Summary
Distinguishing between perivascular cell types remains a hurdle in vascular biology due to overlapping marker expressions and similar functionalities. Clarifying and defining heterogeneities in vitro among perivascular cells could lead to improved cell-based tissue regeneration strategies and a better understanding of human developmental processes. We studied contractile vascular smooth muscle cells (vSMCs), synthetic vSMCs, and pericytes derived from a common human pluripotent stem cell source. Using in vitro cultures, we show unique cell morphology, subcellular organelle organization (namely endoplasmic reticulum, mitochondria, and stress fibers), and expression of smooth muscle myosin heavy chain and elastin for each cell type. While differences in extracellular matrix deposition and remodeling were less pronounced, the multipotency, in vivo, migratory, invasion, and contractile functionalities are distinctive for each cell type. Overall, we define a repertoire of functional phenotypes in vitro specific for each of the human perivascular cell types, enabling their study and use in basic and translational research.
Highlights
-
•
Contractile and synthetic vSMCs and pericytes were derived from a common hiPSC line
-
•
Morphology and organelle organization differ among the perivascular derivatives
-
•
SMMHC and elastin specify the mature contractile vSMC phenotype
-
•
Migration, invasion, and contractility are unique for each perivascular cell type.
The in vitro use of perivascular cells poses unique challenges in tissue engineering owing to overlapping cell characteristics. Using a common human pluripotent stem cell line, Gerecht and colleagues define a repertoire of phenotypes of derived pericytes and synthetic and contractile vascular smooth muscle cells by evaluating cell morphology, marker expression, and functionality.
Introduction
The vasculature is a multicellular system in which each cell type plays an important and indispensible role in its function. The inner lining of endothelial cells (ECs), which are in direct contact with the blood, is surrounded and supported by perivascular cells—either vascular smooth muscle cells (vSMCs) or pericytes. vSMCs surround larger vessels such as arteries and veins, whereas pericytes typically surround smaller microvessels and capillaries (Alberts et al., 2002). The disparate vessel locations for each perivascular cell type suggest that further differences exist that should be investigated and better understood in vitro in order to appropriately rebuild blood vessels for therapeutic applications (Dar and Itskovitz-Eldor, 2013; Wanjare et al., 2013b).
As the vasculature’s support system, perivascular cells are primarily responsible for imparting contractility and producing and depositing extracellular matrix (ECM) proteins. Both cell types migrate to sites of angiogenesis, the growth of blood vessels from preexisting ones, to help stabilize and mature nascent endothelial tubes. Whether pericytes and vSMCs function similarly in these regards and to what extent have been unclear.
Along with the aforementioned functional similarities, perivascular cell types also exhibit overlapping marker expression. Adding to this complexity, neither perivascular cell type can be distinguished by one marker alone; instead, a combination of markers is needed for their identification. For example, both cell types have been demonstrated to express alpha smooth muscle actin (α-SMA). The expression of α-SMA and the transmembrane chondroitin sulfate proteoglycan neuron-glial 2 (NG2) help distinguish pericytes in different vessel types (Crisan et al., 2012); pericytes of the capillaries are NG2+α-SMA−, of the venules are NG2-α-SMA+, and of the arterioles are NG2+α-SMA+. When cultured in vitro, however, pericytes are positive for both of these markers. Other markers that are expressed on both perivascular cell types include calponin and platelet-derived growth factor receptor β (PDGFRβ) (Birukov et al., 1991; Dar et al., 2012).
Examining differences in perivascular cell types is further complicated by added heterogeneities within the subtypes (Hedin and Thyberg, 1987; Kusuma and Gerecht, 2013). Two distinct vSMC phenotypes have been elucidated: synthetic and contractile (Beamish et al., 2010; Hedin and Thyberg, 1987; Wanjare et al., 2013a). Both participate in neovascularization, but synthetic vSMCs predominate in the embryo and in diseased or injured adult vessels while contractile vSMCs predominate in healthy adult vessels.
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced PSCs (hiPSCs), have been widely used to study somatic cell types due to their ability to obtain cell derivatives of identical genetic backgrounds. They are known for their ability to self-renew indefinitely in culture and to differentiate toward every cell type, including perivascular cells (Dar and Itskovitz-Eldor, 2013). hiPSCs are derived from a patient’s own cells and thus can yield derived cell populations that are patient specific, providing a clinically relevant pluripotent cell source for therapeutic use. Indeed, we and others have examined the derivation of both vSMCs (Drukker et al., 2012; Ferreira et al., 2007; Wanjare et al., 2013a) and pericytes (Dar et al., 2012; Kusuma et al., 2013; Orlova et al., 2014).
Using a stepwise differentiation protocol, we have demonstrated the maturation of smooth muscle-like cells (SMLCs) (Vo et al., 2010) to synthetic vSMCs (syn-vSMCs) and contractile vSMCs (con-vSMCs) from both hESCs and hiPSCs (Wanjare et al., 2013a). Using a similar but distinct stepwise differentiation protocol, we have also demonstrated the derivation of pericytes from various hPSC lines (Kusuma et al., 2013).
Building off of our previous studies, we sought to comprehensively define differences among con-vSMCs, syn-vSMCs, and pericytes derived from a common hPSC source in order to uncover cellular and functional differences in vitro, toward the long-term goal of rebuilding vasculature for therapeutic applications. For example, the quality of tissue-engineered blood vessels is dependent on the characteristics of the in vitro perivascular cells used. Current challenges of engineering blood vessels include precise mechanical requirements and tissue-specific cell types (Kumar et al., 2011). The in vitro characterization of our hPSC-derived perivascular cells may mediate the production of tissue-engineered blood vessels that have the patency and mechanical responsiveness equivalent to the native tissue (Chan-Park et al., 2009). Of clinical relevance, the hiPSC-BC1 line is used as the hPSC source for our studies. BC1 is derived without viral integration and has been fully genetically sequenced (Cheng et al., 2012; Chou et al., 2011). Here, we focus on differences in perivascular cells derived from BC1 and hESC H9 cells with respect to cellular characteristics, protein expression, ECM deposition, and remodeling, migration, invasion, and contractility.
Results
Pericytes and vSMCs Differ in Morphological Features and Proliferation Rates
Perivascular cells were derived from hPSCs using differentiation protocols from previous studies (Kusuma et al., 2013; Vo et al., 2010; Wanjare et al., 2013a). Using this protocol, hPSC SMLCs subjected to long-term differentiation and in the presence of high serum and growth factors were guided toward a synthetic fate, whereas deprivation of serum and growth factors yielded a contractile fate (Figure 1A) (Wanjare et al., 2013a). Early pericytes were derived as part of a bicellular population with early ECs named early vascular cells (EVCs) and further differentiated toward mature pericytes by a selective plating strategy (i.e., removal of collagen IV substrate and constrained adhesion time) in the presence of high serum (Figure 1A) (Kusuma et al., 2013). For pericytes, EVCs highly express CD105 and CD146, while pericyte derivatives express NG2, PDGFRβ, CD44, and CD73 (Figure 1A) (Kusuma et al., 2013).
Figure 1.
