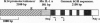Abstract
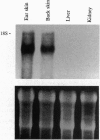
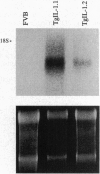
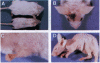
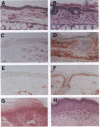
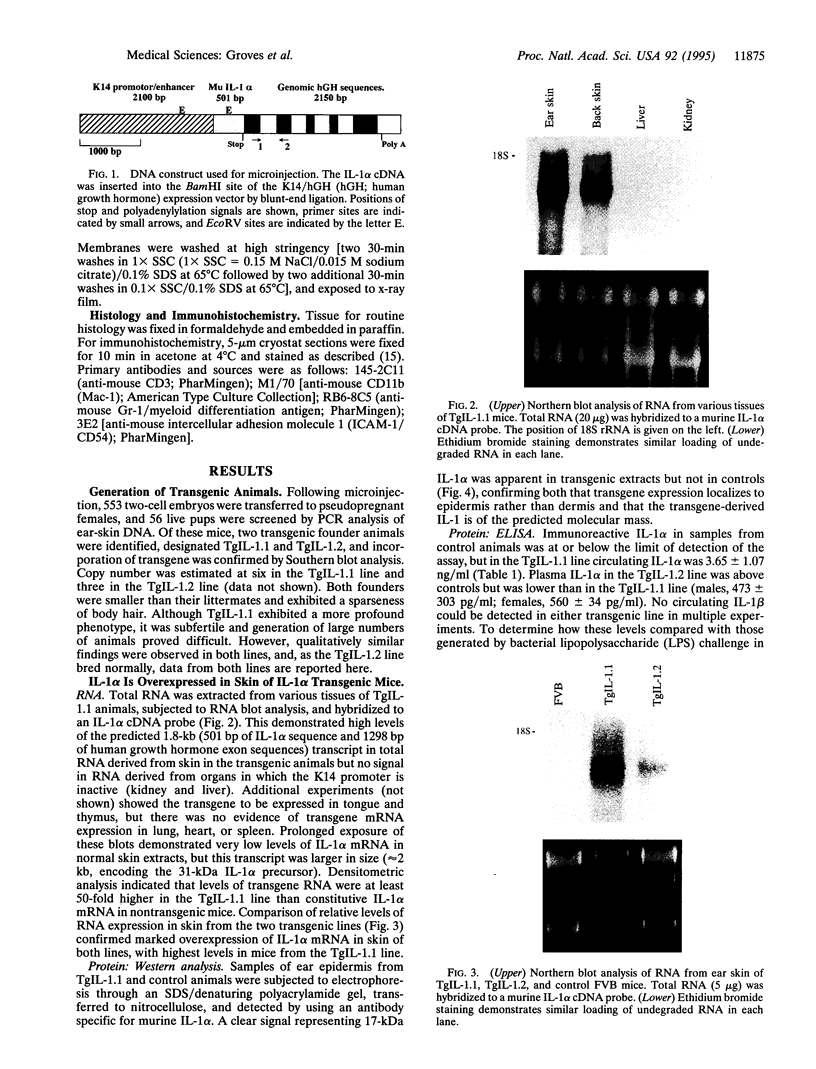
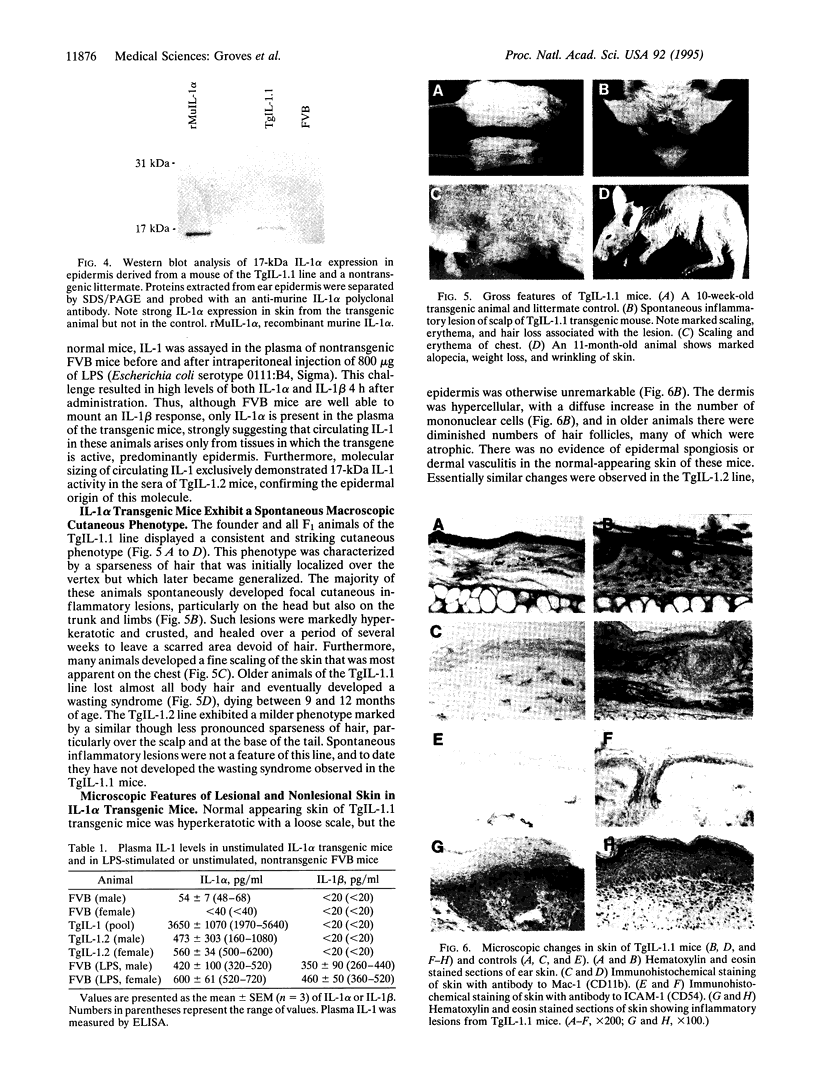
Resting epidermal keratinocytes contain large amounts of interleukin 1 (IL-1), but the function of this cytokine in the skin remains unclear. To further define the role of IL-1 in cutaneous biology, we have generated two lines of transgenic mice (TgIL-1.1 and TgIL-1.2) which overexpress IL-1 alpha in basal keratinocytes. There was high-level tissue-specific expression of transgene mRNA and protein and large quantities of IL-1 alpha were liberated into the circulation from epidermis in both lines. TgIL-1.1 mice, which had the highest level of transgene expression, developed a spontaneous skin disease characterized by hair loss, scaling, and focal inflammatory skin lesions. Histologically, nonlesional skin of these animals was characterized by hyperkeratosis and a dermal mononuclear cell infiltrate of macrophage/monocyte lineage. Inflammatory lesions were marked by a mixed cellular infiltrate, acanthosis, and, in some cases, parakeratosis. These findings confirm the concept of IL-1 as a primary cytokine, release of which is able to initiate and localize an inflammatory reaction. Furthermore, these mice provide the first definitive evidence that inflammatory mediators can be released from the epidermis to enter the systemic circulation and thereby influence, in a paracrine or endocrine fashion, a wide variety of other cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest. 1990 Feb;85(2):605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysk M. M., Bell T., Tyring S. K., Rajaraman S. Cytokines modulate terminal differentiation and the expression of predesquamin in cultured keratinocytes. Exp Cell Res. 1994 Mar;211(1):49–52. doi: 10.1006/excr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Camp R., Fincham N., Ross J., Bird C., Gearing A. Potent inflammatory properties in human skin of interleukin-1 alpha-like material isolated from normal skin. J Invest Dermatol. 1990 Jun;94(6):735–741. doi: 10.1111/1523-1747.ep12874591. [DOI] [PubMed] [Google Scholar]
