanning densitometry (Gel Analyzer 1.0; Biosure, Athens, Greece); the ribosomal gene rp49, rp49-F: 5′-AGCACTTCATCCGCCACC-3′, rp49-R: 5′-ATCTCGCCGCAGTAAACG-3′ (32), was used as normalizer.
Tissue protein extract preparation, immunoblotting analysis, and detection of protein carbonyl groups
Dissected somatic tissues or isolated gonads were homogenized on ice in Nonidet P-40 lysis buffer (see above) containing protease inhibitors, and lysates were immediately cleared with centrifugation for 15 min at 19,000 g (4°C). After adjustment of protein content with the Bradford method (Bio-Rad Laboratories), samples were mixed with reducing Laemmli buffer, and equal protein amounts were fractionated by SDS-PAGE followed by immunoblotting, as described previously (33). Primary and horseradish peroxidase-conjugated secondary antibodies were applied for 1 h at room temperature, and immunoblots were developed by using an enhanced chemiluminescence reagent kit (GE Healthcare Amersham, Pittsburgh, PA, USA). Quantification of blots was performed by scanning densitometry (see above).
Dissected tissue samples were processed for the detection of carbonyl groups using the Oxyblot detection kit (S7150; Merck Millipore, Billerica, MA, USA), as per the manufacturer's specifications.
Proteasome peptidase activity in fly tissue extracts
Dissected somatic tissues or gonads were lysed on ice by using buffers suitable for the isolation of either 26S (0.2% Nonidet P-40, 5 mM ATP, 10% glycerol, 20 mM KCl, 1 mM EDTA, 1 mM DDT, and 20 mM Tris, pH 7.6) or 20S (0.5% Triton X-100, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, and 20 mM Tris, pH 7.6) proteasomes (18). Lysates were cleared with centrifugation at 19,000 g (4°C), and, after protein content adjustment with Bradford, supernatants were immediately used to determine the 3 proteasome proteolytic activities, as described previously (16). Briefly, the CT-L (LLVY), C-L (PGPH; LLE) and T-L (LRR) activities were assayed by recording (excitation, 350 nm; emission, 440 nm) the hydrolysis of the fluorogenic peptides Suc-Leu-Leu-Val-Tyr-AMC, Z-Leu-Leu-Glu-AMC, and Boc-Leu-Arg-Arg-AMC (Enzo Life Sciences, Farmingdale, NY, USA), respectively, at 37°C for 30 min. Proteasome activity was determined as the difference between the total activity of crude extracts and the residual activity in the presence of the proteasome inhibitors MG-132 or Epoxomicin (Enzo Life Sciences).
Proteasome immunoprecipitation
For proteasome immunoprecipitation analyses, somatic tissues or gonads were extracted on ice in a 0.1% Nonidet P-40, 150 mM NaCl, 50 mm Tris/HCl buffer (pH 8.0) containing protease inhibitors. Extracts were centrifuged at 19,000 g (4°C) for 10 min, and equal protein amounts from the supernatant were cleared by adding protein-A Sepharose beads (code no. 17-0974-01, GE Healthcare Amersham) with constant rocking for 1 h at 4°C. The target antigen was then immunoprecipitated at 4°C (overnight constant rocking) with 2 μg of the corresponding antibody, followed by the addition of protein-A Sepharose beads for 2 h at 4°C. Immunoprecipitated protein complexes were collected and washed in extraction buffer and in 50 mM Tris (pH 8.0); they were then eluted from the Sepharose beads by boiling for 3 min in reducing Laemmli buffer and analyzed by immunoblotting. Normal IgGs (instead of the primary antibody against the target antigen) or only the protein-A Sepharose beads were used in control experiments.
In experiments combining immunoprecipitation with measurement of proteasome activity, a 10-fold reduced amount (0.2 μg) of the primary antibody was applied. Immunoprecipitated proteasomes were washed in extraction buffer and in 26S proteasome activity buffer. Peptidase activity was then directly measured without eluting the immunoprecipitated proteasomes from the beads.
Proteasome immunohistochemistry in whole-fly sections
Flies were slightly anesthetized and, following the removal of wings and legs, were fixed in 4% formalin for 24 h. Fixed flies were then embedded in paraffin, according to standard procedures (34). For proteasome immunohistochemistry, whole-fly paraffin sections (∼4 μM thick) were deparaffinized by incubation in xylene and rehydrated in a graded series of ethanol aqueous solutions. Following antigen retrieval by microwave heating in 10 mM citrate buffer for 25 min, sections were immersed for 15 min in 3% H2O2 in TBS in order to block endogenous peroxidase activity. The primary anti-20S-α subunit antibody (1:100 dilution) was applied for 1 h at room temperature and detected by using the visualization system EnVision Plus (Dako, Glostrup, Denmark). The immunoreaction was developed with 3,3-diaminobenzidine tetrahydrochloride (DAB; Dako) followed by section counterstaining with hematoxylin. A microscope (DM LB; Leica Microsystems, Wetzlar, Germany) equipped with a digital camera (DFC320; Leica) and the Application Suite 2.2 software (Leica) was used for image acquisition. Negative controls, in which the primary antibody was omitted, were free of staining.
Antibodies used
Primary antibodies against the 20S-α (sc-65755) and p42A (Rpn7; sc-65750) Drosophila proteasome subunits; the anti-ubiquitin (sc-8017), anti-GAPDH (sc-25778), anti-GFP (sc-9996), anti-actin (sc-1616) antibodies; and the HRP-conjugated secondary antibodies (anti-rabbit-IgG, sc-2004; anti-mouse-IgG, sc-2005; and anti-goat-IgG, sc-2020) used were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). The anti-advanced glycation end product (AGE)-modified protein antibody (KAL-KH001) was from Cosmo Bio Co. Ltd. (Tokyo, Japan). The polyclonal antibody against the Drosophila β5 proteasome subunit was a generous gift from Dr. Maria Figueiredo-Pereira (Department of Biological Sciences, Hunter College, City University of New York, New York, NY, USA).
Statistical analysis
Presented experiments have been performed at ≥2 biological replicates (n=2; in each biological replicate samples were analyzed at least in duplicates unless otherwise indicated). Assays were done in either single-fly tissue preparations or after pooling isolated tissues from 10–20 flies. Data points correspond to the means of the independent experiments; error bars denote sd. Individual protein or gene expression levels were quantified against a reference and presented in comparison to the respective control. For statistical analysis, Microsoft Excel (Microsoft, Redmond, WA, USA) and the Statistical Package for Social Sciences (IBM SPSS 19.0 for Windows; IBM, Armonk, NY, USA; administrated by University of Athens) were used. Significance was evaluated using 1-way ANOVA and was accepted at values of P < 0.05.
RESULTS
Aging of Drosophila somatic tissues is characterized by increased proteome damage, as well as by a sharp decline of proteasome peptidase activity and expression
To analyze the alterations in proteostasis during aging of Drosophila, we initially set the 3 age groups (young, middle-aged, and old) to be studied. Under our culture conditions, although both sexes had similar maximum life span, male flies showed decreased median life span (50±3.33 d) compared to females (60±2.51 d) (P<0.01). Also, all flies (following an initial increase at the age of 10–15 d) showed a significant drop in locomotor performance after the age of 34–36 d (data not shown). On the basis of this initial screening, 1- to 4-d-old mated flies were used as young and 33- to 37-d-old as middle aged, while terminally aged flies were males above the age of 50 d and females above the age of 60 d.
