Abstract
Glutathione (GSH) deficiency and interleukin-1β (IL-1β) upregulation are linked to the progression of vascular inflammation and atherosclerosis. The consumption of sulfide-rich vegetables is known to lower the risk of atherosclerosis. This study examined the hypothesis that hydrogen sulfide (H2S) upregulates the glutamate–cysteine ligase catalytic subunit (GCLC) and GSH and inhibits IL-1β in a monocyte cell model. U937 monocytes were supplemented with H2S (0–12.5 μM) for 2 hr and then exposed to a control or high glucose (HG, 25 mM) for 22 hr. Levels of GCLC and glutamate–cysteine ligase modifier subunit (GCLM) expression were determined by western blotting and GSH using high-performance liquid chromatography (HPLC), and IL-1β using enzyme-linked immunoassay (ELISA). H2S significantly (P<0.05) upregulated expression of GCLC and GCLM, and formation of GSH, and inhibited IL-1β secretion in controls and HG-treated monocytes. This is the first demonstration of H2S upregulation of GCLC and GSH and inhibition of IL-1β levels, which may be what mediates the beneficial effects of H2S-rich compounds in mitigating the pathogenesis of metabolic syndrome and atherosclerosis.
Introduction
Various vegetables, such as garlic, onions, leeks, broccoli, and shallots, are known to be a rich source of hydrogen sulfide (H2S).1,2 Epidemiological studies suggest that consumption of these vegetables may protect against cancer in humans and has the potential to prevent insulin resistance and atherosclerosis in humans and animal models.1,2 However, the mechanism by which H2S in these vegetables provides the beneficial effect in lowering atherosclerosis is not known. Interleukin-1β (IL-1β) is secreted by many tissues and acts both locally and systemically.3–5 The overexpression of the IL-1β receptor or exposure to IL-1β increases the progression of atherosclerosis, whereas knockdown of IL-1β or inhibition of IL-1β reduces the biomarkers and lesions associated with atherosclerosis in animal studies.6,7 Human clinical trials using anakinra, a IL-1 receptor antagonist or those examining specific inhibition of IL-1β using canakinumab, a monoclonal antibody for IL-1β, have shown significant reduction in the biomarkers of vascular inflammation.3–5 Several recent reviews have discussed the potential of IL-1β inhibition as a promising therapy to prevent or halt the progression of atherosclerotic vascular disease.4 This study reports for the first time that H2S upregulates the glutamate–cysteine ligase catalytic subunit (GCLC) and glutamate–cysteine ligase modifier subunit (GCLM), increases cellular glutathione (GSH), and decreases IL-1β levels. The potential of H2S to inhibit the IL-1β levels observed in this study could provide evidence for a novel mechanism by which dietary supplementation with H2S-rich vegetables could lower the progression of metabolic syndrome and atherosclerosis.
Materials and Methods
Treatment with HG and H2S and immunoblotting studies
The U937 monocyte cell line was obtained from American Type Culture Collection (ATCC; Manassas, VA) and maintained as described earlier.8 Cells (106/mL) were pretreated with three different concentrations of H2S (0–12.5 μM) for 2 hr followed by high glucose (HG; 25 mM) exposure for the next 24 hr. Control cells were exposed to media with 7 mM glucose, because glucose gets metabolized but not replaced in cell culture studies, and 7 mM glucose does not lead to a glucose deficiency at 24 hr of incubation. In HG studies, cells were exposed to a HG concentration of 25 mM. In some experiments, cells were exposed instead to 18 mM mannitol (control) because the medium contains 7 mM glucose. After treatment, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer as described earlier.8,9 Lysates were cleared by centrifugation, and total protein concentrations were determined using a bicinchoninic acid (BCA) assay (Pierce/Thermo Scientific, Rockford, IL). Details of immunoblotting are similar to those given in our previous publications.8 The antibodies for GCLC (73 kD), GCLM (31 kD), were purchased from Abcam (Cambridge, MA). The intensity of each immunoblotting band was measured using the histogram tool of Adobe Photoshop CS5. Sodium sulfide (Alfa Aesar, Ward Hill, MA) was used as a source of H2S for treatment of monocytes. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned.
GSH, GR activity, IL-1β, and cell viability assays
The concentrations of GSH were determined using the high-performance liquid chromatography (HPLC) method as described before.10 GSH concentration was expressed per volume of cell suspension. GR activity was determined using a protocol described earlier.8 GR activity was expressed as the rate of decrease in absorbance at 340 nm/min due to the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) by GR, and was normalized per milligram of protein. All appropriate controls and standards as specified by each manufacturer's kit were used for the IL-1β assay performed using enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, MN). In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. Viability of cells was determined using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA).
