Abstract
In the past decade, enormous progress has been made in understanding the role of stem cells in physiologic tissue renewal and in pathologic processes such as cancer. These findings have shed light on the identity and biological properties of such cells and the intrinsic and extrinsic signals that balance stem cell self-renewal with differentiation. With its astonishing self-renewal capacity, the intestinal epithelium has provided a unique model to study stem cell biology, lineage specification, and cancer. Here we review the role of Notch signaling in physiologic cell renewal and differentiation in the intestine as well as during its malignant transformation.
Keywords: Intestine, Stem Cells, Homeostasis, Notch, Colon Cancer
The primary function of the intestinal tract is food digestion, the absorption of nutrients and water, and the cellular defense against pathogens and microorganisms. To withstand the demands of these functions, the epithelium lining of the intestinal tract has developed a remarkable capacity for cell renewal. Every day, thousands of cells are born and as many die in a continuous and highly regulated cycle of birth, differentiation, and death. In the past decade, the biological processes that underlie this astonishing capacity for self-renewal, lineage specification, and epithelial differentiation have been uncovered.1 Defects in these biological processes disrupt normal intestinal homeostasis and architecture and underlie inflammatory disease and cancer.
Development, Cell Types, and Architecture of the Mammalian Intestine
During the early stages of embryonic development, endodermal cells undergo epithelial-to-mesenchymal transition, invaginate, and form the gut tube, which is gradually subdivided into 3 parts along the anterior-posterior axis: the foregut, midgut, and hindgut. The foregut will develop into the pharynx, esophagus, and stomach, while the midgut will give rise to the small intestine and the hindgut to large intestine (colon). Starting from approximately mouse embryonic day 14, through the upward movement of the underlying mesenchyme, the single-layered intestinal epithelium forms finger-like projections into the gut lumen (the villi). After birth, crypts form between villi by invagination into the underlying connective tissue. Reciprocal signaling between the epithelium and the mesenchyme shapes intestinal morphogenesis.2
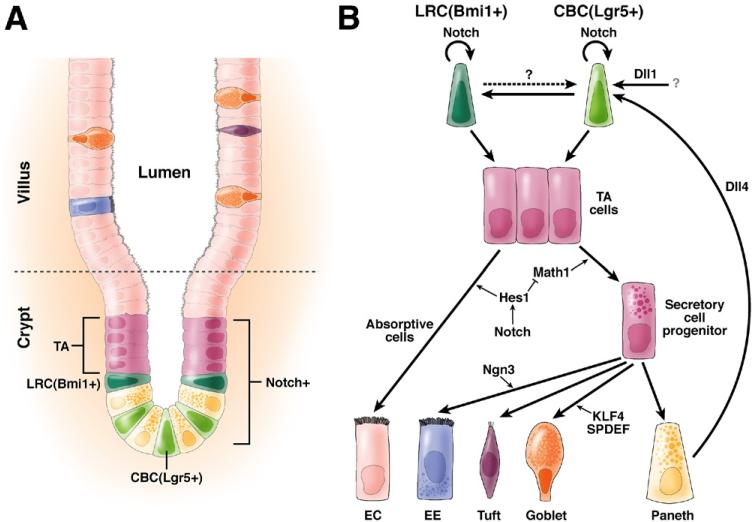
Three weeks after birth, development of the adult gut is complete and can be divided into the small and the large intestine. Along the rostral-caudal axis, the small intestine can be subdivided into duodenum, jejunum, and ileum. The functional unit of the small intestine is a villus containing terminally differentiated cells connected to the crypts of Lieberkühn, which harbor the proliferative compartment. The colon is only composed of crypts and has no extending villi. The intestine is a highly dynamic organ system with a turnover rate of approximately 60 hours for the entire epithelial population.3 The proliferating cells in the crypt are intestinal stem cells (ISCs) and transit amplifying (TA) cells that fuel the continuous production of several differentiated epithelial cell types (Figure 1). The crypt epithelium cycles asynchronously, and new crypts arise though bifurcation (crypt fission) during adult life as the intestinal tract continues to grow.4 Within a week, progenitors migrate upward from the crypt into the villus tip, from which they are shed into the intestinal lumen. To support this high turnover, mouse crypt stem cells are estimated to undergo a thousand divisions during their lifetime. Whereas stem cell self-renewal occurs within the crypt throughout adult life, TA daughters continue to differentiate to produce 2 main types of differentiated cells: the absorptive cells or enterocytes (ECs) and the secretory cells. The ECs are involved in nutrient uptake and secretion of hydrolases and constitute the majority of cells in the villus epithelium. Secretory cells consist of the mucus-producing goblet cells, the hormone-producing enteroendocrine (EE) cells, and the lysozyme-producing Paneth cells. Paneth cells migrate downward into the crypt base, where they live for several weeks. Recently, tuft cells were recognized as a fourth kind of secretory cell, which secrets opioids and cyclooxygenase enzymes.5
Figure 1. The mammalian small intestine and role of Notch in ISC renewal.
(A) Two populations of stem cells, CBCs and label retaining cells (LRCs), characterized by Lgr5 and Bmi1 expression, respectively, are located at the bottom of the crypt. Their daughters, the TA cells, undergo rapid proliferation as they move upwards. Terminal cell differentiation starts at about the upper third of the crypt and gives rise to 5 major cell types: the absorptive ECs and the secretory cell types composed of EE cells, tuft cells, goblet cells, and Paneth cells. Whereas most differentiated cells move and home into the villi, Paneth cells move back into the bottom of the crypt after their birth to form the niche to orchestrate ISC renewal and differentiation. (B) The relationship between the LRC and CBC stem cell populations is not very clear. It is possible that they could give rise to each other (dashed arrows). Although both populations produce TA cells, it has been proposed that CBCs may represent a rapidly cycling population (thick arrow) whereas LRC is a slow cycling one (thin arrow). Notch may be required for the maintenance of both. Although Paneth cells express Dll4 ligand, it remains unclear which cells provide the Dll1 ligands, which play a dominant role in sustaining stem cell self-renewal as well. During cell fate specification, Notch promotes the EC fate and inhibits the secretory cell fates through down-regulation of Math1, a master transcription factor for all secretory cell fates. Math1 controls secretory commitment by activation of cell type-specific transcription factors. For example, Ngn3 specifies the EE cell fates while KLF4 and SPDEF goblet cells.