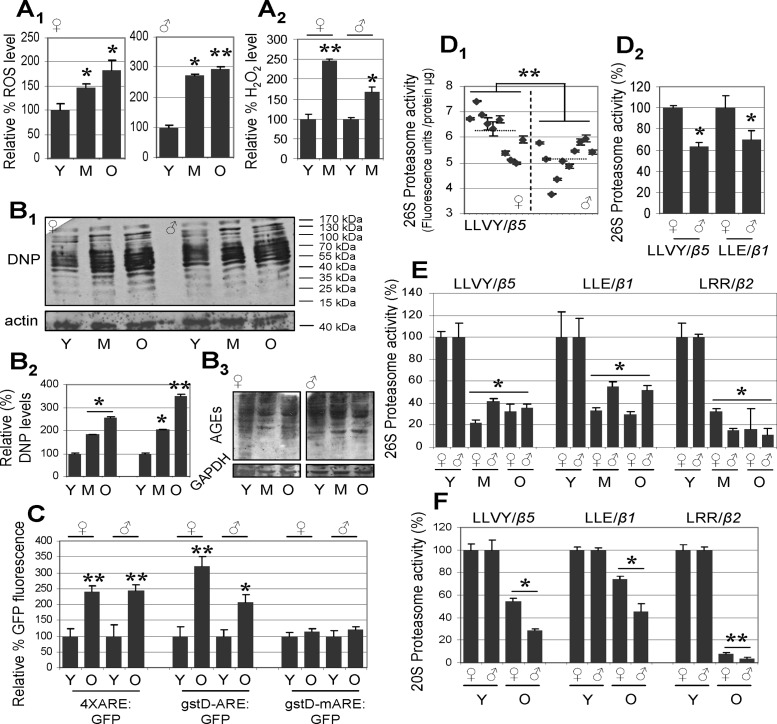
As our preliminary analyses in isolated female abdomen, which consists of both somatic tissues and the reproductive organs (the ovaries), showed a distinct pattern of proteasome regulation compared to somatic tissues from the head or the thorax (data not shown; see below) we initially studied proteome alterations during aging of isolated male and female somatic tissues. In accordance with previous studies (35, 36, 37), our analyses showed that Drosophila somatic tissue aging is accompanied (in both sexes) by higher levels of ROS (Fig. 1A1) and H2O2 (Fig. 1A2). Moreover, somatic tissues aging correlated with sex-independent accumulation of oxidatively damaged (carbonylated) proteins (Fig. 1B1, B2) and of AGE-modified proteins (Fig. 1B3). To investigate the age-dependent functionality of transcription factors that modulate the cellular antioxidant and detoxification responses, we used transgenic flies that express the green fluorescent protein (GFP) under the control of genomic sequences bearing functional or mutated AREs (26, 31). As previously indicated (26, 31), we found that young transgenic flies with functional AREs expressed appreciable levels of GFP (data not shown), indicating a basal activity of the antioxidant response circuit in the young organism under conditions of minimal stress. Somatic tissue aging correlated with further activation of the GFP reporter gene (Fig. 1C), likely reflecting the age-related increase in oxidative stress that can (at least partially) still mobilize specific antioxidant responses and ARE activation.
Figure 1.
Drosophila somatic tissue aging is characterized by accumulation of ROS, H2O2, and oxidized (carbonylated) and AGE-modified proteins, as well as by activation of ARE-mediated transcription and a significant decline of proteasome peptidase activity. A1–B3) Relative percentage of ROS (A1) and H2O2 (A2), protein carbonylation (B1) and quantitative analysis (B2), and AGE-modified protein levels (B3) in somatic tissues of young (Y), middle-aged (M), and old (O) female or male flies. DNP, 1,3-dinitrophenylhydrazone. C) Relative percentage of GFP fluorescence during aging of female and male gstD-ARE:GFP (gstD-ARE:GFP/II), 4XARE:GFP (4XARE:GFP-16(R7)/II), and gstD-mARE:GFP (gstD-mARE:GFP/III) transgenic flies; as is evident, reporter induction required a functional ARE. Median life spans of transgenic flies were as follows: 4XARE:GFP, 41±1.45 d; gstD-ARE:GFP, 39±9.29 d; gstD-mARE:GFP, 52±2.87 d. D1, D2) Comparative analyses of somatic tissue proteasome activity in single female or male fly preparations or after pooling female or male flies. D1) Fluorescence units per microgram of tissue protein following measurement of the 26S proteasome peptidase activity CT-L (LLVY/β5) in single female or male fly preparations; mean value in female flies was 6.195 ± 0.81 U/μg, whereas in males, it was 5.162 ± 0.68 U/μg. D2) Relative activity (%) of 26S CT-L (LLVY/β5) and C-L (LLE/β1) in preparations of pooled (10–12 flies) female or male flies. E, F) Relative activity (%) of 26S (E) and 20S (F) proteasome peptidase in somatic tissues during aging; sex-independent decline of activities is evident. Actin or GAPDH probing in B1, B3 was used as reference for equal protein loading. Bars represent means ± sd; n = 2 (B1–B3, C, D1); n = 3 (A1, A2, D2, E, F). *P < 0.05; **P < 0.01.
As oxidized proteins accumulate in aged somatic tissues despite the activation of transcriptional antioxidant responses, we focused on studying proteasome functionality under conditions of in vivo aging. We initially analyzed single-fly preparations and, in accordance with recent findings (21), we noted interindividual and sex-dependent differences in proteasome activity. Specifically, we found that (compared to females) somatic tissues from young males had significantly lower 26S proteasome activity (Fig. 1D1). These findings were further verified in preparations of pooled young female or male fly samples (Fig. 1D2) indicating that despite interindividual and sex-differences, pooling of large numbers of flies reliably reflects averaged differences in proteasome activity. As the observed differences suggested sex-dependent differential regulation of resources for proteostasis maintenance, we studied age-related proteasome modulation separately in female and male somatic tissues. We found that both the 26S and 20S proteasome peptidase activity declined in the somatic tissues of middle-aged flies and remained at low levels in very old insects (Fig. 1E, F); interestingly, males showed stronger suppression of 20S activity during aging (Fig. 1F).
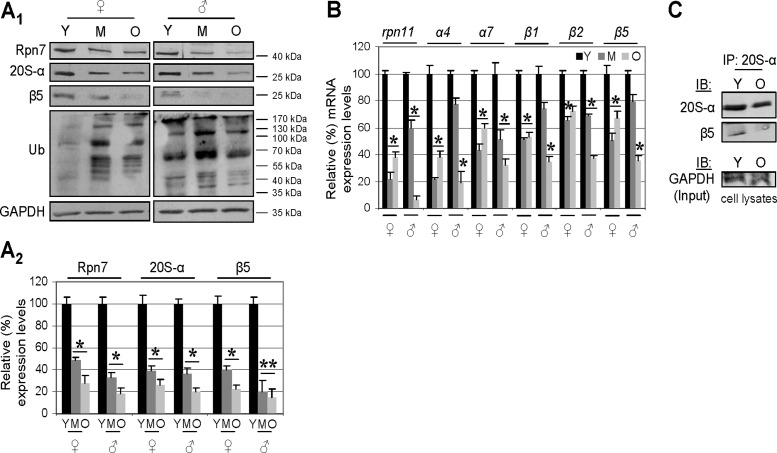
To identify the mechanism underlying proteasome dysfunction during aging, we then studied the protein abundance and mRNA expression of major 19S and 20S proteasome structural components. We noted that aging of Drosophila somatic tissues correlates with down-regulation of protein (Fig. 2A1, A2) and mRNA (Fig. 2B) expression of all the major 19S and 20S proteasome subunits assayed. Furthermore, immunoprecipitation analyses revealed that the low expression levels of proteasome subunits result in reduced amounts of assembled 20S proteasomes in the somatic tissues of aged flies (Fig. 2C). Down-regulation of proteasome genes expression during aging does not appear to represent a global phenomenon for genes modulating proteostasis maintenance, as we noted diverse expression patterns for the autophagy-related atg6 and atg8 genes, cncC (nrf2 homolog in Drosophila; ref. 26), and the antioxidant response-related gene gstD1 (Supplemental Fig. S1A). Thus, the suppression of proteasome gene expression during aging is a major hallmark of deregulation of the proteostasis network.