Results and Discussion
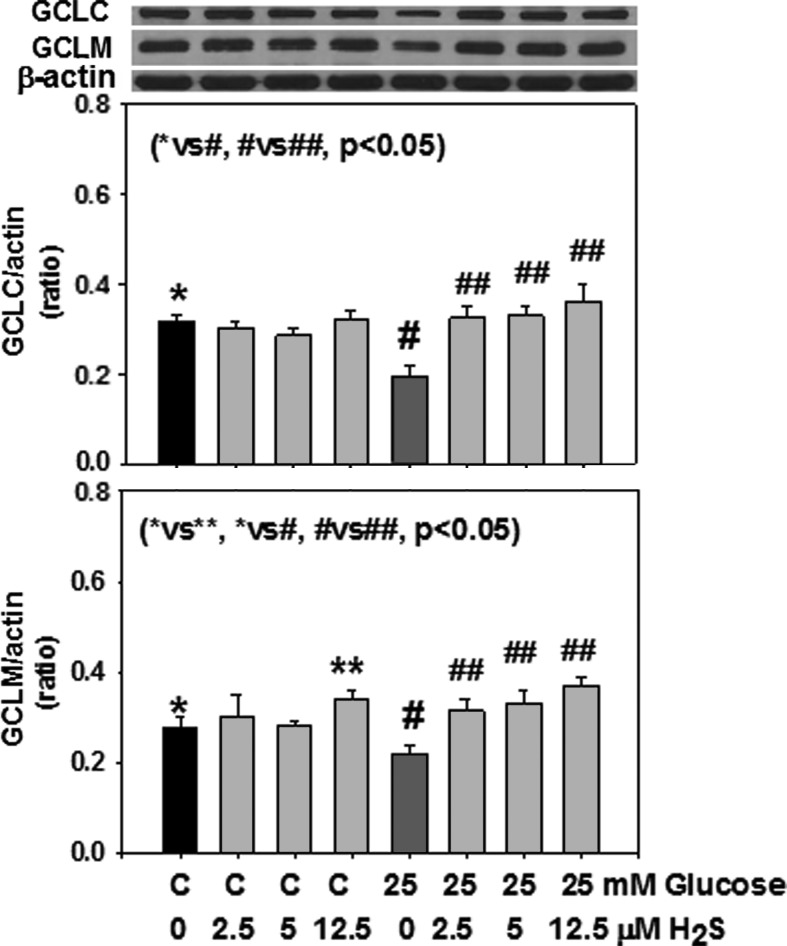
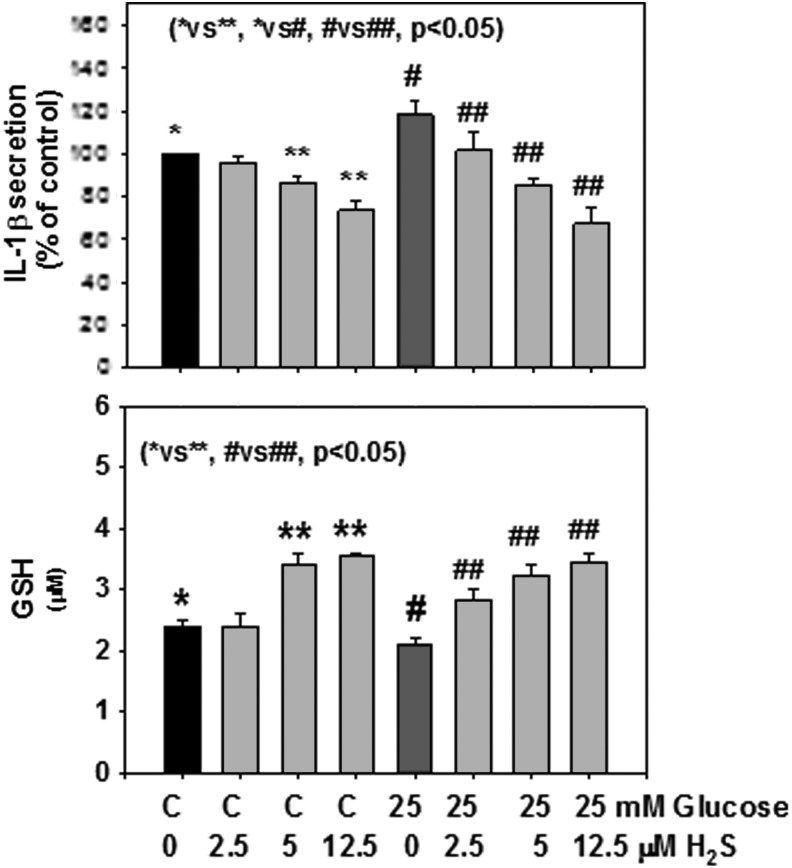
Figure 1 shows that H2S supplementation significantly upregulated GCLC expression in monocytes exposed to HG. The effect of H2S on upregulation of GCLM was also seen when monocytes were exposed to control or HG levels. Similarly, there was significantly more GSH formation in H2S-supplemented monocytes. H2S supplementation had no effect on GR activity (data not shown). Figure 2 shows that the effect of H2S on the upregulation of GCLC was also seen when the GSH level was determined in monocytes exposed to HG levels. Figure 2 also shows that H2S supplementation significantly inhibited secretion of the proinflammatory cytokine IL-1β in HG-treated U937 monocytes. Mannitol treatment had no effect on GCLC, GSH, or cytokine secretion (data not given here). There was no change in cell viability as the result of any of the treatments. The results presented here were analyzed statistically using analysis of variance (ANOVA) with Sigma Stat. A P value of less than 0.05 for a statistical test was considered significant.
FIG. 1.
Effect of hydrogen sulfide (H2S) supplementation on expression of glutamate–cysteine ligase catalytic subunit (GCLC) (top) and glutamate–cysteine ligase modifier subunit (GCLM) (bottom) in monocytes treated with control or high glucose (HG). Values are mean±standard error (SE) of three experiments. HG caused a decrease in GCLC and GCLM expression. Note a significant increase in the upregulation of both GCLC and GCLM expression by H2S.
FIG. 2.
Effect of hydrogen sulfide (H2S) supplementation on glutathione (GSH) formation (bottom) and interleukin-1β (IL-1β) secretion (top) in monocytes treated with control or high glucose (HG). Values are mean±standard error (SE) of three experiments. HG caused a significant increase in secretion of IL-1β and its inhibition by H2S. Similarly, there was a significant improvement in GSH levels in monocytes supplemented with H2S.