Evidence for the Existence of ISCs
Clonal analysis performed several decades ago showed that all differentiated cell types arise from a few stem cells residing in the monoclonal crypts and contribute a column to the polyclonal villi.6-9 Early lineage tracing studies revealed a slow proliferating or quiescent stem cell located about 4 cells above Paneth cells (at the +4 position).10 Lineage tracing studies based on stem cell markers have delineated the location of 2 distinct ISC pools in the crypt11,12: (1) the columnar base cells (CBCs)13 with a high turnover, expressing the leucine-rich repeat membrane protein Lgr5,11 and (2) a quiescent label-retaining population located 4 cells above the Paneth cells, expressing the polycomb protein Bmi1.14,15 A model emerging from these findings is that 2 distinct stem cell populations ([Lgr5+, CBC] and [Bmi1, +4]) act cooperatively to support normal physiologic cell replenishment and tissue repair.16 However, +4 cells are also labeled with Lgr5 lineage markers,12 suggesting that both lineages are derived from CBCs. Coordination between cell renewal, transit amplification, terminal differentiation, and apoptosis requires a precise interplay among several signaling pathways, which is invariably perturbed during intestinal diseases such as cancer. In this review, we will focus on the role of the Notch signaling pathway, a master regulator of cell fate during intestinal homeostasis. We summarize the expression patterns and functions of the core components of the pathway, starting at the membrane with the Notch ligands and receptors and ending with target gene activation in the nucleus. Genetic and chemical gain- and loss-of-function studies are also discussed to illustrate the important roles of the Notch pathway in intestinal homeostasis.
The Core Components of the Notch Signaling Pathway
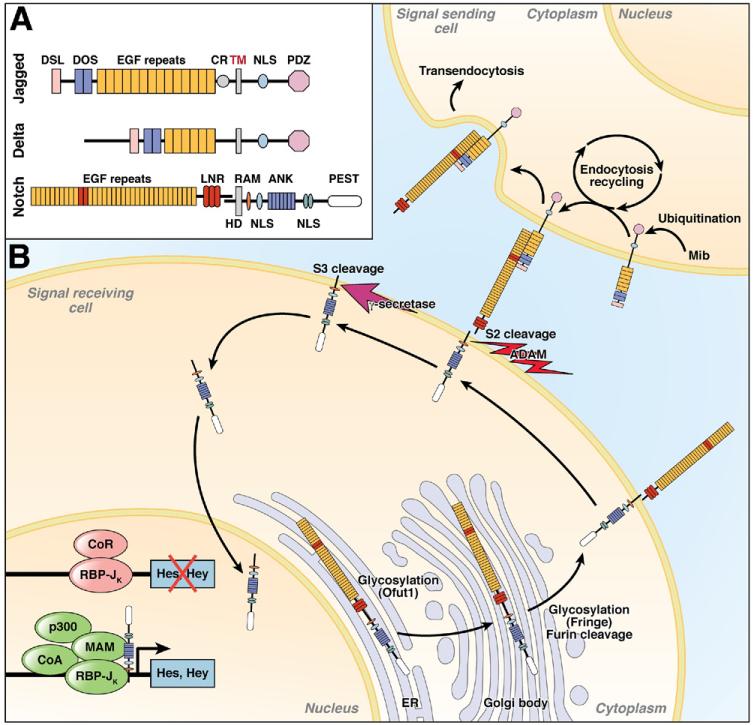
The Notch signaling pathway is a highly conserved short-range communication mechanism used in all metazoans.17,18 Notch genes encode large type I transmembrane receptors that bind to type I ligands on adjacent cells. In the fruit fly (Drosophila melanogaster), the core signaling components are encoded by a single Notch receptor, 2 ligands (Delta and Serrate), a DNA binding protein (Suppressor of Hairless [Su(H)]), and several modifiers. Mammalians have 4 Notch receptors (Notch1-4), 3 Delta-like ligands (Dll1, 3, 4), and 2 Serrate-like ligands termed Jagged (Jagged1 and 2). Notch signaling is initiated by ligand binding to the Notch receptor expressed on adjacent cells (in trans) and can be inhibited when the ligand binds to the receptor present on the same cell (in cis).19 Trans binding triggers unfolding of the juxtamembrane domain and enables ADAM proteases to cleave Notch.20-22 An intramembranous enzyme complex called γ-secretase then cleaves Notch in its transmembrane domain, which results in the release of the cytoplasmic domain from the plasma membrane. The Notch intracellular domain (NICD) shuttles to the nucleus, where it binds to RBP-Jκ (the Su(H) homologue), recruits mastermind-like (MAML) adaptor proteins, and establishes an activator complex, leading to the expression of target genes.18 In mammals, the best-characterized Notch target genes belong to the Hes (Hairy Enhancer of Split) and Herp/Hey (Hes-related repressor proteins with Y-box) family of basic helix-loop-helix (bHLH) transcriptional repressors. Hes and Hey in turn repress the activity of many tissue-specific bHLH transcriptional activators such as Math (mouse homologue of Atonal) and Mash (mouse homologue of Achaete/Scute).23 The core components of the Notch signaling pathway are illustrated in Figure 2. Thus, Notch activation can block a default cell fate among equivalent cells via lateral inhibition with feedback amplification. For example, Hes1 mediates the repression of gut-specific bHLH protein Math1,24 and Math1 often activates the expression of Notch ligand, establishing feedback amplification.25
Figure 2. The core components of the Notch signaling pathway.
(A) Typical domain structure of the vertebrate Notch receptor and ligands. Jagged and Delta ligands share from N-terminus to C-terminus the Delta/Serrate/LAG-2 domain (DSL), the Delta and OSM-11-like protein domain (DOS), a variable number of EGF repeats, transmembrane domain (TM), nuclear localization signal (NLS), and PSD95/Dlga/Zo-1domain (PDZ). Jagged1 and 2 also have a cysteine-rich domain (CR). Notch receptors have 24 to 36 EGF repeats, which mediate ligand interaction (11 and 12, shaded in pink, are in direct contact with the DOS domain of the ligands). Following these EGF repeats are 3 cysteine-rich Lin-12/Notch repeats (LNRs), a heterodimerization domain (HD), transmembrane domain (TD), the RBP-Jκ association module (RAM), 7 NLS-flanked ankyrin repeats (ANK), and the proline/glutamic acid/serine/threonine-rich degradation motif (PEST). (B) Key steps involved in the activation of the Notch signaling pathway. In the absence of Notch activity, RBP-Jκ binds to the promoters of Notch target genes such as Hes and Hey and recruits corepressors to repress transcription. Newly synthesized Notch receptor experiences a series of posttranslational modifications in the ER and Golgi body before reaching the cell surface, including the glycosylation by O-fucose-transferase (Ofut1) and Fringe as well as the S1 cleavage by furin-like convertases. In contrast, the ligands are ubiquitinated in the signaling-sending cell by ubiquitin ligases such as Mib-1, endocytosed, and then recycled back to the cell membrane. Trans-interactions between the Notch receptor and its ligands is mediated by EGF repeat 11-12 of the receptor and the DOS domain of the ligands followed by transendocytosis of the ligand. This causes unfolding of the LNR/HD domain exposing the S2 cleavage site a substrate for the ADAM (A Disintegrin And Metalloprotease) metalloproteases. S2 cleavage leads to shedding of the extracellular domain of the receptor and turns it into the substrate for the γ-secretase complex, which catalyzes the cleavage at the S3 site within the Notch transmembrane domain. This leads to the release of the NICD from the cell membrane and its subsequent translocation into the nucleus, where it displaces corepressor bound to RBP-Jκ and recruits coactivators such as Mastermind and p300 to activate gene expression.