Figure 2.
Aging of Drosophila somatic tissues is characterized by lower proteasome expression and assembly rates. A1, A2) Representative immunoblot (A1) and densitometric analysis (A2) of female and male somatic tissue protein samples probed with antibodies against Rpn7, 20S-α, β5, and ubiquitin. B) RT-PCR densitometric analyses of rpn11, α4, α7, β1, β2, and β5 mRNA expression levels during aging of somatic tissue. C) Immunoprecipitation of the 20S-α and β5 protein interaction (indicating assembled proteasome) in lysates from young and aged Drosophila somatic tissues. Lysates were immunoprecipitated (IP) with antibodies against 20S-α, and immunoprecipitates were probed by immunoblotting (IB) with anti-20S-α and anti-β5 antibodies. GAPDH protein probing was used as a reference for protein loading (A1) and rp49 gene expression as reference for total RNA input (B). Densitometric data indicate relative expression against a loading reference followed by normalization against controls, namely samples from young flies; n = 3 (A2); n = 2 (B). Bars represent means ± sd. *P < 0.05, **P < 0.01 vs. controls.
To better understand proteasome regulation during aging, it is highly desirable to precisely investigate changes at the tissue level. Because of the small size of Drosophila, studies on purified tissues represent a technical challenge; nevertheless, studies of body parts that are greatly enriched in specialized tissues is a useful surrogate. Thus, we studied the effects of aging in different somatic tissues of male and female flies, namely the head (mostly neuronal tissue with minor contributions from fat and muscles), the thorax (mainly muscle tissue and the tracheal system), and the abdomen (muscle and adipose tissues, along with the digestive system) after removal of the gonads. In all somatic tissues, we noted accumulation of carbonylated and ubiquitinated proteins during aging; higher amounts of modified proteins were found to accumulate in the head tissues, which are enriched in neurons (data not shown). Moreover, in all tissues, the expression levels of main 19S and 20S proteasome protein subunits (Supplemental Fig. S2A), along with 26S proteasome peptidase activity (Supplemental Fig. S2B), were found to decrease significantly in middle-aged flies and remained low in very old flies. All distinct body parts studied had similar basal proteasome activity (data not shown), whereas the rate of proteasome catalytic activity down-regulation during aging was significantly lower in female compared to male somatic tissues (Supplemental Fig. S2B). Similarly, in hemolymph preparations, both the expression of proteasome subunits and the CT-L and C-L catalytic activity were found to decline during aging (Supplemental Fig. S2C). Thus, the disruption of proteasome function during aging is a universal phenomenon across the various somatic tissues of Drosophila, albeit with a sex-dependent pattern.
Gonads of aging flies (ovaries and spermathecae) maintain substantial proteasome expression levels and highly active proteasomes
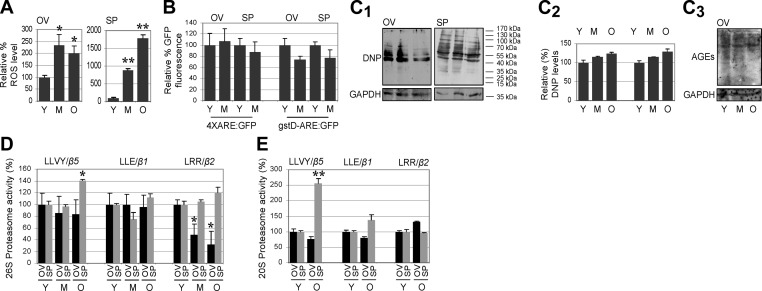
To investigate age-related alterations in proteome maintenance in the gonads, we isolated ovaries and spermathecae from young, middle-aged, and old female and male flies, and we analyzed proteome damage and proteasome regulation. Despite higher ROS levels in the gonads of aged flies (Fig. 3A) and absence of activation of a transcriptional antioxidant response (Fig. 3B), we did not find accumulation of carbonylated proteins or AGE-modified proteins (Fig. 3C). This is most likely due to more active proteasomes in the aged gonads, as we observed that both the 26S and 20S proteasome peptidase activity remained relatively stable (with the exception of T-L activity in ovarian tissue) during aging; interestingly, in some cases, catalytic activity was even found to increase (Fig. 3D, E).
Figure 3.
Gonads of aged organisms retain low levels of carbonylated proteins and relatively stable 26S and 20S proteasome peptidase activity. A) Relative percentage of ROS in the ovaries (OV) and spermathecae (SP), showing higher ROS levels during aging. B) ARE-mediated transcription (quantified as percentage GFP fluorescence) in female and male gonads during aging of transgenic 4XARE:GFP and gstD-ARE:GFP flies. C1–C3) Immunoblotting analyses (C1) and quantitative analysis (C2) of total protein carbonylation and immunoblotting analysis of AGE-modified proteins (C3) in female and male gonads during aging; probing with GAPDH was used as protein loading reference. DNP, 1,3-dinitrophenylhydrazone. D, E) Relative activity (%) of 26S (D) and 20S (E) proteasomes in Drosophila ovaries and spermathecae during aging. Except for T-L activity, which was found to decline in the aged ovarian tissue, in all other cases, proteasome activity was either stable or increased (e.g., CT-L in SP). Bars represent means ± sd; n = 3 (A); n = 2 (B--E). *P < 0.05, **P < 0.01.
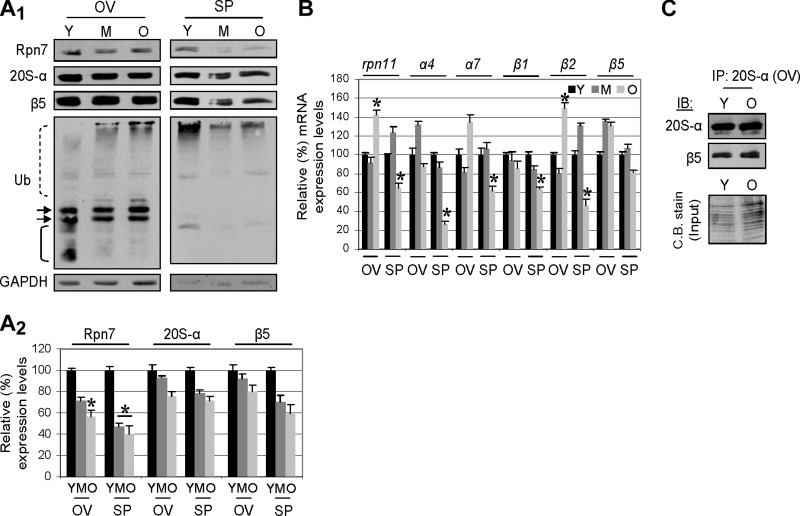
Further analyses corroborated these findings, as we noted that the gonads of aged flies maintained substantial protein (Fig. 4A1, A2) and mRNA (Fig. 4B) expression levels of proteasome subunits and almost unchanged levels of assembled proteasomes (Fig. 4C). Specifically, a decrease in protein expression was noted for the 19S Rpn7 proteasome lid subunit in both the ovaries and the spermathecae (Fig. 4A), which was, however, significantly less pronounced compared to the somatic tissues (see Fig. 2A). The stability of the proteostasis networks in the gonads of the aged flies was also evident by the fact that (with the exception of the atg6 gene, for which we found decreased expression in aged spermathecae), the expression of the atg6, atg8, cncC, and gstD1 genes was either stable or up-regulated (e.g., atg6, and cncC in females) in the gonads of aged organisms (Supplemental Fig. S1B). As further evidence of an active proteasome in the gonads of aged flies, and despite the fact that the pattern of ubiquitinated proteins seemed to change in the ovarian tissue as the flies aged (Fig. 4A1, left panel), there was no significant accumulation of ubiquitinated proteins (Fig. 4A1); a direct correlation between proteasome activity and tissue levels of ubiquitinated proteins was noted following exposure of flies to specific proteasome inhibitors, which, in both the soma and the gonads, induced the accumulation of ubiquitinated proteins (unpublished results). Conclusively, the gonads of the aged organism retain substantial capacity for proteasome-mediated regulation of proteostasis.