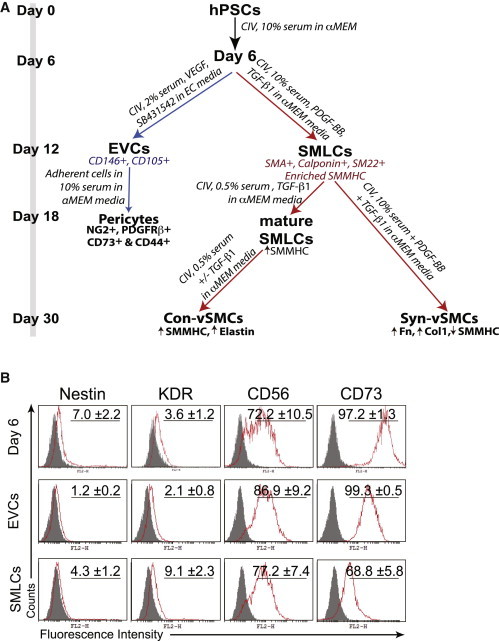
Characterization of Cellular Properties of Perivascular Derivatives
(A) Schema for the differentiation procedure to derive perivascular cells from hPSCs.
(B) Flow cytometry analysis of day 6 differentiating cells, EVCs, and SMLCs. Isotype control in gray. Results shown are representative of three independent experiments. CIV, collagen IV.
See also Figure S1.
To better understand progression in differentiation, we examined marker expression at different time points along the differentiation of hPSCs. Over the first 6 days of differentiation, the three classes of perivascular cells underwent identical differentiation conditions. During embryonic development, vSMCs may arise from a number of precursors from different germ layers (Cheung et al., 2012; Majesky, 2007). We found that expression of mesodermal genes KDR, APLNR, and TCF21 increased over the first 6 days of differentiation in both BC1 and H9 cell lines, whereas expression of neural crest markers SOX1, PAX6, and WNT1 remained stagnant (Figure S1A available online). Additionally, WNT1 was not expressed in BC1 differentiating cells. These data suggest the emergence of a mesodermal population. Furthermore, our day 6 differentiating cells were assessed for the expression of markers indicative of several intermediate lineages: neuroectoderm (nestin), lateral plate mesoderm (KDR), paraxial mesoderm (Pax1) (Cheung et al., 2012), early mesoderm (CD56) (Evseenko et al., 2010), and general mesoderm (CD73) (Boyd et al., 2009; Vodyanik et al., 2010). We found that a small fraction of day 6 differentiating cells was positive for nestin (Figure 1B). KDR was only slightly expressed. Pax1 was not detected via PCR or by immunofluorescence (data not shown). Mesoderm markers CD56 and CD73, however, exhibited more pronounced expression; day 6 differentiating cells were 72% positive for CD56 and >95% positive for CD73. To distinguish the subsequently derived cell populations (i.e., EVCs and SMLCs), we performed flow cytometry analysis for the aforementioned markers. After differentiation toward EVCs, we observed that nestin expression was completely abolished; however, a small fraction of SMLCs remained nestin+ (Figure 1B). Similarly to day 6 differentiating cells, EVCs exhibited >95% positive expression for CD73, whereas CD73 expression decreased to ∼70% in SMLCs. An important differentiator between EVCs and SMLCs is the presence of vascular endothelial cadherin (VEcad). Our previous studies demonstrated that BC1-EVCs exhibited approximately 30% VEcad+ cells (Kusuma et al., 2013). Contrastingly, we could not detect VEcad expression on SMLCs (Vo et al., 2010). A similar trend of marker expression was observed at different time points along the differentiation of hESC line H9 (Figure S1B).
We next evaluated the differences in proliferation rates among the hPSC-derived mature perivascular cells. As expected, hiPSC con-vSMCs exhibited low proliferation rates because they are cultured in media containing low serum (0.5% serum) (Figure 2A) (Wanjare et al., 2013a), while hiPSC syn-vSMCs exhibited high proliferation rates because they are cultured in media containing 10% serum (Figure 2A). Although hiPSC pericytes were cultured using media containing 10% serum, they exhibited contact inhibited proliferation and grew in cell colonies (Figures 2A and 2B). Next, we evaluated the morphological features after culture on 2D surfaces (Figure 2B). While both hiPSC vSMC types spread evenly throughout the Petri dish, hiPSC pericytes arranged themselves into colony-like structures. hiPSC con-vSMCs displayed the largest cell areas and nuclei sizes compared to hiPSC syn-vSMCs and hiPSC pericytes (Figure 2C). hESC derivatives exhibited the same trend (Figure S1C). Indeed, a phenotypic switch from syn-vSMCs to con-vSMCs has been correlated with smaller nuclei and a decrease in proliferation (Chen et al., 1997).
Figure 2.
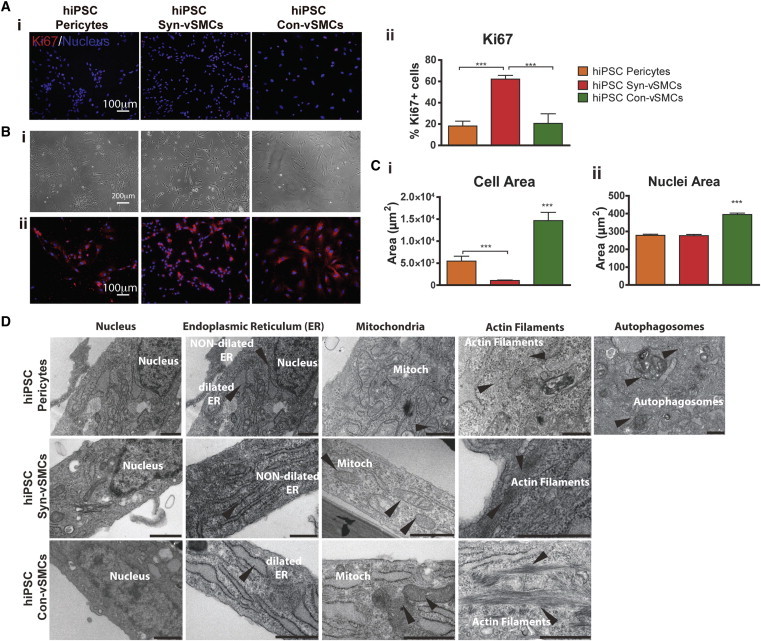
Analysis of hPSC-Derived Perivascular Cells
Derivatives were analyzed for (A) proliferation using (i) stain for Ki67 (red; nuclei in blue) and (ii) corresponding image quantification, (B) morphology using (i) light microscopy and (ii) FM4-64 membrane stain (red) and DAPI (blue), (C) corresponding image quantification of (i) cell and (ii) nuclei area, and (D) subcellular organelle organization using transmission electron microscopy. CIV, collagen type IV; ER, endoplasmic reticulum; mitoch, mitochondria. Scale bars in (D) are 1 μm. Results shown from three independent experiments. All graphical data are reported as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. See also Figure S2.
There were stark differences in endoplasmic reticulum (ER), mitochondria location, stress fibers, and the presence of autophagosomes observed between pericytes and vSMCs. As previously described, hiPSC con-vSMCs contain dilated ER, while hiPSC syn-vSMCs contain nondilated ER (Wanjare et al., 2013a) (Figure 2D). On the other hand, hiPSC pericytes (as well as cell-line placental pericytes) contained both dilated and nondilated ER (Figure 2D; Figure S2A). The mitochondria of hiPSC pericytes were in close proximity to the nucleus in contrast to vSMCs, whose mitochondria were located further away from the nucleus. With respect to stress fibers, only con-vSMCs had stress fibers located throughout the entire cell body. Both hiPSC syn-vSMCs and hiPSC pericytes primarily had stress fibers located at the basal lateral surface (Figure 2D). Pericytes also had autophagosomes present, whereas both hiPSC syn-vSMCs and con-vSMCs did not (Figure 2D; Figure S2).