The Notch Pathway Is Expressed and Active in the Intestine
Notch receptors, ligands, and canonical target genes are expressed in the embryonic and adult gut epithelium. Notch1 and Notch2 receptors show both overlapping and spatially distinct expression patterns. Whereas Notch2 is only expressed in scattered cells within the crypt epithelium of the small intestine and in smooth muscle cells, Notch1 is expressed in the crypt epithelium of the entire gut, in a few differentiated villus epithelial cells, and in the endothelium. Notch3 and Notch4 expression is restricted to the endothelium and the mesenchyme. Notch ligands Jag1 and Jag2 follow mostly the expression pattern of Notch1 in the epithelium26,27 and of Notch2 in the smooth muscle. Dll1 and Dll4 are both expressed during embryogenesis, in the adult gut in the crypt epithelium, and in secretory cells in villus epithelium.26,28,29 Dll4 is also expressed in the endothelium. Antibodies directed to the γ-secretase-generated epitope on NICD1 detect it in the crypt epithelium,30 where Hes1, 5, 6, and 7 are also expressed,26 suggesting that Notch1 signaling is active in the crypt. The expression of Hes1 is coincident with Ki-67 labeling of proliferating cells and absent from EE cells.24 Whereas Hes1, 6, and 7 are expressed exclusively in the crypt epithelium, Hes5 is also expressed in the villus epithelium.24,26 Taken together, these expression studies show that Notch1 and Notch2 are the main receptors in the epithelium and Dll1, Dll4, and Jagged1 are predominantly expressed in crypts.
Genetic Analysis of Notch Function
Notch Receptors
Analysis of mouse chimeras derived from wild-type and LacZ-expressing, Notch1-deficient ES cells shows that Notch1 is dispensable for crypt-villus formation30; conditional removal of Notch1 or Notch2 (using tamoxifen inducible Villin-Cre) also permits normal crypt development.31 These findings suggest that Notch1 and Notch2 function are redundant. Indeed, combined deletion of Notch1 and Notch2 mimics RBP-Jκ deletion and pharmacologic inhibition of γ-secretase (see following text), all leading to loss of stem cells, premature differentiation, and goblet cell hyperplasia.31 In contrast, Notch1-null crypts in chimeric mice produce villi with mildly increased secretory cells and reduced absorptive cells compared with villi from wild-type crypts.30 The discrepancy between these 2 phenotypes may be explained by the timing of Notch inactivation: chronic and widespread (chimeric) versus acute and cell type specific (Villin+ cells only). Thus, we can conclude that Notch2 and Notch1 are redundant in maintaining the crypt-villus structure and that Notch1 has a dominant role in suppressing secretory fate when TA progenitors differentiate.
Consistent with Notch loss-of-function analysis, ectopic expression of NICD1 in the embryonic and adult intestinal epithelium (Rosa26-NICD/Villin- or Fabpl-Cre) causes ectopic proliferation of crypt progenitors as well as immature cycling progenitors detected in the villus epithelium. These cells are characterized by Hes1 (but not Hes5) up-regulation and suppression of Math1 and Ngn3.32,33 This failure in cell cycle exit causes a reduction in terminal differentiation within the secretory lineages and substantial apoptosis in the villus epithelium. The effects of NICD1 expression on the long-term renewal of the crypt epithelium could not be studied because Rosa26-NICD mice die within the first week after birth. Nonetheless, these results show that ectopic expression of Notch perturbs intestinal homeostasis and may leads to uncontrolled proliferation and carcinoma. In contrast to its antigoblet function in the crypt, NICD overexpression in postmitotic villus epithelial cells is accompanied by elevated expression of Hes5 but not Hes1 and leads to increased goblet cell numbers with other secretory cell types unaffected.34 Consistent with these observations, the cleaved, activated form of Notch1 is detected in post-mitotic mucin-producing goblet cells.30 These findings seem contradictory. One explanation may be that whereas Notch is active and sufficient to induce goblet cell fate through Hes5 in a Hes1-Math1–independent manner in postmitotic precursors, the specification of goblet cell fate in normal physiologic context may not rely on Notch activation and is instead default. This default fate in multipotent progenitors is repressed by Notch signaling through Hes1 up-regulation. Loss of Notch1 function specifically in postmitotic villus epithelium is needed to address this.
Posttranslational modifications of Notch receptors with sugar moieties are essential for productive ligand binding and determine receptor-ligand selectivity.35 Mice deficient for the O-fucosyl transferase1 (Pofut1) are embryonic lethal and phenocopy mice lacking RBP-Jκ.36 Similarly, intestinal-specific deletion of Pofut1 leads to hyperplastic crypts composed of secretory cells and resembles intestines lacking both Notch1 and 2 or RBP-Jκ.31,37,38
Notch Ligands
As single mutants, lack of Dll1, but not loss of Dll4 or Jagged1, causes increased goblet cell numbers in the adult intestine, suggesting that Dll1 is the most important Notch receptor ligand in the crypt. Nevertheless, simultaneous inactivation of Dll4, but not Jagged1, with Dll1 leads to enhanced massive goblet metaplasia and proliferative arrest,39 further suggesting that Dll4 can compensate for Dll1 loss whereas Jagged1 is not signaling in the niche. Endocytosis of Notch ligands is crucial for their function,40 and Mindbomb-1 (Mib1) is an ubiquitin ligase that acts in endocytosis of Notch ligands.41 Zebrafish lacking Delta (DeltaD) or Mib129 and intestine-specific deletion of Mib1 in mice all show precocious secretory differentiation,42 whereas Mib2 mutants are viable.43 Mib1 mutant mice have an additional phenotype, which is the mislocalization of Paneth cells to the villus epithelium. NICD overexpression in Mib-null intestines reverses endocrine metaplasia and restores the localization of Paneth cells, showing that both phenotypes are Notch dependent. Because Paneth cell positioning is Ephrin/Eph signaling dependent and regulated by the Wnt-β-catenin cascade,44 Notch may affect Paneth cell localization thus in 2 ways: directly by inducing the expression of Ephrin-B1 and indirectly by down-regulating EphB2 through β-catenin/TCF4 inactivation.42 Alternatively, the combination of Notch-dependent and Mib-dependent processes produced a phenotype not seen in Notch pathway mutant only.