Figure 4.
Gonads from aged flies retain substantial proteasome expression levels and assembled proteasomes. A1, A2) Representative immunoblotting (A1) and densitometric (A2) analysis of ovary (OV) and spermatheca (SP) protein samples probed with antibodies against Rpn7, 20S-α, β5 and ubiquitin. Down-regulation of protein expression mostly refers to the 19S subunit Rpn7, and it is less pronounced than in somatic tissues (see Fig. 2A). Dashed line bracket indicates polypeptides showing increased ubiquitination in aged ovaries; arrows indicate polypeptides with similar ubiquitination levels; solid line bracket indicate polypeptides with reduced ubiquitination. B) RT-PCR densitometric analyses of the rpn11, α4, α7, β1, β2, and β5 mRNA expression levels in the gonads during aging. C) Immunoprecipitation of the 20S-α and β5 protein interaction (indicating assembled proteasomes) in lysates from young and aged Drosophila ovaries. Immunoprecipitation (IP) was performed with an anti-20S-α antibody, and immunoprecipitates were probed by immunoblotting (IB) with anti-20S-α and anti-β5 antibodies; Coomassie blue (C.B.) stain depicts total protein input. GAPDH protein probing was used as a reference for equal protein loading (A1) and rp49 gene expression as reference for total RNA input (B). Densitometric analysis (n=2) was performed as in Fig. 2. Bars represent means ± sd. *P < 0.05.
Gonads of young flies possess more abundant and more active proteasomes than somatic tissues
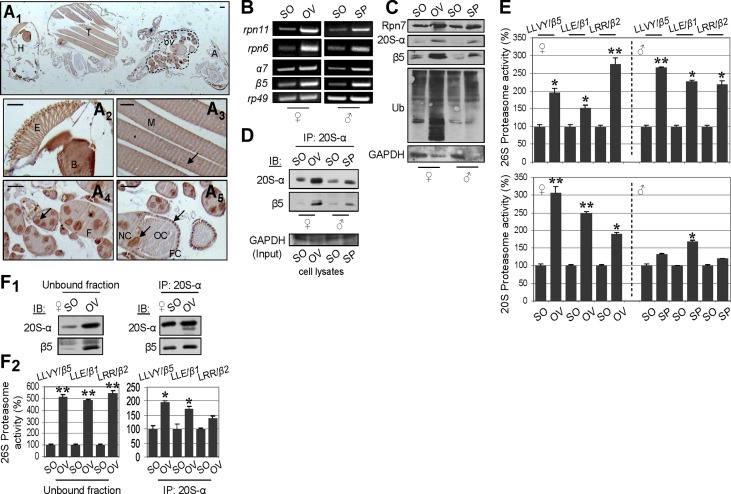
As our analyses have indicated that gonads exhibited a superior proteasome-mediated capacity for proteostasis maintenance and resistance to aging-related damage, we also compared proteasome regulation and content between the somatic tissues and the gonads of young male and female flies. Immunohistochemical staining of whole female fly paraffin sections with an antibody against the 20S-α proteasome subunit showed generalized cytoplasmic and nuclear staining in all tissues (Fig. 5A1); more intense staining was seen in the neural tissues of the head (e.g., the brain and the eye), in the muscles, and in the ovaries (Fig. 5A2–A5). In ovarian tissue, positive 20S-α immunostaining was observed at all stages of oogenesis and in all cell types of the follicles (i.e., the oocyte, the nurse cells, and the follicle cells), where 20S-α was distributed in both the cytoplasm and the nucleus (Fig. 5A4, 5A5); proteasome nuclear staining may imply a role of proteasomes at sites of active gene transcription and/or in nuclear protein maintenance (38). Subsequent comparative analyses of proteasome expression, assembly, and peptidase activity between the somatic tissues and the gonads of young male and female flies revealed that 19S and 20S proteasome mRNAs (Fig. 5B) and protein subunits (Fig. 5C) were more highly expressed in the gonads of both sexes than in the soma; the elevated levels of ubiquitinated proteins in the gonads (Fig. 5C) most likely reflect the fact that these tissues are mainly composed of mitotic cells, and thus the normal short-lived ubiquitinated proteins (e.g., cyclins) are expected to be more abundant than in the somatic postmitotic cell lineages.
Figure 5.
Gonads of young flies possess more abundant and more active proteasomes than somatic tissues. A1–A5) Representative immunohistochemical staining of whole female fly sections with an anti-20S-α proteasome subunit antibody. The antigen was distributed in head (H), thorax (T), and abdomen (A) tissues (A1). More intense staining was seen in the ovarian tissue (ov; A1, dashed line), in the neural tissues of the head [e.g., the eye (E) and the brain (B); A2], and in muscle (M; A3). In the ovarian follicles (F; A4), the 20S-α proteasome subunit was distributed in the nurse cells (NC), the oocyte (OC), and the follicular epithelium (FC) (A5). In all cell types, (including muscle cells; A3) both cytoplasmic and nuclear (arrows) staining was seen. Scale bars = 50 μm. B) Comparative RT-PCR analyses of mRNA expression levels of indicated proteasome subunits in somatic (SO) tissues and gonads (OV, ovary; SP, permathecae) of female and male flies. C) Immunoblotting analysis of proteasome subunit expression levels in gonads and somatic tissues of female and male flies. D) Immunoprecipitation (IP) of 20S-α and β5 proteasome subunit interaction (indicating assembled proteasomes) in lysates from Drosophila gonads and somatic tissues. Immunoprecipitation was performed with the anti-20S-α antibody, and immunoprecipitates were probed by immunoblotting (IB) with anti-20S-α and anti-β5 antibodies. E) Relative activity (%) of 26S (top panel) and 20S (bottom panel) proteasomes in somatic tissues and gonads of Drosophila. In nearly all cases, significantly higher proteasome peptidase activity was found in the gonads. F1, F2) Immunoprecipitation of the 20S-α proteasome subunit followed by immunoblotting analysis of 20S-α and β5 subunits. A minimal amount of the anti-20S-α antibody was used to achieve the immunoprecipitation of equal amounts of proteasomes from different tissues. F1) Indeed, although the unbound ovarian fraction contained (compared to somatic tissues) higher amounts of proteasomes, we detected similar amounts of assembled proteasomes in the eluate. F2) Relative activity (%) of 26S proteasome in the unbound fraction and immunoprecipitated proteasomes in the female soma and ovaries. Note higher endogenous proteasome catalytic activity in the immunoprecipitated ovarian proteasomes. All assays were done in 10- to 13-d-old flies. GAPDH and rp49 were used as references for equal total protein and RNA input, respectively. Bars represent means ± sd; n = 2. *P < 0.05, **P < 0.01.
The tissue-dependent proteasome regulation in young flies was also evident in the higher amounts of assembled proteasomes (Fig. 5D) and the increased 26S and 20S peptidase activity (Fig. 5E) that were noted in the gonads of both sexes vs. the soma. We also asked whether gonad proteasomes are enzymatically overactive compared to those in somatic tissues. To address this issue, we immunoprecipitated equal amounts of proteasomes from female somatic and ovarian tissues by using minimal amounts of the anti-20S-α antibody (see Materials and Methods). As shown in Fig. 5F1, this approach resulted in the coimmunoprecipitation of similar amounts of assembled proteasomes from the two target tissues. Notably, immunoprecipitated ovarian proteasomes showed higher catalytic activity as compared to those from somatic tissues (Fig. 5F2), suggesting the existence of either specific activators that facilitate effective substrate access or specific structural modifications that render ovarian proteasomes highly active.