Differential Expression of Perivascular Markers
We next examined the expression and localization of specific cytoskeleton proteins that have been reported to distinguish vSMCs and pericytes. Stress fibers are bundles of actin filaments that are important in mechanotransduction of adherent cells by anchoring to substrates and creating isometric tension (Deguchi et al., 2006). Perivascular cell contraction is associated with a more filamentous cytoskeleton within the cells. Accordingly, the hiPSC con-vSMCs demonstrated elevated stress fibers per cell compared to both hiPSC syn-vSMCs and hiPSC pericytes, which had significantly fewer stress fibers per cell (Figure 3A, i and ii; additional fields of view in Figure S3). While differences in α-SMA expression and organization could not be observed, calponin expression was upregulated in hiPSC pericytes (Figure 3A, i and iii; Figure S3).
Figure 3.
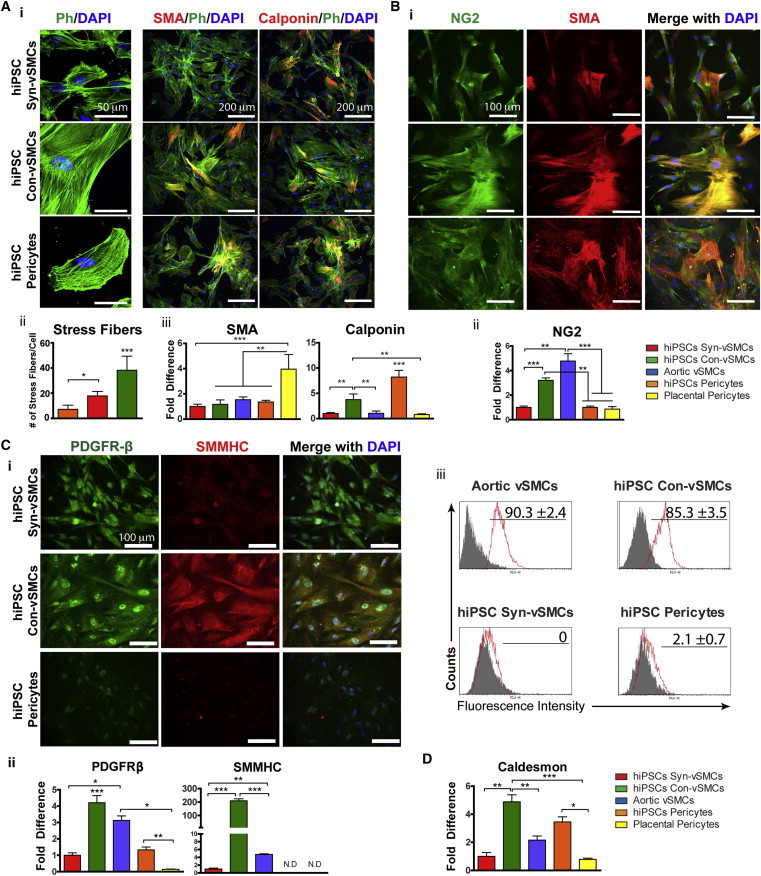
Differences in Stress Fiber and Contractile Marker Expression
Perivascular derivatives were assessed for (A) (i) organization of stress fibers (confocal z-stacks), α-SMA, and calponin (in red; phalloidin in green; nuclei in blue); (ii) stress fiber number and (iii) α-SMA and calponin expression via quantitative real time RT-PCR; (B) (i) organization of NG2 (green) and α-SMA (red; nuclei in blue) and (ii) NG2 expression using quantitative real-time RT-PCR; (iii) (C) (i) PDGFRβ (green) and SMMHC (red; nuclei in blue) and (ii) PDGFRβ and SMMHC expression using quantitative real-time RT-PCR; (iii) flow cytometry analysis of SMMHC (isotype control in gray); and (D) caldesmon expression via quantitative real-time RT-PCR. Results shown from three independent experiments; each RT-PCR sample was run with three technical replicates. All graphical data are reported as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. See also Figures S3 and S4.
The cell-surface proteins NG2 and PDGFRβ are also commonly associated with perivascular cells. The proteoglycan NG2 has been shown to be widely expressed by perivascular cells in both vasculogenic and angiogenic vasculature (Ozerdem et al., 2001). While NG2 is an appropriate marker for identifying pericytes in microvessels, it is merely a supplemental vSMC identifier considering the variety of vSMC markers (Ozerdem et al., 2001). Interestingly, profuse stress fibers were observed with the expression of NG2 protein only in hiPSC con-vSMC cultures (Figure 3B, i). Indeed, NG2 mRNA expression in hiPSC con-vSMCs was significantly elevated compared to hiPSC syn-vSMCs and hiPSC pericytes (Figure 3B, ii). Similarly, aortic vSMCs exhibited NG2 expression with profuse stress fibers compared to placental pericytes (Figure 3B, ii; Figure S4A). We observed differences in the expression of PDGFRβ in our hiPSC derivatives. hiPSC con-vSMCs exhibited elevated PDGFRβ mRNA expression that had a punctuate membrane expression as well as nuclear expression (Figure 3C, i and ii) comparable to aortic vSMCs (Figure S4B).
The mature vSMC marker SMMHC is associated with the contractile vSMC phenotype (Babu et al., 2000; Patel et al., 2006). hiPSC con-vSMCs exhibited elevated SMMHC expression compared to hiPSC syn-vSMCs and pericytes (Figure 3C). Correspondingly, aortic vSMCs exhibited elevated SMMHC expression and SMMHC stress fibers, while placental pericytes did not (Figure 3C; Figure S4B). We further evaluated mRNA and protein expression of SMMHC on our perivascular cell derivatives. hiPSC con-vSMCs exhibited the greatest expression of SMMHC mRNA compared to all other cell types tested (Figure 3C, ii). At the protein level, SMMHC was only detected on control aortic vSMCs and hiPSC con-vSMCs (Figure 3C, iii); hiPSC syn-vSMCs were negative for SMMHC. Interestingly, SMMHC was not detected in either hiPSC pericytes or control placental pericytes (Figure 3C, ii and iii). Finally, the expression of caldesmon, which plays an important role in the perivascular contraction function, was assessed in the different types of perivascular cells. We found that caldesmon was elevated in con-vSMCs compared to syn-vSMCs and pericyte derivatives (Figure 3D). We note that the expression of perivascular markers in aortic vSMCs cultured in low serum (0.5%) conditions was slightly altered yet exhibited a similar trend of aortic vSMCs cultured in 10% serum and hiPSC con-vSMCs (Figure S4C).