Intramembrane Proteolysis: γ-Secretase
The γ-secretase complex is the sole enzyme mediating intramembrane proteolysis of Notch receptors following ectodomain shedding, and several drugs (γ-secretase inhibitors [GSIs]) can inhibit its activity. Because many oncogenic forms of Notch receptors are ligand independent but still require γ-secretase processing, multiple groups used GSIs in the intestine of normal or cancer-prone mouse models and found these drugs induce goblet metaplasia of the proliferative crypt epithelium,37,45-47 phenocopying the intestinal phenotypes of mice lacking Dll1/Dll4, Mib1, or Pofut. Thus, both genetic and pharmacologic disruption of the Notch pathway reveals its essential role in intestinal homeostasis. These exiting findings have led to the concept that pharmacologic disruption with GSIs may be used as differentiation therapy to treat colorectal cancer. However, because GSI treatment depletes ISCs, there remains only a limited therapeutic window. Novel findings discussed in the following text may open this window wider.
The Common Effector RBP-Jκ
To directly address the role of canonical Notch signaling in the gut, van Es et al analyzed the phenotype of mice lacking RBP-Jκ, the common effector of all 4 Notch receptors, and found that induced loss of RBP-Jκ in the adult intestine also caused a massive goblet metaplasia in the proliferative crypt epithelium and villi.37 Collectively, these data point to the canonical axis as important for stem cell maintenance and multipotency; in the absence of Notch signaling, no self-renewal can occur and all the cells assume a single postmitotic default fate (the goblet cell). Interestingly, and similar to the response to NICD in postmitotic villus cells,34 Notch activation also leads to goblet metaplasia in lung epithelium.48 These contradicting behaviors underscore another important aspect of Notch signaling: Notch signaling is used reiteratively in lineage specification, but the outcome is context specific. How does Notch regulate intestinal homeostasis?
Activation of Notch in ISCs
Consistent with the fact that Dll1 and Dll4 are the key ligands for Notch1 receptors in the crypt epithelium that control intestinal homeostasis,39 Paneth cells, which directly abut CBC cells in the crypt base, express Dll4 and support the growth of Lgr5+ CBCs in vitro and regulate their numbers in vivo.49 Long-term renewal of ISCs in vitro is promoted by Notch agonists50 and abrogated on Notch inhibition by GSIs.51 Paneth cells are also required for the stem cell niche because both in vitro and in vivo experiments show that Paneth cell ablation affects stem cell number and long-term survival.49 Whether Paneth cells also support renewal of their Bmi1+ neighbors through Notch-Delta signaling is not known.14 These experiments now uncovered the ISC niche. Initially, the niche was believed to be mesenchymal; instead, it is composed by the stem cell progeny, the Paneth cells, which produce Wnt (Wnt3a) and Notch ligands (Dll4). A role for Paneth cells in the renewal of CBCs was also hypothesized decades ago based on the anatomic proximity of the Paneth cells to the CBC stem cells at the crypt base.52 Because Notch signaling requires cell-cell contact and endocytosis to unfold the juxtamembrane domain, the proximity of the Paneth cells to the CBCs now makes perfect mechanistic sense. Indeed, lineage tracing with a Notch1-specific activity reporter line NIP1::Cre (Notch1 intramembrane proteolysis Cre) already suggested long-term repopulation and multilineage specification of crypt cells with Notch1 activity.30 Recent pulse-chase experiments with a tamoxifen-controlled version of NIP::Cre (NIP::CreERT2) confirms this, showing that a single tamoxifen injection labeled progeny even after 8 months.39
Whether stem cell renewal in the colon is similarly regulated through Dl-Notch signaling remains unexplained, because there are no Paneth cells in the colon to support the ISCs. Sato et al propose that CD24+-expressing cells in the colon crypt base adjacent to Lgr5+ ISCs may function similarly to the CD24+ Paneth cell niche in the small intestine.49 Feedback signaling from TA to stem cells or from a differentiated goblet cell back to the ISCs may also activate Notch signaling. Furthermore, histologic examination failed to detect CBCs and Paneth cells in the gut of some mammalian species, suggesting that other ISC paradigms may exist as well.15 Finally, with Paneth cells at the center stage of host-microbe interactions, inflammatory disease and neoplastic growth in the intestine are hardwired.
Hes1
One of the first functional clues about the role of Notch signaling in intestine development comes from the analysis of Hes1 knockout mice. Hes proteins are transcriptional repressors that are direct targets of NICD/RBP-Jκ and expressed in the proliferative CBCs in the crypt base.53 Hes1 deletion causes perinatal lethality and a modest increase in all endocrine lineages in the intestinal epithelium at the expense of ECs compared with complete absence of Notch.24,37 This suggests that other Hes/Hey family members may partially compensate for Hes1 loss. For example, Hes5, normally not expressed in the crypts, is induced in Hes1-deficient intestines, like the expression of Hes3 and Hes7. At present, however, nothing is known about the function of these proteins in the intestine, but they likely play overlapping roles in suppressing endocrine differentiation. Hes1 deficiency also leads to derepression of Hes1 targets such as Atonal/Math1.24
Math1, Master of Secretory Fate
Math1 is an activator class bHLH gene required for the differentiation of all secretory cell lineages in intestine, including tuft cells.5 Math1 deletion leads to perinatal lethality and the expansion of epithelial progenitors at the expense of all secretory lineages, showing that Math1 is required also for the cell cycle exit of crypt progenitors.54,55 Conversely, overexpression of Math1 promotes premature secretory cell fate induction and proliferative arrest at the expense of absorptive cells.56 γ-Secretase inhibition or RBP-Jκ deletion does not cause goblet metaplasia in Math1-deficient intestine, showing that the key to Notch function is its ability to suppress Math1 in uncommitted TA progenitors.57,58 Thus, a conserved mechanism of lateral inhibition observed first in the fly ectoderm also controls the vertebrate endoderm lineage.59
Achaete/Scute Complex Maintaining Stemness
Mammalian homologues of the fly Achaete/Scute Complex (AS-C) genes are bHLH proteins implicated in cell fate choices controlled by canonical (Delta-Notch-RBPjk-Hes1) signaling.60 Achaete Scute-like 2 (Ascl2) is expressed in Lgr5+ columnar base ISCs.61 Unlike in the fly nervous system, Ascl2 does not act as an antiproliferative, differentiation-promoting proneural bHLH in the gut. Disruption of Ascl2 expression causes loss of Lgr5+ ISCs, whereas overexpression causes crypt hyperplasia and expansion of Lgr5+ populations and expansion of immature progenitors into the villus epithelium. Ascl2 is a direct transcriptional target of TCF4, a member of the Wnt pathway that maintains the stemness of Lgr5+ cells.62 Dl-Notch signaling is also required for stem cell maintenance and for Ascl2 expression.39 In the epidermis, Notch signals engage in both positive and negative regulation of Acl2 via an incoherent feedback loop: Ascl2 is repressed by Hes1 as well as activated by RBP-Jκ.63 It is probable that similarly complex interactions between Notch and Ascl2 also occur in the intestine; however, a formal demonstration of this is lacking.61,62
Neurogenin3, EE Lineage Specification
Atoh5/Neurogenin3 (Ngn3) is another bHLH protein expressed in proliferating endocrine progenitors located at the crypt base and strongly up-regulated in the absence of Hes124; NGN3 is required for EE differentiation in the intestine.64 ECs and goblet, Paneth, and tuft cells develop normally in the absence of Ngn3, and Math1 expression is unaffected. In contrast, markers for neuroendocrine cells (Chromogranin-A, NeuroD) are lacking. Lineage tracing experiments in NGN3 knockouts also show a role for NGN3 in the establishment of EE precursor.64,65 Significantly, in the absence of Ngn3, goblet cell numbers are increased 3-fold. These results reinforce the notion that the goblet cell fate is a default secretory program of multipotent progenitors and that commitment to other secretory sublineages requires additional cues.