Compared to somatic tissues, gonads possess proteasomes more resistant to oxidative stress and retain (independently of age) active antioxidant responses
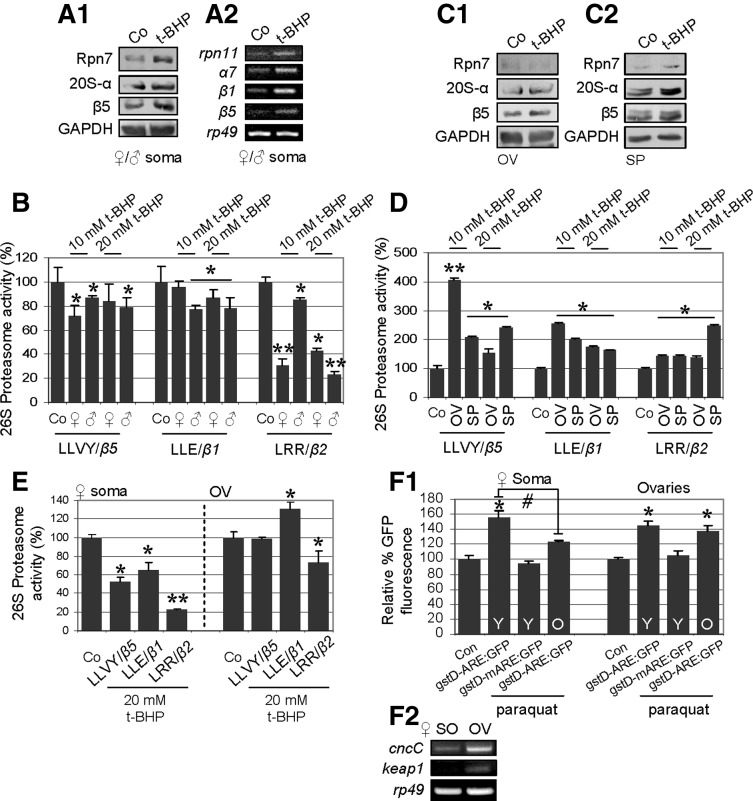
We then asked whether somatic tissues and gonads also display differential responses to exogenous oxidative stress. To address this issue, we exposed eclosed flies to oxidants, namely t-BHP and H2O2. As reported previously (39) and validated herein, exposure of flies to different concentrations of t-BHP or H2O2 resulted in marked dose-dependent reduction of life span and locomotor performance; similar effects were seen in flies exposed to paraquat (data not shown). In addition, exposure of flies (10–15 d old) to different doses of t-BHP for various periods induced higher levels of ROS and AGEs in both the somatic tissues and the ovaries of females (Supplemental Fig. S3A). Interestingly, carbonylated and ubiquitinated proteins were found to accumulate mostly in the somatic tissues of the females but not in the ovaries (Supplemental Fig. S3B, C). This observation was explained by our finding that although exposure of flies to t-BHP induced proteasome subunit expression in both the somatic tissues and in the gonads (Fig. 6A1, A2, C1, C2), proteasome catalytic activity declined in the soma but increased in the gonads (Fig. 6B, D). Moreover, gonads retained their capacity to respond to oxidants, even in aged organisms, as it was observed that treatment of aged flies with t-BHP (under identical conditions to those used in young flies) reduced somatic proteasome activity by ∼50% but had minor effects on gonad proteasome activity (Fig. 6E). To address whether this property of gonads is also reflected in the activation of transcriptional antioxidant responses, we exposed young and old flies bearing the gstD-ARE:GFP reporter transgene to paraquat. As shown in Fig. 6F1, aged somatic tissues showed reduced capacity (compared to young tissues) to activate AREs in response to exogenous oxidative insult. In support of our aforementioned findings showing that the gonads maintain youthful proteostasis-regulating networks, it was observed that the paraquat-treated aged ovaries could activate AREs almost as effectively as the young ovarian tissue (Fig. 6F1); interestingly, as for the proteasome genes, the ovarian tissue expressed higher basal cncC and keap1 levels compared to the soma (Fig. 6F2). Therefore, gonads retain their capacity to maintain proteasome activity and antioxidant response in response to oxidative stress in the aged organism.
Figure 6.
Gonad proteasome activity is more resistant to oxidative stress than that of somatic tissues and retains active antioxidant responses, in an age-independent manner. A1, A2) Immunoblot (A1) and RT-PCR (A2) analyses showing stable or increased protein (A1) and mRNA (A2) expression of proteasome subunits in somatic tissues of young (mixed population) flies exposed to 10 mM t-BHP for 8–13 d. B) Relative activity (%) of 26S proteasome in somatic tissues of young Drosophila following treatment with 10 mM t-BHP as in A or with 20 mM t-BHP for 3 or 4 d; in most cases, proteasome activity declined. C) Immunoblot analyses of the indicated proteasome protein subunits, showing stable or increased expression levels in ovaries (OV; C1) and spermathecae (SP; C2) of young flies treated with 10 mM t-BHP as in A. D) Relative activity (%) of 26S proteasome, showing proteasome activation in the gonads of young Drosophila exposed to 10 to 20 mM t-BHP as in B. E) Relative activity (%) of 26S proteasome in aged female fly somatic and ovarian tissues following exposure to 20 mM t-BHP for 3–4 d. F1, F2) Relative percentage of GFP fluorescence in young (Y) and old (O) female somatic and ovarian tissues of gstD-ARE:GFP and gstD-mARE:GFP transgenic flies exposed to 20 mM paraquat for 15 h (F1), and relative cncC and keap1 mRNA expression levels (F2) in somatic and ovarian tissues of 10- to 13-d-old female flies. GAPDH and rp49 were used as references for equal total protein and RNA input, respectively. Bars represent means ± sd; n = 3. *,#P < 0.05, **P < 0.01.
Sustained activation of CncC in transgenic flies maintains youthful proteasome gene expression patterns in aged somatic tissues, whereas RNAi-mediated cncC knockdown promotes a soma-like down-regulation of proteasome gene expression profile in aged gonads
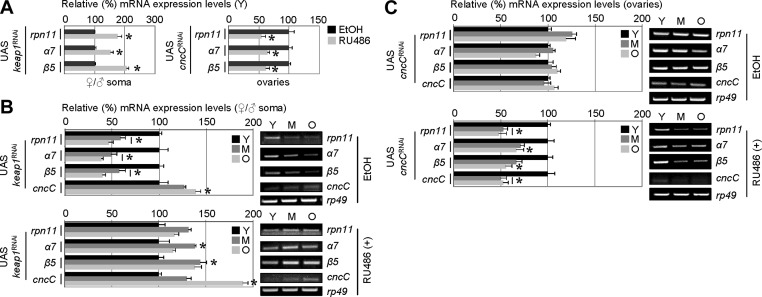
Considering that the mechanism by which basal expression of proteasome genes is down-regulated during cellular senescence or in vivo aging remained virtually unknown, we sought to functionally analyze the molecular pathway involved in this phenotype. As we have found that ovaries (compared to soma) express high levels of CncC/Keap1 and retain active antioxidant response during aging, we asked whether CncC/Keap1 signaling is implicated in the differential age-related proteasome expression in the soma vs. the gonads. We initially noted that CncC activation by keap1 RNAi enhanced proteasome gene expression in young somatic tissues, whereas RNAi-mediated cncC knockdown suppressed endogenous proteasome gene expression in ovarian tissues from young flies (Fig. 7A). Thus, we assayed proteasome gene expression in young, middle aged, and aged tissues of transgenic flies under conditions of either CncC activation by keap1 RNAi or after RNAi-mediated cncC knockdown. As shown in Fig. 7B, keap1 knockdown by RNAi resulted in aged somatic tissues with youthful proteasome gene expression levels, whereas RNAi-mediated cncC knockdown (Fig. 7C) induced a soma-like downregulation of proteasome gene expression in ovaries from aged flies (a detailed analysis of the Nrf2/Keap1-mediated modulation of fly health span and proteasome activity will be reported elsewhere). Together with the noted differences in the induction of antioxidant response in the soma and gonads of aged flies (see Fig. 6F), these findings highlight the Nrf2/Keap1 module as a key factor in the differential regulation of proteostasis in the somatic vs. the reproductive tissues during aging.