ECM Protein Production
A primary function of perivascular cells is the deposition of ECM proteins to help stabilize vasculature. Because the ECM composition of various vessel types differs, we next assessed the different perivascular cell types for expression and production of ECM proteins collagen I, collagen IV, fibronectin, laminin, and elastin in vitro (Figure 4). We found that both phenotypes of vSMCs exhibited concentrated perinuclear collagen I expression, while hiPSC pericytes demonstrated diffuse expression of collagen I around the cytoplasm (Figure 4A, i). hiPSC syn-vSMCs and hiPSC pericytes exhibited similar extracellular and globular expression of collagen IV, while hiPSC con-vSMCs had abundant fibrous intracellular and extracellular collagen IV (Figure 4A, ii). hiPSC con-vSMCs deposited abundant fibronectin extracellularly (Figure 4A, iii); though abundant fibronectin production was detected by hiPSC syn-vSMCs and pericytes, we observed fibronectin production was primarily intracellular (Figure 4A, iii). Laminin appeared perinuclearly around the three perivascular derivatives (Figure 4A, iv). However, hiPSC pericytes exhibited a punctate expression compared to the diffuse laminin protein expression in vSMC derivatives (Figure 4A, iv). Comparable deposition of ECM proteins was observed in aortic vSMCs and placental pericytes (Figure S5A).
Figure 4.
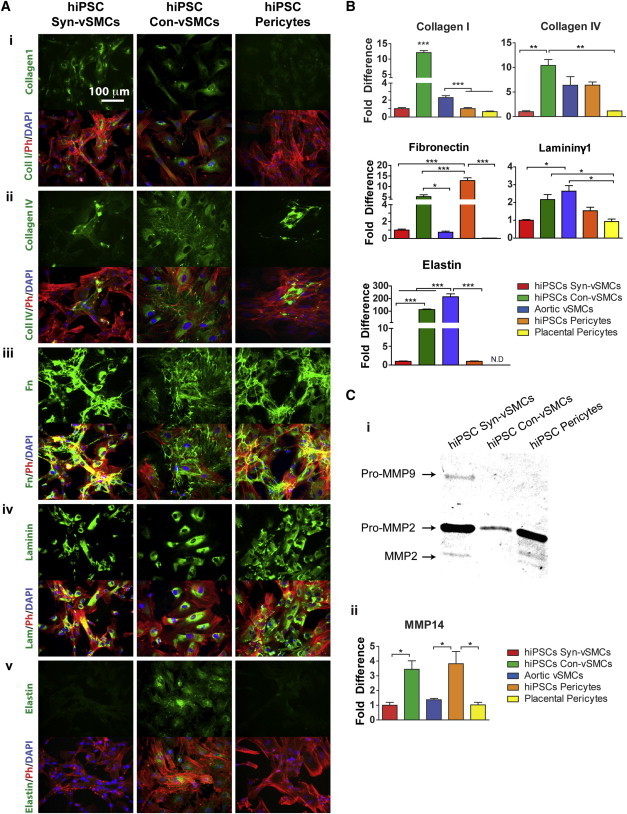
Differential ECM and MMP Expression by Perivascular Derivatives
(A and B) Perivascular derivatives were examined for the production of ECM proteins (i) collagen I, (ii) collagen IV, (iii) fibronectin, (iv) laminin, and (v) elastin (all in green; phalloidin in red; nuclei in blue) after 6 days in culture (A), and their relative expression via quantitative real-time RT-PCR is shown (B).
(C) Perivascular derivatives were compared for (i) the production of MMP2 and MMP9 using zymography, and (ii) the relative expression of MMP14 using quantitative real-time RT-PCR is shown.
Results from three independent experiments are shown; each RT-PCR sample was run with three technical replicates. All graphical data are reported as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
See also Figure S5.
A mature vSMC marker implicated in the mechanical responsiveness of vSMCs (Babu et al., 2000; Patel et al., 2006), elastin was expressed primarily intracellularly and diffusely within con-vSMCs, with some cells exhibiting perinuclear characteristic disordered elastin expression (Figure 4A, v). Correspondingly, aortic vSMCs exhibited elastin expression with both intracellular and extracellular deposition (Figure S5B). We could not detect elastin in either hiPSC syn-vSMCs, or hiPSC pericytes (Figure 4A, v).
Corroborating our immunofluorescence data, we performed RT-PCR analysis on the tested perivascular cells (Figure 4B). We found an increased expression of collagens I and IV and elastin by hiPSC con-vSMCs. Surprisingly, hiPSC pericytes demonstrated the highest expression of fibronectin and its extra domain A (ED-A fibronectin; Figure S5C), suggesting the propensity of the derived pericytes to produce fibronectin under amenable in vitro culture conditions. hiPSC con-vSMCs had higher levels of both fibronectin and ED-A fibronectin than syn-vSMCs. Con-vSMCs and aortic vSMCs exhibited the greatest expression of both laminin and elastin compared to the other tested perivascular cell types. Interestingly, aortic vSMCs had lower expression of collagen I compared to hiPSC con-vSMCs; placental pericytes exhibited lower expression of collagen IV and fibronectin compared to hiPSC pericytes (Figure 4B; Figure S5C). Finally, the hiPSC con-vSMCs expressed elastin mRNA 100-fold more than the hiPSC syn-vSMCs and hiPSC pericytes (Figure 4B). We note that elastin expression in aortic vSMCs was higher compared to hiPSC con-vSMCs and increased when aortic vSMCs were cultured in low serum, demonstrating the importance of culture conditions for the derivation of perivascular cell types from hiPSCs (Figure 4B; Figure S5D).
In the vasculature, there exists a wide range of matrix metalloproteinases (MMPs), which are proteolytic enzymes that degrade the ECM and remodel the architecture of associated vessels. The degradation of ECM allows perivascular cells to migrate and proliferate (Raffetto and Khalil, 2008). Via zymography analysis, we found that only hiPSC syn-vSMCs produce pro-MMP9. Both hiPSC syn-vSMCs and hiPSC pericytes produce pro-MMP2 and its active form, while hiPSC con-vSMCs produce only the pro-MMP2 (Figure 4C, i). We could not detect MMP1 expression using zymography (data not shown). Molecular analysis revealed that hiPSC pericytes have greater MMP14 mRNA expression, while syn-vSMCs have the lowest expression (Figure 4C, ii).
Functionality
In the body, the cellular dynamics of perivascular cells can provide information regarding whether a vessel is emergent, pathogenic, remodeling, or at a stable steady state. Of particular functional importance are multipotency, in vivo behavior, migration, invasion, and contractility of perivascular cells.
Multipotency
A major feature of pericytes is their ability to behave as mesenchymal precursors (Crisan et al., 2008). Indeed, our previous studies have demonstrated that pericyte derivatives could be differentiated to adipocytes and osteoblasts (Kusuma et al., 2013). Contrastingly, neither hiPSC con-vSMCs nor syn-vSMCs demonstrated the potential to differentiate toward adipogenic or osteogenic lineages (Figure 5A).
Figure 5.