KLF-4 and SPDEF Specify the Goblet Cell
The goblet cell fate is driven by additional transcription factors; chief among them are Krüppel-like factor 4 (KLF4)66 and the SAM pointed domain ETS transcription factor (SPDEF).67 Hes1 represses KLF4 directly; inhibition of Notch signaling by GSIs causes goblet cell metaplasia in part due to the loss of KLF4 repression.47,68,69 These data have recently been challenged because intestinal disruption of KLF4 in the adult intestine does not affect secretory differentiation and absence of KLF4 does not block goblet cell metaplasia in RBP-Jκ-deficient or GSI-treated intestines.39 Because goblet cell metaplasia does not occur in compound Notch/Math1-deficient adult intestines,58 KLF4 acts downstream of Math1 and probably in a redundant manner. These seemingly contradictory observations may be reconciled by the findings of Kim, who studied compound RBP-Jκ/Math1 mutant intestines. Absence of Math1 in Notch-deficient intestines led to the up-regulation of Hes1, suggesting that Hes1 may act both upstream and downstream of Math1 to regulate KLF4.57 Activation of SPDEF in Math1-expressing cells is sufficient to induce goblet differentiation,70 a function that requires the downstream transcription factor Gfi1.67,71 These findings place SPDEF and Gfi1 downstream of Math1 in the selection of EE versus goblet/Paneth cell fate. In the absence of Gfi1, NGN3 is more active and EE fate expands at the expense of goblet/Paneth cells. SPEDF is not absolutely required for goblet cells because some still form in SPDEF-deficient intestines.67 In the absence of SPDEF, committed secretory cells fail in terminal differentiation toward goblet and Paneth cell fate, leading to accumulation of Math1/Dll1-expressing cells in both SPDEF-deficient crypt progenitors and the villi. This supports the notion that differentiated, Dll1-expressing committed secretory cells signal back to Notch-expressing progenitors to gate their fate choice in the proliferative crypt environment. This provides an elegant feedback mechanism to control cell number and fate by signaling from differentiated cells to progenitors, analogous to the Paneth-CBC interaction in the crypt. Altogether, these findings suggest a tightly controlled program of Notch-Math1 axis to suppress secretory cell differentiation.
The Drosophila midgut72 has provided an interesting model system for ISC homeostasis via lateral inhibition; however, significant differences exist, which are described in the next section.
Notch and ISCs in Flies
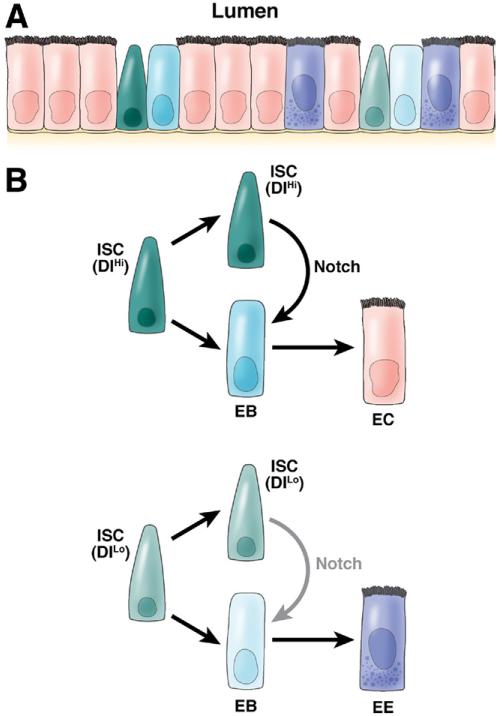
In Drosophila melanogaster, the posterior midgut and the hindgut are the functional equivalents of the small and large intestine, respectively.73,74 In the midgut, large polyploid ECs make up the majority of cells in this simple pseudostratified epithelium and are interspersed with diploid EE cells and enteroblasts (EBs). Using lineage tracing, ISCs were identified, which are multipotent, proliferate vigorously, and produce without further division EC and EE cells.73,74 When ISCs divide, this produces 2 daughters: one that remains undifferentiated and another, the EB, that further differentiates into either an EE cell or EC. The EB is the equivalent of a bipotent progenitor in mammalian intestine except that it is postmitotic.73,74 The diploid ISCs resemble the wedge-shaped CBC/Lgr5+ stem cells in the mammalian intestine.11,73,74 Also, more dispersed clones composed of differentiated cells were observed that dissipate and turnover within a week due to apoptotic cell loss, reminiscent of the shedding in the mammalian villus epithelium. Thus, the fly midgut is a simplified version of the mammalian small intestine lacking TA cells (Figure 3).
Figure 3. Drosophila posterior midgut and role of Notch in ISC renewal.
(A) The Drosophila posterior midgut is a relatively simple pseudostratified epithelium, with only 2 differentiated cell types: ECs and EE cells. In addition, ISCs and their nonproliferative daughters, EBs, are scattered in between. (B) Two populations of ISCs exist: one with high Delta expression (ISC [DlHi]) and one with no or low Delta expression (ISC [DlLo]). When they divide, both give one daughter ISC and one daughter EB cell. However, the daughter ISC from ISC (DlHi) will activate a high level of Notch signaling in its sister EB cells, which drives its differentiation into EC cells; in contrast, the daughter ISC from ISC (DlLo) elicits a low level of Notch signaling in its sister EB cells, which will differentiate into EE cells. Thus, different levels of Notch activation elicit different cell fates.