Figure 7.
CncC activation (by RNAi-mediated keap1 knockdown) in adult flies results in youthful proteasome gene expression patterns in the aged soma, whereas RNAi-mediated cncC knockdown induces a soma-like down-regulation of proteasome genes expression in ovaries of aged flies. A) Densitometric RT-PCR analyses of rpn11, α7, and β5 proteasome gene expression levels in somatic (left panel) or ovarian (right panel) tissues of young (Y) UAS keap1 (left panel) or UAS cncC (right panel) RNAi flies. RU486 was administered to flies for ∼7 d; control flies were treated with ethanol (EtOH). UAS, upstream activation sequence. B, C) RT-PCR densitometric analyses of the rpn11, α7, β5, and cncC gene expression levels (left panels) and representative agarose gels (right panels) in young (Y), middle-aged (M), and old (O) somatic tissues (B) or ovaries (C) of UAS keap1 (B) or UAS cncC (C) RNAi flies; in all cases, control flies were fed with EtOH. Eclosed flies were constantly fed with RU486 in order to induce transgene expression. As either keap1 or cncC RNAi affected maximum and median life span of flies (data to be reported elsewhere), in all cases, young flies used in these analyses were at the age of ≤15% of their life span, middle-aged at ∼45–55% of their life span, and old flies at ≥80% of their life span. Bars represent means ± sd; n = 2. Controls were arbitrarily set to 100%. *P < 0.05.
DISCUSSION
Aging relates to a progressive shrinkage of homeodynamics that promotes multiple cellular, molecular, and biochemical changes; these changes induce a vicious circle of increasing accumulation of molecular damage and of reducing stress tolerance that leads to increased probability of diseases and eventually to death (1, 2, 4, 7). We report herein that in Drosophila, the main hallmarks of aging relevant to proteostasis and proteasome regulation appear in a sex-independent manner mainly in somatic tissues, whereas gonads age at a significantly lower rate. The expression of the somatic 19S and 20S proteasome genes and protein subunits was found to be down-regulated (along with proteasome assembly rates) already in middle-aged flies (Supplemental Fig. S4A), when signs of aging first appeared (e.g., reduced muscle strength). Notably, aging of the somatic tissues affected mostly 19S proteasome component expression and 26S peptidase activity, which were significantly lower than 20S activity in very old female flies. This finding may relate to the fact that 20S proteasome is more critical for the removal of damaged proteins (8, 12), and it is also more resistant to oxidative stress (40). In addition, somatic tissues of young females had higher proteasome activity compared to males, and female soma showed lower rates of proteasome activity decline during aging. These observations suggest the existence of sex-dependent modulation of the proteostasis network functionality during aging, which may contribute to the greater ability of females to cope with stress and to their increased longevity (present study and ref. 41). Notably, there may also be species-specific adaptations in relation to endogenous proteasome peptidase activity, as our preliminary analyses have revealed that normal human cycling cells have higher proteasome activity as compared to Drosophila somatic cells; proteasome activities in human cells seem to further increase during advanced tumorigenesis (data to be presented elsewhere).
Consistent with our findings and in contrast to transcriptome data showing higher proteasome gene expression in Drosophila during aging (24), two previous studies (using whole-body mixed populations of female and male Drosophila) showed decreased proteasome expression and a decline of 26S proteasome activities in middle-aged flies (18, 19), which, reportedly, and in agreement with our data, related to impaired assembly of the 26S proteasome (19). The finding reported in those two studies that 20S activity did not change significantly in middle-aged flies may be due to the fact that whole-body (including the gonads) preparations were used, as well as that, according to our present work, 20S activity mostly declines in terminally aged somatic tissues. Thus, the previously described observations of lower proteasome activity during senescence of cultured human somatic cells (16) are extended by our in vivo studies in the setting of Drosophila somatic (mostly postmitotic) tissues.
Interestingly, there is a sex-independent difference between the proteostasis network regulation in the gonads and the somatic tissues during Drosophila aging. Specifically, we have found that increased ROS levels in the gonads of aged organisms do not correlate with higher amounts of modified proteins or ARE activation and that the gonads of aged organisms maintain substantial expression levels and high catalytic activity of proteasomes. In addition, the gonads of young flies possess more abundant and more active proteasomes than somatic tissues. Consistent with these findings, which indicate that the gonad proteome is maintained at a high-quality level to safeguard reproduction, it has been previously demonstrated that cytokinesis in yeast encompasses a sirtuin sir2p gene-dependent asymmetrical segregation of carbonylated and aggregated proteins that were retained in mother cells and were not inherited by daughter cells (42). Such segregation and uneven partitioning of damaged proteins is required for old mother cells to develop a rejuvenated, germ-like, daughter cell lineage (43, 44), suggesting that an evolutionarily conserved mechanism may have evolved to avoid clonal senescence by establishing an aging (soma-like) and rejuvenated (germ-like) lineage. During Caenorhabditis elegans reproduction, removal of carbonylated proteins depends on proteasome activity (45), and elimination of damaged proteins during differentiation of embryonic stem cells coincided with considerably elevated activity of the 20S proteasome (46). In C. elegans, it was very recently reported that forced reinvestment of resources from the germ line to the soma resulted in elevated somatic proteasome activity, clearance of damaged proteins, and increased longevity (47). This activity was associated with increased expression of rpn-6, a gene also found to be overexpressed in human embryonic stem cells (48) and found by us herein to be overexpressed in Drosophila reproductive tissues. Finally, Fredriksson et al. (49) reported recently that Drosophila eggs exhibit high capacity to prevent protein aggregation, and possess higher proteasome activity and reduced levels of carbonylated proteins compared to the soma. Also, similar to what we report herein, the researchers observed that 26S somatic proteasome capacity declined in middle-aged flies, whereas proteasome activity was maintained in the eggs of aged females; their observation that 20S activity does not decline in the soma may relate to our finding that 20S peptidase activity is mostly suppressed in terminally aged flies. Our study adds substantial new information and mechanistic insights to the findings of Fredriksson et al. (49) by demonstrating, in a detailed fashion, sex-, tissue-, stress-, and age-dependent regulation of proteasome and antioxidant responses during Drosophila aging, by extending these novel concepts also to the male reproductive tissue and by implicating the Nrf2 pathway (see below) in the differential age-related proteostasis modulation in somatic vs. reproductive tissues.