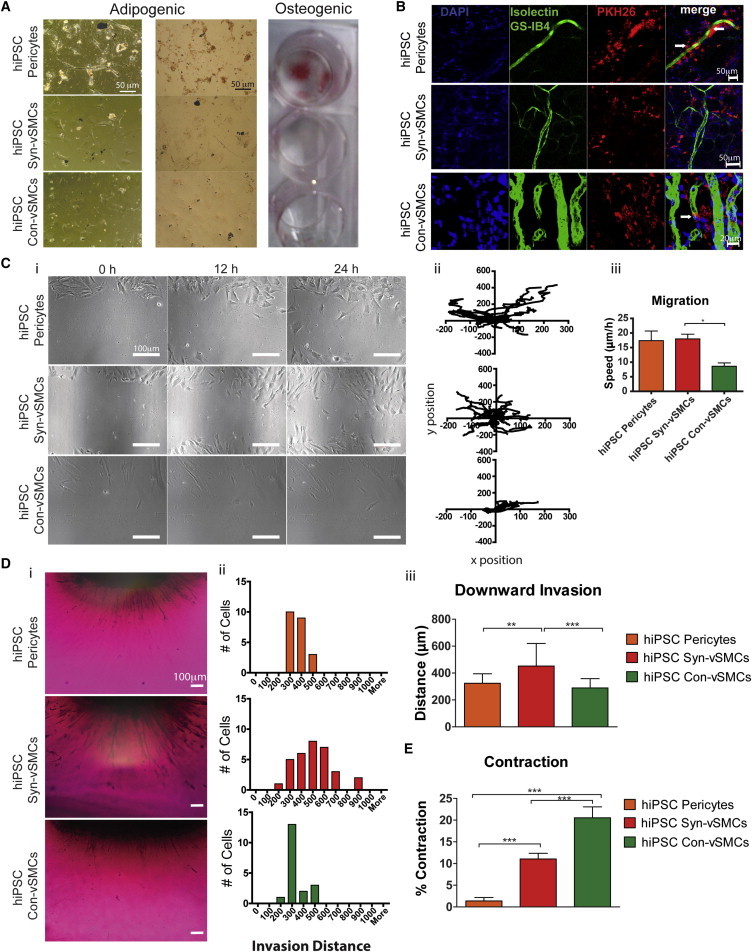
Comparison of Functionalities Demonstrated by Perivascular Derivatives
(A) Differentiation potential of perivascular derivatives into mesenchymal lineages including adipocytes (oil red O stain) and osteoblasts (alizarin red S stain).
(B) One-week subcutaneously transplanted perivascular derivatives (in red; PKH26) migrated to newly formed host blood vessels (mouse ECs in green [Alexa 488-conjugated isolectin IB4]) within Matrigel, as indicated by representative confocal images. White arrows indicate occasions of circumferential wrapping of vasculature by the transplanted hiPSC derivatives; nuclei are indicated in blue (DAPI).
(C) Migration potential via a wound-healing assay. Shown are (i) phase-contrast images quantified for (ii) cell trajectories and (iii) speed.
(D) Downward invasion of hiPSC perivascular cells through collagen gels toward ECs was examined after 48 hr via (i) cross section of toluidine blue-dyed cells and (ii) quantification of the cell number, distance of invasion, and (iii) the average distance traveled.
(E) Contraction was induced by 10−5 M carbachol, and percent contraction was quantified.
Results shown from at least three biological replicates using three independent fields of view in each. All graphical data are reported as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
In Vivo Integration
To compare in vivo functionality, we employed a Matrigel plug assay using our hiPSC perivascular cells. After 1 week of subcutaneous transplantation, all three types of perivascular cells aligned next to the host’s growing functional vasculature, with occasional circumferential wrapping observed by con-vSMCs and pericytes (Figure 5B) and vascular tube narrowing observed only by con-vSMCs (Kusuma et al., 2013; Wanjare et al., 2013a).
Migration
Mechanisms that induce cell motility include chemokinesis, chemotaxis, responses to interactions with ECM, and random increases such as in wound healing (Louis and Zahradka, 2010). In a wound-healing assay, hiPSC pericytes and hiPSC syn-vSMCs migrated inward from the wound margin (Figure 5C, i). hiPSC pericytes exhibited a significant number of trajectories that were not perpendicular to the wound margin, whereas hiPSC con-vSMCs followed oriented trajectories predominantly toward the right. Indeed, after 24 hr, wound closure was observed only with the hiPSC syn-vSMCs (Figure 5C, i and ii). Overall, the hiPSC con-vSMCs exhibited the slowest migration speed (Figure 5C, iii).
Invasion
Invasion is the cell motility associated with ECM degradation. To assess the ability of perivascular cells to invade toward ECs, we cultured a monolayer of ECs beneath a 3D collagen gel. Each perivascular cell type was cultured atop the collagen gel, and migration was measured after 48 hr. Human iPSC syn-vSMCs exhibited increased invasion toward ECs after 48 hr compared to hiPSC con-vSMCs and hiPSC pericytes (Figure 5D, i). Quantification of this dynamic behavior further revealed not only that more hiPSC syn-vSMCs invaded the collagen gels compared to the other perivascular cells but also that they invaded to a deeper distance (Figure 5D, ii and iii).
Contractility
In healthy blood vessels, perivascular cells provide stability to vessels by contracting to counteract the pulsatile force generated by heartbeats. Examining the contractility of the three perivascular derivatives in response to the cholinergic agonist drug carbachol, we found that hiPSC con-vSMCs contracted significantly more than both hiPSC pericytes and hiPSC syn-vSMCs (Figure 5E).
Discussion
The major function of both pericytes and vSMCs is to stabilize blood vessels, and thus both exhibit a great deal of similarities. Distinguishing among the three perivascular cells will facilitate their use in tissue engineering applications. Because pericytes are found in capillaries (<10 μm diameter) and microvessels (10–100 μm diameter), while vSMCs are found in larger vessels (>100 μm diameter), we sought to investigate methods that could elucidate similarities and differences between pericytes and vSMCs in vitro.
In previous studies, we derived pericytes (Kusuma et al., 2013) and both hiPSC syn-vSMCs and hiPSC con-vSMCs (Wanjare et al., 2013a). In performing direct comparisons between these perivascular cell derivatives, we observed numerous differences that enable the study of human perivascular development and functionality and may shed light on means to not only distinguish between them but also clearly define their functionality for future use in tissue-regenerative strategies. A summary of the key features compared among the perivascular derivatives from hiPSC-BC1 is shown in Table 1.
Table 1.
Summary of Features Compared among hPSC-Derived Perivascular Cells
| Cell Type/Features | Con-vSMCs | Syn-vSMCs | Pericytes |
|---|---|---|---|
| Morphology | large, spindle-like | small, spindle-like | colony-like, flat, polygonal |
| Proliferation rate | + | +++ | + |
| Cell size | +++ | ++ | + |
| Endoplasmic reticulum | dilated | nondilated | dilated+ nondilated |
| Markers | SMA, calponin, NG2, PDGFRβ, SMMHC | SMA, calponin, NG2, PDGFRβ, SMMHClow | SMA, calponin NG2, PDGFRβ |
| ECM mRNA expression | collagen Ihigh, collagen IVhigh, fibronectin, laminin | collagen I, collagen IV, fibronectin, laminin | collagen I, collagen IV, fibronectinhigh, laminin |
| ECM deposition | collagen I, collagen IVfibrous, fibronectin, laminindiffuse, elastin | collagen Ilow, collagen IVglobular, fibronectin, laminindiffuse | collagen IVglobular, fibronectin, lamininpunctuate |
| MMPs | 14 (MT-1) | 2, 9 | 2, 14 |
| Mesenchymal differentiation | — | — | adipogenic, osteogenic |
| In vivo integration | alignment, circumferential wrapping, tube narrowing | alignment | alignment, circumferential wrapping |
| Migration | −/+ | +++ | ++ |
| Invasion | −/+ | +++ | + |
| Contractility | +++ | + | −/+ |
Note that values are relative to the three cell types examined.
By assessing marker expression from day 6 differentiating cells compared to derived EVCs and SMLCs, we were able to label our day 6 cells as early mesoderm, characterized by expression of CD56 and CD73. Via EVC differentiation, day 6 cells differentiate into VEcad+ and PDGFRβ+ cells (Kusuma et al., 2013); in SMLC differentiation, day 6 cells were induced to differentiate into cells positive for SMMHC, SM22, and calponin (Vo et al., 2010).