GSH is a cofactor of many enzymes involved in the detoxification of oxygen radicals, and its deficiency is implicated in the etiology and progression of a number of human diseases, including cardiovascular, immune, diseases of aging, and diabetes.11,12 Recent studies report lower blood levels of H2S and GSH in various disease states, such as those of asthmatic patients, hypertensive patients, and diabetic animals and patients.13–18 Hyperglycemia and diabetes is associated with increased oxidative stress, and blood levels of GSH are lower in diabetic patients.12,13,19,20 Oxidative stress and inflammation appear to be causatively linked and play a key role in the progression of vascular inflammation and atherosclerosis. HG can increase levels of IL-1β, and IL-1β can further stimulate generation of oxidative stress.21 Enrichment of GSH provides protection against inflammatory reactions and inhibition of GSH promotes inflammatory reactions when cells are treated with IL-1β.22 Many studies support the hypothesis of a link between low GSH status and biomarkers of atherosclerosis.11 H2S is emerging as an important signaling molecule.23 Recent studies support the idea that H2S or L-cysteine (LC), a precursor of H2S, is beneficial and can lower atherosclerotic lesions in animal models.23 On the other hand, recent reviews have discussed the potential of IL-1β inhibition as a promising therapy to prevent or halt the progression of atherosclerotic vascular disease.4,5
This study reports a novel link between H2S supplementation and an improvement in cellular GSH levels and inhibition of IL-1β levels in cell culture studies. This study demonstrates that H2S can upregulate GCLC and GCLM and increases cellular GSH formation in cultured monocytes. GSH is formed from LC by the enzymatic action of glutamate cysteine ligase.24 Glutamate cysteine ligase consists of two subunits, GCLC and another modulatory subunit, GCLM. Previous studies report that H2S can regenerate cellular GSH.25 However, no previous study has demonstrated that H2S can upregulate GCLC and GCLM. GSH is a water-phase sulfhydryl antioxidant with the ability to scavenge reactive oxygen species (ROS), which may explain the decreased levels of IL-1β in H2S-supplemented cells observed in this study.