Specifically, we have found that the relatively unchanged proteasome functionality of aged gonads also correlated with differential response (compared to soma) to exogenous oxidative stress. We report that, although exposure of young flies to oxidants induced higher proteasome expression levels in all tissues, it was only in the gonads that these responses correlated with higher proteasome catalytic activity. Notably, in aged flies, somatic proteasome activity was increasingly sensitive to oxidants, whereas ovaries maintained their proteasome activity. In addition, although the capacity of antioxidant response (e.g., ARE activation) was reduced in the aged soma, it was largely retained in the gonads. The increased resistance of gonads to oxidative stress-mediated proteasome dysfunction may be attributed to either the higher intrinsic proteasomes peptidase activity of the gonads or to more intense responses to oxidants in the reproductive tissues vs. the soma. Previous studies have shown that proteasome activity can be directly regulated by the molecular chaperone Hsp90 (50), while it was recently found that the immunoproteasome, the 20S proteasome, and the proteasome regulator PA28αβ are oxidative-stress-adaptive proteolytic complexes (51). It is also possible that somatic proteasomes are more exposed to oxidants (as compared to gonad proteasomes) and are thus more strongly affected by oxidative modifications (e.g., carbonylation, HNE adduct formation, and S-glutathionylation of their subunits; ref. 52) that directly affect their catalytic activities. In support, we have found that exposure of isolated Drosophila proteasomes to low concentrations of oxidants suppressed their peptidase activity (data not shown). More studies are needed however to understand in vivo proteasome regulation by oxidative stress, as our preliminary analyses (unpublished results) indicate that it involves multiple regulatory pathways and also depends on several parameters, including age, sex, tissue, and subcellular organelle affected, as well as the type of oxidant, dose, and duration of exposure.
At the in vivo setting of the ARE-GFP reporter transgenic lines, it was evident that the endogenous age-related stress is “sensed” by the (at least partially) functional network of somatic antioxidant defenses, which increased ARE activity. Moreover, in relation to other genes encoding components of the proteostasis and antioxidant response machinery, we noted reduced basal expression levels only for the autophagy-related atg8 gene (as also reported in ref. 53), whereas atg6, cncC, and gstD1 (a prototypical oxidative stress response gene and a direct CncC target; ref. 26) expression levels were found either unchanged or even increased in the aged soma. In light of these findings, the significant age-dependent somatic suppression of basal proteasome gene expression was intriguing. We report herein novel data indicating that the differential expression of proteasome genes in somatic vs. reproductive aged tissues depends on the Nrf2/Keap1 signaling pathway, as knocking down keap1 resulted in aged somatic tissues with youthful proteasome gene expression levels, whereas cncC knockdown promoted a soma-like proteasome gene expression profile in aged gonads. These findings suggest that keap1-knockdown-mediated sustained elevation of Nrf2 signaling counteracts an age-dependent decline of endogenous Nrf2-positive regulation of basal proteasome gene expression and also provide a novel mechanistic explanation for the age-related somatic loss of proteasome function. The positive Nrf2 regulation of proteasome activation can be also viewed as an indicator of stress-induced compensation, which is a well-known phenomenon of hormesis (17). As it was recently found that Nrf2 occupies an alternate ARE site in the liver in old rats, indicating an age-specific adaptation to maintain gene expression (54), it is plausible that gonads may exploit similar mechanisms to maintain youthful proteasome gene expression via Nrf2; alternatively, the reduced proteome oxidation in aged gonads may spare Nrf2 from critical deleterious modifications that impair its functionality. Additional questions that emerge and warrant further investigation relate to whether these phenotypes also involve other (than Nrf2) proteasome-specific transcriptional regulators or age-dependent chromatin modifications, as well as whether additional genomic modulators contribute to ARE activation (e.g., in genes like gstD1) during aging. Loss of the ability of somatic tissues to maintain proteostasis networks is a hallmark of aging and is most probably causative, as it is anticipated that the gradual dysfunction of somatic antioxidant response and proteasomes will form a vicious circle (Supplemental Fig. S4B) that, apart from fueling the accumulation of more proteotoxic stress, will also affect the degradation of normal short-lived ubiquitinated proteins, resulting in the deregulation of several physiological cellular processes.
In summary, this work provides novel evidence supporting differential regulation of proteostasis maintenance and proteome clearance in the young organism, during aging, or in response to oxidative challenge in the somatic tissues and the gonads of Drosophila (Supplemental Fig. S4). It also supports the notion that, given the finite resources that are available during the lifetime, the distinction between somatic and reproductive tissues necessitates that the reproductive cell lineage (the “immortal” germ line) is maintained at a functional level that preserves viability across generations, whereas investment in the “mortal” soma is more limited, as it is destined merely to support the survival of a single generation.
Supplementary Material
Acknowledgments
The authors thank Maria Figueiredo-Pereira (Hunter College, City University of New York, New York, NY, USA) for the Drosophila β5 proteasome subunit antibody; Eirini Komseli for immunostaining of paraffin-embedded flies; Triantafyllia Ntouroupi for technical support during the early phase of this project; and Marianna Antonelou for helpful suggestions.
This work was supported by a European Union FP7 Capacities grant INsPiRE (REGPOT-CT-2011–284460) to I.P.T.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AGE
- advanced glycation end product
- ARE
- antioxidant response element
- C-L
- caspase-like
- CncC
- cap‘n’collar isoform C
- CT-L
- chymotrypsin-like
- DNP
- 1,3-dinitrophenylhydrazone
- GFP
- green fluorescent protein
- gstD1
- glutathione S-transferase Delta-1
- Keap1
- Kelch-like ECH-associated protein 1
- Nrf2
- NFE2-related factor 2
- PCR
- polymerase chain reaction
- ROS
- reactive oxygen species
- RT-PCR
- reverse transcription-polymerase chain reaction
- t-BHP
- tert-butyl hydroperoxide
- T-L
- trypsin-like
- UPS
- ubiquitin-proteasome system
REFERENCES
- 1. Carnes B. A. (2011) What is lifespan regulation and why does it exist? Biogerontology 12, 367–374 [DOI] [PubMed] [Google Scholar]
- 2. Rattan S. I. (2012) Biogerontology: from here to where? The Lord Cohen Medal Lecture-2011. Biogerontology 13, 83–91 [DOI] [PubMed] [Google Scholar]
- 3. Wensink M. (2012) Age-specificity and the evolution of senescence: a discussion. [E-pub ahead of print] Biogerontology doi: 10.1007/s10522-012–9410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirkwood T. B. L., Austad S. N. (2000) Why do we age? Nature 408, 233–238 [DOI] [PubMed] [Google Scholar]
- 5. Rattan S. I. (2006) Theories of biological aging: genes, proteins, and free radicals. Free Rad. Res. 40, 1230–1238 [DOI] [PubMed] [Google Scholar]
- 6. Rattan S. I. (2008) Increased molecular damage and heterogeneity as the basis of aging. Biol. Chem. 389, 267–272 [DOI] [PubMed] [Google Scholar]
- 7. Vijg J., Campisi J. (2008) Puzzles, promises and a cure for aging. Nature 454, 1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breusing N., Grune T. (2008) Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 389, 203–209 [DOI] [PubMed] [Google Scholar]
- 9. Sykiotis G. P., Bohmann D. (2010) Stress-activated cap‘n'collar transcription factors in aging and human disease. Sci. Signal. 3, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hubbard V. M., Valdor R., Macian F., Cuervo A. M. (2012) Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 13, 21–35 [DOI] [PubMed] [Google Scholar]
- 11. Navon A., Ciechanover A. (2009) The 26S proteasome: from basic mechanisms to drug targeting. J. Biol. Chem. 284, 33713–33718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pickering A. M., Davies K. J. (2012) Degradation of damaged proteins: the main function of the 20S proteasome. Prog. Mol. Biol. Transl. Sci. 109, 227–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickart C. M., Cohen R. E. (2004) Proteasomes and their kin: proteases in the machine age. Nat. Rev. Mol. Cell Biol. 5, 177–187 [DOI] [PubMed] [Google Scholar]