In vivo perivascular cell characteristics are dependent on the local 3D cellular environment, which is composed of cell-cell interactions, surrounding ECM, local mechanical conditions, and chemical cues. While contractile vSMCs are quiescent in the body, synthetic vSMCs display increased proliferation in order to remodel vessels in response to growth factors and cytokines released during vessel injury or disease (Sprague and Khalil, 2009). The in vitro cell culture supplement, serum, contains numerous cytokines and growth factors among other chemokines such as hormones, lipids, attachment factors, etc. (Brunner et al., 2010). Therefore, hiPSC syn-vSMCs responded in vitro just as the synthetic phenotype in the body with high proliferation rates as they were exposed to these different chemokines in serum. Although endothelial-pericyte interactions have been studied extensively, pericyte-pericyte interactions are not well understood. Here, we show the colony-like growth of hiPSC pericytes, suggesting that profound gap junction interactions are needed to activate contact inhibited proliferation of our hiPSC pericytes (Li et al., 2003).
The ER is a major organelle involved in cell protein synthesis. Although in vivo synthetic rat vSMCs have been shown to increase their secretory apparatus that included extensive active ER and Golgi complexes compared to contractile vSMCs, in vitro we observe significant differences in ER size (Thyberg et al., 1997). An in vivo study using mice with knockout miR-143 and miR-145, microRNAs that promote the contractile vSMC phenotype, reported that mice aortas exhibited synthetic vSMC phenotypes and dilated ER containing flocculent material (Elia et al., 2009). In contrast, we show in vitro that hiPSC con-vSMCs have dilated ER, hiPSC syn-vSMCs have nondilated ER, and hiPSC pericytes have both types of ER. Expansion of the ER in mammalian cells has been reported to be necessary in order to accommodate increasing luminal content (mostly unfolded proteins) as a result of ER stress or improper ER function (Görlach et al., 2006; Schönthal 2012). Consequently, the observed ER expansion may indicate that serum deprivation activates vSMC contraction signaling while halting ER-dependent protein synthesis, folding, and transport signaling, thus creating a bottleneck in the ER machinery and giving rise to the dilated appearance. Because their ER machinery is a low priority, hiPSC con-vSMCs therefore have accumulated unfolded proteins in their dilated ER and are functionally dedicated to cell contractility. Likewise, ER stress in the endothelium has also been linked to the increased contraction of isolated mouse aortas (Liang et al., 2013). Liang et al. demonstrated that there was greater phenylephrine-induced vasoconstriction in the aortas of AMP-activated protein kinase knockout mice (AMPKα2−/−), which exhibit aberrant ER stress in ECs compared to WT mice (Liang et al., 2013). The study may suggest that vSMCs not only functionally contract more as a result of their own ER stress, but may also respond in a similar manner in response to EC ER stress. This correlation between ER stress and vSMC contractility may additionally have a role in vessel diseases such as atherosclerosis in which vSMCs take on a synthetic phenotype and contraction is greatly reduced (Rzucidlo et al., 2007). For instance, apoptosis of vSMCs as a result of vSMC ER stress has been implicated in collagen production in atherosclerotic lesions (Tabas, 2010). Therefore, differences in ER signaling cues as well ER structure in our hiPSC con-vSMCs and hiPSC syn-vSMCs may be helpful in studying the diseased syn-vSMCs in vitro. Finally, the proximity of mitochondria to the nucleus as well as the presence of autophagosomes in both placental and hiPSC pericytes, but not in hiPSC vSMCs, may also indicate differences in the cellular machinery of pericytes.
We also found clear differences in the amount of stress fibers between the perivascular derivatives. Abundant stress fibers were found in con-vSMCs located throughout the entire cell body. While hiPSC syn-vSMCs had fewer stress fibers, pericyte derivatives demonstrated the lowest levels of stress fibers per cell and had stress fibers located at the basal lateral surface. All perivascular cells expressed α-SMA in similar levels while calponin was found to be highly expressed in hiPSC pericytes, suggesting that this typical early vSMC marker may also be helpful to identify pericytes. Our findings also indicate that SMMHC is a distinctive marker for con-vSMCs. Upregulated expression and stress fiber organization of SMMHC were observed in hiPSC con-vSMCs. The markers NG2 (or CSPG4) and PDGFRβ are widely utilized to identify pericytes; however, vSMCs also express these markers, making it difficult to distinguish which cell type is actually represented (Murfee et al., 2005). NG2 has been observed to be expressed by both pericytes and vSMCs in arterioles and capillaries, but not beyond postcapillaries (along venules), in rats (Murfee et al., 2005). Here, we showed that con-vSMCs can be distinguished from pericytes and syn-vSMCs by colocalization of NG2 with stress fibers.
The in vitro production and expression of ECM proteins collagens I and IV and laminin differed between the perivascular derivatives. In the body, pericytes produce ECM in the subendothelial basement membrane of capillaries, while both vSMCs and pericytes produce ECM in the tunica media layer of larger blood vessels (Niland, 2009). Collagen I, a fibrillar collagen, is a substantial component of the interstitial connective tissue in contrast to collagen IV, which is present in all basal lamina, forming the basic irregular fibrous 2D network of vasculature (Eble and Niland, 2009). Similarly, laminin is an indispensible component of the vascular basement membrane, the primary site where collagen IV and laminin form an interdependent network (Eble and Niland, 2009). In human arteries, collagen I, deposited between vSMCs, was reported to exhibit sparse thin fiber morphology in the media of small arteries while being organized in fibrillar structures in larger arteries. We report that in vitro, hiPSC pericytes, associated with small vasculature, have a greatly diminished collagen I expression compared to both hiPSC syn-vSMCs and hiPSC con-vSMCs, found in larger vessels (Shekhonin et al., 1987). Both our in vitro findings and in vivo studies illustrate that perivascular cells associated with larger vessels express more collagen I. From our in vitro study, we also observed that a morphologically distinct high density globular collagen IV expression is deposited by both hiPSC syn-vSMCs and pericytes, while a more fibrous collagen IV deposition as well as increased collagen IV expression is exhibited by hiPSC con-vSMCs. Similarly, in vivo, collagen IV deposition varies between the two phenotypes of vSMCs. For instance, fibrous plaques of atherosclerotic human arteries, known to mainly contain syn-vSMCs, have been reported to have greatly decreased collagen IV deposition and increased collagen I deposition around vSMCs compared to healthy arteries containing contractile vSMCs (Rekhter, 1999; Shekhonin et al., 1985, 1987). However, the loci of the plaques have been shown to have large quantities of collagen IV, correlating with the vSMCs being surrounded by layers of basement membrane material in this region (Rekhter, 1999; Shekhonin et al., 1987). Additionally, the in vitro laminin expression was different in hiPSC pericytes compared to both phenotypes of hiPSC vSMCs. hiPSC con-vSMCs had diffuse cytoplasmic expression of laminin, while hiPSC pericytes had punctate expression around the cell membrane. hiPSC syn-vSMCs had mostly diffuse cytoplasmic expression of laminin with few instances of punctate expression. In vivo, rat synthetic vSMCs lost the ability to produce laminin unlike contractile vSMCs (Thyberg et al., 1997). Fibronectin was expressed and deposited by all tested perivascular cell types, with hiPSC pericytes expressing the highest fibronectin mRNA levels. Human iPSC con-vSMCs expressed higher levels of fibronectin mRNA compared to hiPSC syn-vSMCs. Similarly, the hiPSC con-vSMCs also expressed higher levels of ED-A fibronectin compared to the hiPSC syn-vSMCs, while in vivo, ED-A fibronectin was suggested to be associated with the synthetic phenotype (Glukhova et al., 1989). Examination of the expression pattern of ED-A fibronectin in differentiating stem cells highlights the need for further investigation of stem cell derivatives and the conceivable differences between in vitro and reported in vivo phenotypes, thus warranting additional studies correlating fibronectin slice variants to perivascular phenotypes both in vitro and in vivo. Elastin was also highly expressed by con-vSMCs compared to all other perivascular cell types. Not only is elastin production characteristic of the contractile phenotype, but also, interestingly, in vivo studies demonstrated that increasing elastin production itself promoted a contractile vSMC phenotype by inhibiting vSMC proliferation (Karnik et al., 2003; Urbán et al., 2002).