IL-1β is an important mediator of the inflammatory responses that lead to the progression of various diseases such as atherosclerosis.3–5 Various studies indicate a positive association between IL-1β gene polymorphism and the severity of atherosclerosis.4 Animal studies have demonstrated that overexpression of the IL-1β receptor or exposure to IL-1β increases the progression of atherosclerosis, and that knockdown of IL-1β or inhibition of IL-1β reduces biomarkers and lesions associated with atherosclerosis.6,7 Human plaques contain IL-1β mRNA and protein.5 Human clinical trials using anakinra, a IL-1 receptor antagonist or specific inhibition of IL-1β using canakinumab, a monoclonal antibody for IL-1β, have shown a significant reduction in the biomarkers of vascular inflammation.3–5 Recent reviews have discussed the potential of IL-1β inhibition as a promising therapy to prevent or halt the progression of atherosclerotic vascular disease.3–5 The potential of H2S in the upregulation of GSH and inhibition of IL-1β levels observed in this study provide evidence for a novel mechanism by which H2S supplementation may reduce oxidative stress and thereby lower biomarkers of vascular inflammation and the progression of metabolic syndrome and atherosclerosis.
Acknowledgments
S.K.J. is supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Office of Dietary Supplements of the National Institutes of Health (RO1 DK 072433) and the Malcolm Feist Endowed Chair in Diabetes. The authors thank Ms. Georgia Morgan for excellent editing of this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adams MR, Golden DL, Chen H, Register TC, Gugger ET. A diet rich in green and yellow vegetables inhibits atherosclerosis in mice. J Nutr 2006;136:1886–1889, [DOI] [PubMed] [Google Scholar]
- 2.Li W, Tang C, Jin H, Du J. Effect of onion extract on endogenous vascular H2S and adrenomedulin in rat atherosclerosis. Curr Pharm Biotechnol 2011;12:1427–1439 [DOI] [PubMed] [Google Scholar]
- 3.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 4.Qamar A, Rader DJ. Effect of interleukin 1β inhibition in cardiovascular disease. Curr Opin Lipidol 2012;23:548–553 [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012;126:2739–2748 [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 7.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: Role of the interleukin-1 receptor antagonist. Circulation 2008;117:2577–2579 [DOI] [PubMed] [Google Scholar]
- 8.Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun 2013;437:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manna P, Jain SK. Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem 2011;286:39848–39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290–292 [PubMed] [Google Scholar]
- 11.Wu G, Fang YZ, Yang S, et al. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–492 [DOI] [PubMed] [Google Scholar]
- 12.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011;34:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain SK, McVie R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism 1994;43:306–309 [DOI] [PubMed] [Google Scholar]
- 14.Jain SK, Bull R, Rains JL, et al. Low levels of hydrogen sulfide in the blood of diabetic patients and streptozotocin-treated rats cause vascular inflammation. Antiox Redox Signal 2010;12:1333–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain SK, Manna P, Micinski D, et al. In African American type 2 diabetic patients, is vitamin D deficiency associated with lower blood levels of hydrogen sulfide and cyclic adenosine monophosphate, and elevated oxidative stress? Antioxid Redox Signal 2013;18:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Ingrid S, Ding Y, et al. Imbalance of endogenous homocyteine and hydrogen sulfide metabolic pathway in essential hypertension children. Chin Med J 2007;120:389–393 [PubMed] [Google Scholar]
- 17.Whiteman M, Gooding KM, Whatmore JL, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulfide. Diabetologia 2010;53:1722–1726 [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Yao WZ, Gao GZ, et al. Serum hydrogen sulfide as a novel marker predicting bacterial involvement in patients with community-acquired lower respiratory tract infections. Respirology 2009;14:746–752 [DOI] [PubMed] [Google Scholar]
- 19.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem 1989;264:21340–21345 [PubMed] [Google Scholar]
- 20.Jain SK, McVie R, Duett J, et al. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989;38:1539–1543 [DOI] [PubMed] [Google Scholar]
- 21.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1 beta expression in human monocytes: Mechanistic insights. Am J Physiol Endocrinol Metab 2007;293:E337–E346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutkute K, Asmis RH, Nikolova-Karakashian MN. Regulation of neutral sphingomyelinase-2 by GSH: A new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res 2007;48:2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani S, Untereiner A, Wu L, et al. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal 2014;20:805–817 [DOI] [PubMed] [Google Scholar]
- 24.Franklin CC, Backos DS, Mohar I, et al. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 2009. l;30:86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 2010;12:1–13 [DOI] [PubMed] [Google Scholar]