In the fly posterior midgut, Notch is expressed in the ISC and EB but not in the mature EE cell and EC74; in contrast, one of Notch ligands, Dl, is only expressed in the ISC.72 After asymmetrical division of the ISC, Dl is retained in the newly formed ISC and activates the Notch signaling pathway in the newly formed EBs. There are 2 populations of ISCs with a different expression level of Dl. Consequently, EBs derived from different ISCs experience different levels of Notch activation. Interestingly, the difference in the activation level of Notch in EBs is further translated into different cell fates. A high level of Notch activation drives EBs to differentiate into ECs; in contrast, a low level of Notch activation promotes an EE cell fate.72 Consistently, pharmacologic and genetic inhibition of Notch activity causes increased endocrine differentiation and a reduction of ECs. In addition, loss of Notch function further induces continuous proliferation and expansion of ISC cells, suggesting that Notch signaling prevents ISCs/EBs from proliferating. This pro-differentiation role of Notch in Drosophila ISCs contrasts with its important role in self-renewal of the Lgr5+ ISCs in the mammalian gut. In summary, Notch has a dual context-dependent function; in committed (EB) cells it suppresses the EE differentiation, whereas in the ISCs/EBs it appears to block proliferation. Not only is Notch signaling important in the maintenance of the stem cell niche, but it may also be necessary to establish the stem cell niche.75 Mathur et al have shown that all intestinal lineages arise from adult midgut progenitors (AMPs) during larval development. AMPs divide symmetrically and produce diploid Dl+ cells. At some point, AMPs divide and produce 2 daughters: one that expresses Dl and the other Notch/Su(H). Whether the division is asymmetric (ie, producing daughters with different fates due to distribution of Notch components before cytokinesis) or whether asymmetry is established after division is not yet known. This Notch receptor-expressing cell develops as the peripheral cell (PC), which wraps around the Dl+ cells and through dpp/BMP signaling suppresses the differentiation of progenitors expanding in the so-called AMP islands. At later stages, the PC breaks down and undergoes apoptosis. Most Dl+ AMPs differentiate into ECs, but a single AMP per island remains undifferentiated, maintains Dl expression, and is marked to become the future ISC.75 In contrast, BMP inhibition via expression of Noggin in the adult villus mesenchyme of mice leads to the formation of ectopic hyperproliferative crypts.76 Although both in flies and mammals, ISCs generate their own niche (Paneth cell vs PC), the direction of Notch signaling between the niche and ISC is reversed (Figure 3).
Wnt and Notch: An Essential Pair
The Wnt-β-catenin signaling cascade is a master regulator of embryonic development and adult tissue homeostasis in all animals and frequently deregulated in pathological conditions such as cancer.77 Like Notch signaling, Wnt signaling is context dependent and can promote proliferation and block differentiation but also induce and maintain differentiated cell fates.78 There is significant cross talk between these pathways during intestinal homeostasis. Overexpression of NICD1 in intestinal epithelia results in a complete block of goblet cell differentiation and excessive expansion of mouse crypt progenitors.33 Whereas the proliferative effect of NICD depends on canonical Wnt signaling, suppression of secretory cell differentiation still occurs in the Tcf4-deficient intestine.79 The expression of TCF4/Lef1 is not affected by overexpression of Notch1.33 Thus, these knockout studies show Wnt signaling is more important in controlling proliferation while Notch is more important in suppressing cell fate. However, a more complex picture is emerging; genetic interactions between Wnt and Notch in fly development are well documented (reviewed by Nakamura et al80). The most compelling evidence in support of a direct biochemical interaction between Wnt-Notch signaling pathways is provided by GSK3β, a kinase, which phosphorylates β-catenin and marks it for degradation. GSK3β has also been shown to phosphorylate NICD, leading to its degradation in some contexts.81 Conversely, Notch has also been shown to activate GSK3β, leading to β-catenin degradation in other contexts.42 Therefore, GSK3β may function as a hub between Notch and Wnt. Further, Delta and Jagged ligands are transcriptionally regulated by Wnt-β-catenin through multiple TCF/Lef binding sites in their promoters.82-84 Because Wnt signaling is not only required for Paneth cell specification but also for its maintenance,85 active Wnt signaling may drive Dll4 ligand expression. Additional regulation of the Notch pathway by Wnt in intestinal cells may occur through direct transcriptional regulation of the Wnt target gene Musashi (Msi1) expressed in CBCs.86,87 Msi1 is activated in intestinal epithelial cells by Wnt3a ligand, which in turn activates both Lgr5 and/or Bmi1 expression as well as β-catenin and may affect Notch signaling in mammalian cells as it does in flies.88 In mammals, a mechanistic understanding of how Msi1 interacts with Notch is lacking.
Linking Notch and Colorectal Cancer
Notch signaling is frequently deregulated in human cancers, and activating mutations are common in acute T-cell leukemias.89,90 Notch1 receptor, ligands, and target genes are expressed in adenomas from Apc (adenomatous polyposis coli) mutant mice37 in colorectal cancer cell lines and primary carcinomas.83,91,92 Elaborate expression studies in human adenocarcinomas revealed that Notch1, Jag1, and Hes1 expression were often present in colorectal cancers, but there was no significant difference in survival between Hes1-expressing tumors and nonexpressing tumors.93 Other studies report reduced Hes1 expression in colon carcinomas and corresponding metastases compared with adenomas.79,94 Interestingly, Notch1 and Notch2 expression seemed to have an opposite but interdependent prognostic value in colon cancer survival. High Notch1 expression is associated with poor survival, whereas high Notch2 expression is associated with better survival.95,96 Lineage tracing in Apcmin mice has shown that the tumor-initiating cells in Apc−/− adenomas arise from Notch1 marked clones.30 Indeed, CBC/Lgr5+ cells highly express Notch1 receptors49 and have properties of tumor-initiating cells.97 Collectively, these data argue that Notch activation is an initiating event during colorectal cancer progression, but whether activation of the Notch pathway is required for maintenance and spread of tumors is uncertain. Unlike in T-ALL, mutations in Notch receptors are not common in colorectal cancers.98,99 Thus, alternative mechanisms must exist to explain the frequent involvement of Notch signaling in colon cancer. One important mechanism is the ability of Wnt/β-catenin to activate Notch ligands. Notch ligands are direct targets of β-catenin-TCF4 and therefore also commonly expressed in colorectal adenomas from Apcmin mice.31,79,100 Activation of Notch ligands by Wnt is critical for the growth of colorectal adenomas and cancers with activated Wnt/β-catenin. Moreover, reducing the dosage of Jagged1 reduces the incidence of adenoma.83,92 Notch activation is also important in vitro for expansion of normal ISCs (Lgr5+) as well as tumor-initiating cells from colorectal cancer specimens.50,101 More recently, Sonoshita showed that the expression of AES (Amino terminal Enhancer of Split) protein, a repressor of Notch activity, is frequently down-regulated in human colon liver metastases.102 Also, in the corresponding primary tumors, AES expression was low, particularly at the “invasive front,” a region characterized by high Wnt-β-catenin-TCF signaling, loss of adhesion receptors such as E-cadherin, and mesenchymal transformation.103,104 Loss of AES also promotes the formation of highly invasive adenomas in Apc mutant mice. How AES acts to repress Notch is unclear and how AES itself is regulated is not known. Thus, AES is a colorectal cancer metastasis suppressor acting most likely through Notch. Taken together, these results may point to a continued requirement of Notch ligand signaling to sustain the growth of cancer cells with ISC properties. This hypothesis would be consistent with the observed heterogeneity in Notch1 activity in carcinomas because they are largely composed of differentiated cells not relying on Notch signaling.