Pericytes and vSMCs have been known to express the gelatinases MMP2 and MMP9 needed to degrade basement membranes during vessel remodeling (Candelario-Jalil et al., 2009; Newby, 2006; Virgintino et al., 2007). Accordingly, in vitro, hiPSC syn-vSMCs expressed MMP2 and MMP9, corresponding to previous observations that this phenotype is associated with vessel remodeling, which includes ECM degradation. The expression of MMP2 and MMP9 in hiPSC pericytes coincided with pericytes’ close contact with basement membranes of vessels. A membrane-associated MMP, MMP14, was more greatly expressed by derived pericytes compared to control placental pericytes. MMP14 is known for its ability to degrade various ECM proteins; thus, we had expected that control pericytes would express this MMP type more greatly given the abundance of these ECM proteins in microvessels and capillaries. We suspect the discrepancy may be due to a loss of this site-specific feature due to in vitro culture of harvested pericytes, emphasizing the advantages of derived perivascular cell types over primary cells.
Not surprisingly, only hiPSC-derived pericytes had the potential to differentiate to mesenchymal lineages, including adipogenic and osteogenic, while neither hiPSC nor vSMC types could differentiate. In vivo, all transplanted perivascular cells aligned next to the host vasculature, with both pericytes and con-vSMCs occasionally wrapping the microvasculature. These differences correlated with in vivo phenotypes of these various perivascular cell types; both pericytes and con-vSMCs support vasculature in vivo and are thus closely associated with the endothelial lining, providing support. Syn-vSMCs, alternatively, are known for their role in remodeling vasculature. Differences in cellular mechanics may also be used to distinguish among the three different perivascular cell types. hiPSC syn-vSMCs were able migrate in response to wounding and invade through ECM toward ECs, whereas hiPSC con-vSMCs were not. Interestingly, while the hiPSC pericytes migrated in response to wounding, they failed to invade through ECM toward ECs, indicative of their short-distance migratory nature. This result coincides with the fact that pericytes have a close spatial relation to ECs in vessels (Díaz-Flores et al., 2009).
Finally, hiPSC con-vSMCs can be characterized by their elevated cellular contractility (as demonstrated by both carbachol treatment and increased expression of contractile protein [caldesmon]), while hiPSC syn-vSMCs are characterized by increased speed, migration, and invasion. These results are in agreement with previous observations that vSMCs switch to a synthetic phenotype in the body in response to injury to aid in tissue repair (Louis and Zahradka, 2010).
In summary, these perivascular derivatives demonstrate an important building block toward not only reconstructing physiologically relevant vasculature but also the study of developmental processes and diseases implicating these cell types. Important elements of our system are the several noted discrepancies between our in vitro results and in vivo phenotypes, alluding to the complexity of the field. A major challenge of correlating in vitro results to phenotypes observed in vivo is the presence of heterogeneous vSMC subpopulations of unknown origin that lie between the contractile and synthetic phenotypic spectrum in vivo. Unanswered questions, particularly those regarding the in vivo synthetic phenotype, still remain. For instance, ECM deposition of syn-vSMCs located in vastly different environments such as normal developing or remodeling vessels compared to diseased or injured vessels remains unknown. To further add to the complication, by utilizing SMMHC lineage tracing, Tang et al. reported that synthetic vSMCs actually arise from multipotent stem cells lying within vessels and not phenotypic switching by vSMCs in vivo (Drukker et al., 2012; Tang et al., 2012). The advantage of our study is the derivation and comparison of synthetic and contractile vSMCs from a known common early smooth muscle precursor. In fact, some of our study’s in vitro results actually yield a more useful phenotype for engineering blood vessels, such as increased ECM production from the hiPSC derivatives compared to control cell lines; however, other discrepancies, such as lower expression of fibronectin splice variant ED-A in derived syn-vSMCs compared to con-vSMCs, drive the need for continued study on the derivation of specialized cell types to rebuild tissue. Additionally, studies in a 3D environment would allow further investigation of morphological features such as nucleic size that may better match in vivo properties.
Overall, the ability to generate human perivascular cells including contractile vSMCs, synthetic vSMCs, and pericytes with identical genetic backgrounds offers unprecedented opportunities to study the development and functionality of well-defined human perivascular derivatives from healthy and disease hiPSCs. Furthermore, by employing a viral integration-free and fully genetically sequenced hiPSC line, BC1, we anticipate that these findings hold translational importance.
Experimental Procedures
Complete experimental procedures are available online (see the Supplemental Experimental Procedures).
Cell Culture
hiPSC Line and Differentiation
The hiPSC line BC1 (Cheng et al., 2012; Chou et al., 2011; kindly provided by Dr. Cheng, SOM JHU) and the hESC line H9 were expanded and differentiated to perivascular cells as described previously (Kusuma et al., 2013; Wanjare et al., 2013a).
Stress Fiber Quantification
The number of stress fibers per cell was quantified using line intensity profiles of cells in ImageJ (Wei et al., 2011). Stress fibers were labeled with fluorescent Alexa 488 phalloidin and imaged at 20× and 40×. A line-intensity profile across a single cell was generated, with each peak representing a single stress fiber.
Invasion toward ECs
A downward invasion assay was used to assess invasion of perivascular cells. Human umbilical vein ECs were seeded on 16-well detachable wells (Fisher). After 24 hr, 150 μl of collagen gel (150 μl) was added on top of the ECs. After 1 hr, hiPSCs perivascular cells were seeded on top of the gels. After 48 hr, the gels were fixed and stained with toluidine blue dye and cross sections of the gels were imaged and quantified.