- Cheng J., Turksen K., Yu Q. C., Schreiber H., Teng M., Fuchs E. Cachexia and graft-vs.-host-disease-type skin changes in keratin promoter-driven TNF alpha transgenic mice. Genes Dev. 1992 Aug;6(8):1444–1456. doi: 10.1101/gad.6.8.1444. [DOI] [PubMed] [Google Scholar]
- Cooper K. D., Hammerberg C., Baadsgaard O., Elder J. T., Chan L. S., Sauder D. N., Voorhees J. J., Fisher G. IL-1 activity is reduced in psoriatic skin. Decreased IL-1 alpha and increased nonfunctional IL-1 beta. J Immunol. 1990 Jun 15;144(12):4593–4603. [PubMed] [Google Scholar]
- Dowd P. M., Camp R. D., Greaves M. W. Human recombinant interleukin-1 alpha is proinflammatory in normal human skin. Skin Pharmacol. 1988;1(1):30–37. doi: 10.1159/000210749. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R. W., Allen M. H., Ross E. L., Barker J. N., MacDonald D. M. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995 Mar;132(3):345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Groves R. W., Giri J., Sims J., Dower S. K., Kupper T. S. Inducible expression of type 2 IL-1 receptors by cultured human keratinocytes. Implications for IL-1-mediated processes in epidermis. J Immunol. 1995 Apr 15;154(8):4065–4072. [PubMed] [Google Scholar]
- Groves R. W., Ross E., Barker J. N., Ross J. S., Camp R. D., MacDonald D. M. Effect of in vivo interleukin-1 on adhesion molecule expression in normal human skin. J Invest Dermatol. 1992 Mar;98(3):384–387. doi: 10.1111/1523-1747.ep12499816. [DOI] [PubMed] [Google Scholar]
- Groves R. W., Sherman L., Mizutani H., Dower S. K., Kupper T. S. Detection of interleukin-1 receptors in human epidermis. Induction of the type II receptor after organ culture and in psoriasis. Am J Pathol. 1994 Nov;145(5):1048–1056. [PMC free article] [PubMed] [Google Scholar]
- Hammerberg C., Arend W. P., Fisher G. J., Chan L. S., Berger A. E., Haskill J. S., Voorhees J. J., Cooper K. D. Interleukin-1 receptor antagonist in normal and psoriatic epidermis. J Clin Invest. 1992 Aug;90(2):571–583. doi: 10.1172/JCI115896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Martin G., Van Le L., Morris J., Peace A., Bigler C. F., Jaffe G. J., Hammerberg C., Sporn S. A., Fong S. cDNA cloning of an intracellular form of the human interleukin 1 receptor antagonist associated with epithelium. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3681–3685. doi: 10.1073/pnas.88.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C., Saurat J. H., Schmitt A., Jaunin F., Dayer J. M. Interleukin 1 is present in normal human epidermis. J Immunol. 1986 May 1;136(9):3317–3323. [PubMed] [Google Scholar]