Targeting Notch in Colon Cancer
Pharmacologic blockade of Notch signaling using GSI (preventing Notch intramembrane cleavage) or genetic ablation in rodent intestine induces a proliferative arrest and goblet cell metaplasia.45-47,83,92 This prompted the exploration of GSIs in the treatment of colorectal cancers. Indeed, GSI treatment of Apcmin adenoma-bearing mice results in reduced adenoma proliferation and induced their differentiation along the secretory lineage.37 Nevertheless, the following facts challenge the common view and hope that GSIs may be used as differentiation therapy in the treatment of colon cancer.
First, GSI treatment causes ISC depletion and massive goblet conversion, an irreversible and unwanted side effect of GSI, caused by derepression of Math1 and subsequent induction of KLF4.69,105,106 Goblet cell metaplasia can be suppressed by glucocorticoid treatment, which blocks KLF4 activity independent of Notch/Hes. This elegant finding may be applied to limit gut toxicity during GSI treatment while maintaining the effect of GSI on Notch effectors.69
Second, some patients with colon cancer may be refractory to GSI treatment. HATH1 (the human Homologue of Math1/Atonal) expression is frequently lost in colorectal cancer by methylation or genomic deletion.106-108 Therefore, GSIs would be expected to be ineffective in tumors lacking HATH1. Furthermore, there are also subgroups of mucinous colorectal tumors in which expression of HATH1 is maintained.109 The most likely explanation is that Notch signaling is lost or inactive in a subpopulation of these tumors and therefore GSIs may also be ineffective.
Third, whereas RBP-Jκ activity is essential for normal crypt epithelium, it appears dispensable for the transformation of Apc-deficient cells.110 One possible explanation for this interesting finding is that Hes1 is still expressed in the RBP-Jκ/Apc mutant intestine, possibly activated by β-catenin/TCF.110 Although Math1 is required for GSI-induced goblet metaplasia in wild-type intestines,58 blocking Notch may not suffice to restore Math1 activity in colon cancers because it is posttranscriptionally repressed by activated Wnt/β-catenin signaling independent of Notch activity.111 A direct role for canonical Wnt signaling in secretory cell fate specification (through Math1) had already been observed in mice with intestinal expression of the Wnt inhibitor Dickkopf, which showed decreased secretory cell numbers.112 If true, these findings have important implications for the use of GSIs in colon cancer treatment and suggest that targeting Math1 would be more beneficial.
Finally, systemic inhibition of Notch signaling can lead to the development of epidermal and endothelial tumors.113-116 Thus, there is an urgent need for refinement of drugs targeting Notch signaling in cancers. For example, monoclonal antibodies against Notch1 or Notch2 effectively block adenoma growth in mouse models without inducing intestinal toxicity.117 Nonetheless, such Notch1-specific antibodies, like those that target Notch ligand Dll4, may still induce endothelial tumors after long-term administration.116,118
In summary, further insight into the repertoire of receptors and ligands characterizing specific tumors and their microenvironment is needed to identify unique combinations from which tumor specificity may be attained, but targeting Notch may after all not be that straightforward.
Conclusions
Intestinal biology has provided a wealth of insight into the role of cell fate signaling through Notch receptors. Surprisingly, the mammalian endoderm resembles the fly ectoderm in the manner in which Notch signaling is used. Notch signaling is both the landscape architect and the stem cell guardian. The close interplay between Notch and Wnt signaling sculpts the crypt-villus system and maintains control over self-renewal during normal homeostasis. Gain of Wnt invariably leads to gain of Notch via ligand activation to drive colon cancer development. Although much is known, we are still short of developing effective Notch-based therapies due to its critical and sometimes opposing functions within tissues. A detailed understanding of this context is essential in the design of cell type-specific drugs targeting the Notch pathway.
Acknowledgments
The authors thank B. Wouters (Ontario Cancer Institute, Toronto, Canada) and A. Begg (NKI, Amsterdam, The Netherlands) for their helpful comments on the manuscript.
Funding: R.K. and Z.L. were supported by National Institutes of Health grant R01 DK066408, and M.V. was supported by the Dutch Cancer Society (UU2006-3623) and the European Research Council under the European Community Seventh Framework Programme (FP7/2007-2013/ERC grant 208259).
Abbreviations used in this paper
- AES
Amino terminal Enhancer of Split
- AMP
adult midgut progenitor
- bHLH
basic helix-loop-helix
- CBC
columnar base cell
- Dll
Delta-like ligand
- EB
enteroblast
- EC
enterocyte
- EE
enteroendocrine
- GSI
γ-secretase inhibitor
- Hes
Hairy Enhancer of Split
- ISC
intestinal stem cell
- KLF4
Krüppel-like factor 4
- Mib1
Mindbomb-1
- NICD
Notch intracellular domain
- PC
peripheral cell
- Pofut
O-fucosyl transferase1
- SPDEF
SAM pointed domain ETS transcription factor
- Su(H)
Suppressor of Hairless
- TA
transit amplifying
Footnotes
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Vries RG, Huch M, Clevers H. Stem cells and cancer of the stomach and intestine. Mol Oncol. 2010;4:373–384. doi: 10.1016/j.molonc.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Bjerknes M. Cell production in mouse intestinal epithelium measured by stathmokinetic flow cytometry and Coulter particle counting. Anat Rec. 1983;207:427–434. doi: 10.1002/ar.1092070305. [DOI] [PubMed] [Google Scholar]
- 4.Totafurno J, Bjerknes M, Cheng H. The crypt cycle. Crypt and villus production in the adult intestinal epithelium. Biophys J. 1987;52:279–94. doi: 10.1016/S0006-3495(87)83215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 7.Ponder BA, Schmidt GH, Wilkinson MM, et al. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt GH, Wilkinson MM, Ponder BA. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985;40:425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 9.Winton DJ, Blount MA, Ponder BA. A clonal marker induced by mutation in mouse intestinal epithelium. Nature. 1988;333:463–466. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- 10.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 14.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 18.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprinzak D, Lakhanpal A, Lebon L, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol. 2009;29:5679–5695. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Tetering G, van Diest P, Verlaan I, et al. Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J Biol Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiyanont K, Wales TE, Aste-Amezaga M, et al. Evidence for increased exposure of the notch1 metalloprotease cleavage site upon conversion to an activated conformation. Structure. 2011;19:546–554. doi: 10.1016/j.str.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 24.Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 25.Heitzler P, Bourouis M, Ruel L, et al. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development. 1996;122:161–171. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- 26.Schroder N, Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002;2:247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 27.Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004;52:509–516. doi: 10.1177/002215540405200409. [DOI] [PubMed] [Google Scholar]