Acknowledgments
We gratefully acknowledge support for this work by a NIH predoctoral award from NIH grant F31HL112644 (to S.K.) and NIH grant R01HL107938 (to S.G).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Fourth Edition. Garland Science; New York: 2002. Molecular Biology of the Cell. [Google Scholar]
- Babu G.J., Warshaw D.M., Periasamy M. Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microsc. Res. Tech. 2000;50:532–540. doi: 10.1002/1097-0029(20000915)50:6<532::AID-JEMT10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Beamish J.A., He P., Kottke-Marchant K., Marchant R.E. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng. Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov K.G., Stepanova O.V., Nanaev A.K., Shirinsky V.P. Expression of calponin in rabbit and human aortic smooth muscle cells. Cell Tissue Res. 1991;266:579–584. doi: 10.1007/BF00318599. [DOI] [PubMed] [Google Scholar]
- Boyd N.L., Robbins K.R., Dhara S.K., West F.D., Stice S.L. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng. Part A. 2009;15:1897–1907. doi: 10.1089/ten.tea.2008.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D., Frank J., Appl H., Schöffl H., Pfaller W., Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E., Yang Y., Rosenberg G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Park M.B., Shen J.Y., Cao Y., Xiong Y., Liu Y., Rayatpisheh S., Kang G.C.-W., Greisler H.P. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J. Biomed. Mater. Res. A. 2009;88:1104–1121. doi: 10.1002/jbm.a.32318. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H., Chen Y.-L., Lin S.-J., Chou C.-Y., Mar G.-Y., Chang M.-S., Wang S.-P. Electron microscopic studies of phenotypic modulation of smooth muscle cells in coronary arteries of patients with unstable angina pectoris and postangioplasty restenosis. Circulation. 1997;95:1169–1175. doi: 10.1161/01.cir.95.5.1169. [DOI] [PubMed] [Google Scholar]
- Cheng L., Hansen N.F., Zhao L., Du Y., Zou C., Donovan F.X., Chou B.K., Zhou G., Li S., Dowey S.N., NISC Comparative Sequencing Program Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C., Bernardo A.S., Trotter M.W.B., Pedersen R.A., Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou B.K., Mali P., Huang X., Ye Z., Dowey S.N., Resar L.M.S., Zou C., Zhang Y.A., Tong J., Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Crisan M., Corselli M., Chen W.C., Péault B. Perivascular cells for regenerative medicine. J. Cell. Mol. Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A., Itskovitz-Eldor J. Therapeutic potential of perivascular cells from human pluripotent stem cells. J. Tissue Eng. Regen. Med. 2013 doi: 10.1002/term.1698. Published online January 31, 2013. [DOI] [PubMed] [Google Scholar]
- Dar A., Domev H., Ben-Yosef O., Tzukerman M., Zeevi-Levin N., Novak A., Germanguz I., Amit M., Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- Deguchi S., Ohashi T., Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 2006;39:2603–2610. doi: 10.1016/j.jbiomech.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., Madrid J.F., Varela H., Valladares F., Acosta E., Martín-Vasallo P., Díaz-Flores L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Drukker M., Tang C., Ardehali R., Rinkevich Y., Seita J., Lee A.S., Mosley A.R., Weissman I.L., Soen Y. Isolation of primitive endoderm, mesoderm, vascular endothelial and trophoblast progenitors from human pluripotent stem cells. Nat. Biotechnol. 2012;30:531–542. doi: 10.1038/nbt.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble J.A., Niland S. The extracellular matrix of blood vessels. Curr. Pharm. Des. 2009;15:1385–1400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- Elia L., Quintavalle M., Zhang J., Contu R., Cossu L., Latronico M.V.G., Peterson K.L., Indolfi C., Catalucci D., Chen J. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko D., Zhu Y., Schenke-Layland K., Kuo J., Latour B., Ge S., Scholes J., Dravid G., Li X., MacLellan W.R., Crooks G.M. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2010;107:13742–13747. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L.S., Gerecht S., Shieh H.F., Watson N., Rupnick M.A., Dallabrida S.M., Vunjak-Novakovic G., Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ. Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- Glukhova M.A., Frid M.G., Shekhonin B.V., Vasilevskaya T.D., Grunwald J., Saginati M., Koteliansky V.E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J. Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach A., Klappa P., Kietzmann D.T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Hedin U., Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Karnik S.K., Brooke B.S., Bayes-Genis A., Sorensen L., Wythe J.D., Schwartz R.S., Keating M.T., Li D.Y. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- Kumar V.A., Brewster L.P., Caves J.M., Chaikof E.L. Tissue engineering of blood vessels: functional requirements, progress, and future challenges. Cardiovasc. Eng. Technol. 2011;2:137–148. doi: 10.1007/s13239-011-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma S., Gerecht S. Recent progress in the use of induced pluripotent stem cells in vascular regeneration. Expert Rev. Cardiovasc. Ther. 2013;11:661–663. doi: 10.1586/erc.13.54. [DOI] [PubMed] [Google Scholar]
- Kusuma S., Shen Y.-I., Hanjaya-Putra D., Mali P., Cheng L., Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. USA. 2013;110:12601–12606. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.-F., Sato T., Haimovici R., Okamoto T., Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest. Ophthalmol. Vis. Sci. 2003;44:5376–5382. doi: 10.1167/iovs.03-0360. [DOI] [PubMed] [Google Scholar]
- Liang B., Wang S., Wang Q., Zhang W., Viollet B., Zhu Y., Zou M.-H. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-α2 in vivo. Arterioscler. Thromb. Vasc. Biol. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S.F., Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp. Clin. Cardiol. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- Majesky M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Murfee W.L., Skalak T.C., Peirce S.M. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Newby A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Niland J.A.E.S. The extracellular matrix of blood vessels. Curr. Pharm. Des. 2009;15:1385–1400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- Orlova V.V., Drabsch Y., Freund C., Petrus-Reurer S., van den Hil F.E., Muenthaisong S., ten Dijke P., Mummery C.L. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler. Thromb. Vasc. Biol. 2014;34:177–186. doi: 10.1161/ATVBAHA.113.302598. [DOI] [PubMed] [Google Scholar]
- Ozerdem U., Grako K.A., Dahlin-Huppe K., Monosov E., Stallcup W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Patel A., Fine B., Sandig M., Mequanint K. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc. Res. 2006;71:40–49. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Raffetto J.D., Khalil R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhter M.D. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc. Res. 1999;41:376–384. doi: 10.1016/s0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- Rzucidlo E.M., Martin K.A., Powell R.J. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 2007;45(Suppl A):A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schönthal A.H. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhonin B.V., Domogatsky S.P., Muzykantov V.R., Idelson G.L., Rukosuev V.S. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll. Relat. Res. 1985;5:355–368. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- Shekhonin B.V., Domogatsky S.P., Idelson G.L., Koteliansky V.E., Rukosuev V.S. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987;67:9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- Sprague A.H., Khalil R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Wang A., Yuan F., Yan Z., Liu B., Chu J.S., Helms J.A., Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat. Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J., Blomgren K., Roy J., Tran P.K., Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J. Histochem. Cytochem. 1997;45:837–846. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- Urbán Z., Riazi S., Seidl T.L., Katahira J., Smoot L.B., Chitayat D., Boyd C.D., Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am. J. Hum. Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D., Girolamo F., Errede M., Capobianco C., Robertson D., Stallcup W.B., Perris R., Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Vo E., Hanjaya-Putra D., Zha Y., Kusuma S., Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev. 2010;6:237–247. doi: 10.1007/s12015-010-9144-3. [DOI] [PubMed] [Google Scholar]
- Vodyanik M.A., Yu J., Zhang X., Tian S., Stewart R., Thomson J.A., Slukvin I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Kuo F., Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Kusuma S., Gerecht S. Perivascular cells in blood vessel regeneration. Biotechnol. J. 2013;8:434–447. doi: 10.1002/biot.201200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Gao X., Du J., Su J., Xu Z. Angiogenin enhances cell migration by regulating stress fiber assembly and focal adhesion dynamics. PLoS ONE. 2011;6:e28797. doi: 10.1371/journal.pone.0028797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