- Konnikov N., Pincus S. H., Dinarello C. A. Elevated plasma interleukin-1 levels in humans following ultraviolet light therapy for psoriasis. J Invest Dermatol. 1989 Feb;92(2):235–239. doi: 10.1111/1523-1747.ep12276763. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Thorner J. Secretion of peptides and proteins lacking hydrophobic signal sequences: the role of adenosine triphosphate-driven membrane translocators. Endocr Rev. 1992 Aug;13(3):499–514. doi: 10.1210/edrv-13-3-499. [DOI] [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest. 1990 Dec;86(6):1783–1789. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990 Jun;94(6 Suppl):146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- Köck A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J. C., Luger T. A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990 Dec 1;172(6):1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siders W. M., Klimovitz J. C., Mizel S. B. Characterization of the structural requirements and cell type specificity of IL-1 alpha and IL-1 beta secretion. J Biol Chem. 1993 Oct 15;268(29):22170–22174. [PubMed] [Google Scholar]
- Taketo M., Schroeder A. C., Mobraaten L. E., Gunning K. B., Hanten G., Fox R. R., Roderick T. H., Stewart C. L., Lilly F., Hansen C. T. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K., Kupper T., Degenstein L., Williams I., Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski A., Schwarz T., Neuner P., Krutmann J., Kirnbauer R., Köck A., Luger T. A. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990 Jun;94(6):808–811. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- Vassar R., Rosenberg M., Ross S., Tyner A., Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. R., Kupper T. S. Epidermal expression of intercellular adhesion molecule 1 is not a primary inducer of cutaneous inflammation in transgenic mice. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9710–9714. doi: 10.1073/pnas.91.21.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]