- 28.Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Crosnier C, Vargesson N, Gschmeissner S, et al. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- 30.Vooijs M, Ong CT, Hadland B, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanger BZ, Datar R, Murtaugh LC, et al. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 34.Zecchini V, Domaschenz R, Winton D, et al. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691. doi: 10.1101/gad.341705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol. 2003;4:786–97. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- 36.Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A. 2003;100:5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 38.Guilmeau S, Flandez M, Bancroft L, et al. Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology. 2008;135:849–860. 860 e1–6. doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1- and Dll4-mediated Notch signaling is required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. 1240 e1–7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song R, Kim YW, Koo BK, et al. Mind bomb 1 in the lymphopoietic niches is essential for T and marginal zone B cell development. J Exp Med. 2008;205:2525–2536. doi: 10.1084/jem.20081344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh M, Kim CH, Palardy G, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 42.Koo BK, Lim HS, Chang HJ, et al. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology. 2009;137:145–155. 155 e1–3. doi: 10.1053/j.gastro.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Koo BK, Yoon MJ, Yoon KJ, et al. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS One. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 45.Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 46.Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 47.Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 48.Guseh JS, Bores SA, Stanger BZ, et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 51.Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat. 1981;160:51–63. doi: 10.1002/aja.1001600105. [DOI] [PubMed] [Google Scholar]
- 53.Potten CS, Booth C, Tudor GL, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 54.Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 55.Shroyer NF, Helmrath MA, Wang VY, et al. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 56.VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim TH, Shivdasani RA. Genetic evidence that intestinal notch functions vary regionally and operate through a common mechanism of math1 repression. J Biol Chem. 2011;286:11427–11433. doi: 10.1074/jbc.M110.188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Es JH, de Geest N, van de Born M, et al. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1:18. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- 60.Jan YN, Jan LY. Genetic control of cell fate specification in Drosophila peripheral nervous system. Annu Rev Genet. 1994;28:373–393. doi: 10.1146/annurev.ge.28.120194.002105. [DOI] [PubMed] [Google Scholar]
- 61.van der Flier LG, van Gijn ME, Hatzis P, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 62.Van der Flier L, Sabates-Bellver J, Oving I, et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 63.Moriyama M, Durham AD, Moriyama H, et al. Multiple roles of Notch signaling in the regulation of epidermal development. Dev Cell. 2008;14:594–604. doi: 10.1016/j.devcel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Katz JP, Perreault N, Goldstein BG, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregorieff A, Stange DE, Kujala P, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 68.Ghaleb AM, Aggarwal G, Bialkowska AB, et al. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6:1920–1927. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noah TK, Kazanjian A, Whitsett J, et al. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shroyer NF, Wallis D, Venken KJ, et al. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 73.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 74.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 75.Mathur D, Bost A, Driver I, et al. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 77.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 78.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 79.Fre S, Pallavi SK, Huyghe M, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- 81.Espinosa L, Ingles-Esteve J, Aguilera C, et al. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 82.Galceran J, Sustmann C, Hsu SC, et al. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 2004;18:2718–2723. doi: 10.1101/gad.1249504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodilla V, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, et al. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 2004;18:2712–2717. doi: 10.1101/gad.1248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Es JH, Jay P, Gregorieff A, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 86.Kayahara T, Sawada M, Takaishi S, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 87.Rezza A, Skah S, Roche C, et al. The overexpression of the putative gut stem cell marker Musashi-1 induces tumorigenesis through Wnt and Notch activation. J Cell Sci. 2010;123:3256–3265. doi: 10.1242/jcs.065284. [DOI] [PubMed] [Google Scholar]
- 88.Okabe M, Imai T, Kurusu M, et al. Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature. 2001;411:94–98. doi: 10.1038/35075094. [DOI] [PubMed] [Google Scholar]
- 89.Koch U, Radtke F. Notch signaling in solid tumors. Curr Top Dev Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- 90.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez-Majada V, Aguilera C, Villanueva A, et al. Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer. Proc Natl Acad Sci U S A. 2007;104:276–281. doi: 10.1073/pnas.0606476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guilmeau S, Flandez M, Mariadason JM, et al. Heterogeneity of Jagged1 expression in human and mouse intestinal tumors: implications for targeting Notch signaling. Oncogene. 2010;29:992–1002. doi: 10.1038/onc.2009.393. [DOI] [PubMed] [Google Scholar]
- 93.Reedijk M, Odorcic S, Zhang H, et al. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33:1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veenendaal LM, Kranenburg O, Smakman N, et al. Differential Notch and TGFbeta signaling in primary colorectal tumors and their corresponding metastases. Cell Oncol. 2008;30:1–11. doi: 10.1155/2008/839076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu D, Li Y, Wang W, et al. High level of Notch1 protein is associated with poor overall survival in colorectal cancer. Ann Surg Oncol. 2010;17:1337–1342. doi: 10.1245/s10434-009-0893-7. [DOI] [PubMed] [Google Scholar]
- 96.Chu D, Zhang Z, Zhou Y, et al. Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol. 2011;22:2440–2447. doi: 10.1093/annonc/mdq776. [DOI] [PubMed] [Google Scholar]
- 97.Merlos-Suarez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 98.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 99.Lee SH, Jeong EG, Yoo NJ. Mutational analysis of NOTCH1, 2, 3 and 4 genes in common solid cancers and acute leukemias. APMIS. 2007;115:1357–1363. doi: 10.1111/j.1600-0463.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 100.van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med. 2005;11:496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Sikandar SS, Pate KT, Anderson S, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sonoshita M, Aoki M, Fuwa H, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Christofori G. Metastatic colon cancer cells negotiate the intravasation Notch. Cancer Cell. 2011;19:6–8. doi: 10.1016/j.ccr.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Brabletz T, Jung A, Hermann K, et al. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 105.Pannequin J, Bonnans C, Delaunay N, et al. The wnt target jagged-1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells. Cancer Res. 2009;69:6065–6073. doi: 10.1158/0008-5472.CAN-08-2409. [DOI] [PubMed] [Google Scholar]
- 106.Leow CC, Romero MS, Ross S, et al. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–6057. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- 107.Bossuyt W, Kazanjian A, De Geest N, et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7:e39. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng X, Tsuchiya K, Okamoto R, et al. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis. 2011;17:2251–2260. doi: 10.1002/ibd.21611. [DOI] [PubMed] [Google Scholar]
- 109.Park ET, Oh HK, Gum JR, Jr, et al. HATH1 expression in mucinous cancers of the colorectum and related lesions. Clin Cancer Res. 2006;12:5403–5410. doi: 10.1158/1078-0432.CCR-06-0573. [DOI] [PubMed] [Google Scholar]
- 110.Peignon G, Durand A, Cacheux W, et al. Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–176. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peignon G, Durand A, Cacheux W, et al. Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–176. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rangarajan A, Talora C, Okuyama R, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 116.Liu Z, Turkoz A, Jackson EN, et al. Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J Clin Invest. 2011;121:800–808. doi: 10.1172/JCI43114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 118.Yan M, Callahan CA, Beyer JC, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]