- 14. Friguet B. (2006) Oxidized protein degradation and repair in aging and oxidative stress. FEBS Lett. 580, 2910–2916 [DOI] [PubMed] [Google Scholar]
- 15. Chondrogianni N., Petropoulos I., Franceschi C., Friguet B., Gonos E. S. (2000) Fibroblast cultures from healthy centenarians have an active proteasome. Exp. Gerontol. 35, 721–728 [DOI] [PubMed] [Google Scholar]
- 16. Chondrogianni N., Stratford F. L., Trougakos I. P., Friguet B., Rivett A. J., Gonos E. S. (2003) Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J. Biol. Chem. 278, 28026–28037 [DOI] [PubMed] [Google Scholar]
- 17. Beedholm R., Clark B. F., Rattan S. I. (2004) Mild heat stress stimulates 20S proteasome and its 11S activator in human fibroblasts undergoing aging in vitro. Cell Stress Chaperones 9, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vernace V. A., Arnaud L., Schmidt-Glenewinkel T., Figueiredo-Pereira M. E. (2007) Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 21, 2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tonoki A., Kuranaga E., Tomioka T., Hamazaki J., Murata S., Tanaka K., Miura M. (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol. 29, 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen A., Honarvar A., Zetterberg M. (2010) Changes in activity and kinetic properties of the proteasome in different rat organs during development and maturation. Curr. Gerontol. Geriatr. Res. 230697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen T. O., Sarup P., Loeschcke V., Rattan S. I. (2012) Age-related and sex-specific differences in proteasome activity in individual Drosophila flies from wild-type, longevity-selected and stress resistant strains. Biogerontology 13, 429–438 [DOI] [PubMed] [Google Scholar]
- 22. Szlanka T., Haracska L., Kiss I., Deák P., Kurucz E., Andó I., Virágh E., Udvardy A. (2003) Deletion of proteasomal subunit S5a/Rpn10/p54 causes lethality, multiple mitotic defects and overexpression of proteasomal genes in Drosophila melanogaster. J. Cell Sci. 116, 1023–1033 [DOI] [PubMed] [Google Scholar]
- 23. Cook C., Gass J., Dunmore J., Tong J., Taylor J., Eriksen J., McGowan E., Lewis J., Johnston, J., Petrucelli L. (2009) Aging is not associated with proteasome impairment in UPS reporter mice. PLoS One. 4, e5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Girardot F., Lasbleiz C., Monnier V., Tricoire H. (2006) Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villeneuve N. F., Lau A., Zhang D. D. (2010) Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 13, 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sykiotis G. P., Bohmann D. (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grimberg K. B., Beskow A., Lundin D., Davis M. M., Young P. (2011) Basic leucine zipper protein Cnc-C is a substrate and transcriptional regulator of the Drosophila 26S proteasome. Mol. Cell. Biol. 31, 897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edelmann M. J., Nicholson B., Kessler B. M. (2011) Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases. Expert Rev. Mol. Med. 13, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim S. K. (2007) Common aging pathways in worms, flies, mice and humans. J. Exp. Biol. 210, 1607–1612 [DOI] [PubMed] [Google Scholar]
- 30. Nickell S., Beck F., Scheres S. H., Korinek A., Förster F., Lasker K., Mihalache O., Sun N., Nagy I., Sali A., Plitzko J. M., Carazo J. M., Mann M., Baumeister W. (2009) Insights into the molecular architecture of the 26S proteasome. Proc. Natl. Acad. Sci. U. S. A. 106, 11943–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chatterjee N., Bohmann D. (2012) A versatile ΦC31-based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS One 7, e34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurapati R., Passananti H. B., Rose M. R., Tower J. (2000) Increased hsp22 RNA levels in Drosophila lines genetically selected for increased longevity. J. Gerontol. A Biol. Sci. Med. Sci. 55, B552–B559 [DOI] [PubMed] [Google Scholar]
- 33. Trougakos I. P., Margaritis L. H. (1998) The formation of the functional chorion structure of Drosophila virilis involves inercalation of the “middle” and “late” major chorion proteins: a general model for chorion assembly in Drosophilidae. J. Struct. Biol. 123, 97–110 [DOI] [PubMed] [Google Scholar]
- 34. Kucherenko M. M., Marrone A. K., Rishko V. M., Yatsenko A. S., Klepzig A., Shcherbata H. R. (2010) Paraffin-embedded and frozen sections of Drosophila adult muscles. J. Vis. Exp. 46, pii: 2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferguson M., Mockett R. J., Shen Y., Orr W. C., Sohal R. S. (2005) Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sohal R. S., Orr W. C. (2012) The redox stress hypothesis of aging. Free Radic. Biol. Med. 52, 539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacobson J., Lambert A. J., Portero-Otín M., Pamplona R., Magwere T., Miwa S., Driege Y., Brand M. D., Partridge L. (2010) Biomarkers of aging in Drosophila. Aging Cell 9, 466–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bader N., Jung T., Grune T. (2007) The proteasome and its role in nuclear protein maintenance. Exp. Gerontol. 42, 864–870 [DOI] [PubMed] [Google Scholar]
- 39. Golden T. R., Hinerfeld D. A., Melov S. (2002) Oxidative stress and aging: beyond correlation. Aging Cell 1, 117–123 [DOI] [PubMed] [Google Scholar]
- 40. Reinheckel T., Sitte N., Ullrich O., Kuckelkorn U., Davies K. J., Grune T. (1998) Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem. J. 335, 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tower J. (2006) Sex-specific regulation of aging and apoptosis. Mech. Aging Dev. 127, 705–718 [DOI] [PubMed] [Google Scholar]
- 42. Aguilaniu H., Gustafsson L., Rigoulet M., Nyström T. (2003) Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 [DOI] [PubMed] [Google Scholar]
- 43. Erjavec N., Nyström T. (2007) Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 104, 10877–10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Erjavec N., Cvijovic M., Klipp E., Nyström T. (2008) Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc. Natl. Acad. Sci. U. S. A. 105, 18764–18769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goudeau J., Aguilaniu H. (2010) Carbonylated proteins are eliminated during reproduction in C. elegans. Aging Cell 9, 991–1003 [DOI] [PubMed] [Google Scholar]
- 46. Hernebring M., Brolén G., Aguilaniu H., Semb H., Nyström T. (2006) Elimination of damaged proteins during differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 7700–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vilchez D., Morantte I., Liu Z., Douglas P. M., Merkwirth C., Rodrigues A. P., Manning G., Dillin A. (2012) RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 489, 263–268 [DOI] [PubMed] [Google Scholar]
- 48. Vilchez D., Boyer L., Morantte I., Lutz M., Merkwirth C., Joyce D., Spencer B., Page L., Masliah E., Berggren W. T., Gage F. H., Dillin A. (2012) Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 489, 304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fredriksson A., Johansson Krogh E., Hernebring M., Pettersson E., Javadi A., Almstedt A., Nyström T. (2012) Effects of aging and reproduction on protein quality control in soma and gametes of Drosophila melanogaster. Aging Cell 11, 634–643 [DOI] [PubMed] [Google Scholar]
- 50. Imai J., Maruya M., Yashiroda H., Yahara I., Tanaka K. (2003) The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J. 22, 3557–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pickering A. M., Koop A. L., Teoh C. Y., Ermak G., Grune T., Davies K. J. (2010) The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 432, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aiken C. T., Kaake R. M., Wang X., Huang L. (2011) Oxidative stress-mediated regulation of proteasome complexes. Mol. Cell. Proteomics 10, R110.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simonsen A., Cumming R. C., Brech A., Isakson P., Schubert D. R., Finley K. D. (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4, 176–184 [DOI] [PubMed] [Google Scholar]
- 54. Shenvi S. V., Smith E., Hagen T. M. (2012) Identification of age-specific Nrf2 binding to a novel antioxidant response element locus in the Gclc promoter: a compensatory means for the loss of glutathione synthetic capacity in the aging rat liver? Aging Cell 11, 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.