NKT cells undergo a phase of IL-4 secretion as they mature, with the potential to condition surrounding cells as they develop in the thymus.
Keywords: thymus, G4 mice, KN2 mice, α-galactosylceramide
Abstract
We assessed the production of the canonical Th2 cytokine IL-4 by NKT cells directly in vivo using IL-4-substituting strains of reporter mice that provide faithful and sensitive readouts of cytokine production without the confounding effects of in vitro stimulation. Analysis in naïve animals revealed an “innate” phase of IL-4 secretion that did not need to be triggered by administration of a known NKT cell ligand. This secretion was by immature NKT cells spanning Stage 1 of the maturation process in the thymus (CD4+ CD44lo NK1.1− cells) and Stage 2 (CD4+ CD44hi NK1.1− cells) in the spleen. Like ligand-induced IL-4 production by mature cells, this innate activity was independent of an initial source of IL-4 protein and did not require STAT6 signaling. A more sustained level of innate IL-4 production was observed in animals on a BALB/c background compared with a C57BL/6 background, suggesting a level of genetic regulation that may contribute to the “Th2-prone” phenotype in BALB/c animals. These observations indicate a regulated pattern of IL-4 expression by maturing NKT cells, which may endow these cells with a capacity to influence the development of surrounding cells in the thymus.
Introduction
NKT cells are glycolipid-reactive T lymphocytes restricted to the MHC class I-like antigen-presenting molecule CD1d that have the capacity to influence immune responses associated with infection, allergy, autoimmunity, and cancer. Type I NKT cells are defined by a biased TCR repertoire characterized by an invariant Vα14Jα281 chain coupled with Vβ8.2, Vβ7, or Vβ2 in mice or Vα24-Jα18 in conjunction with Vβ11 in humans [1–3]. Loss of type I NKT cells (hereafter referred to simply as “NKT cells”) is a common feature of a variety of autoimmune conditions, suggesting that these cells play a natural role in suppressing self-reactivity. Conversely, NKT cells can also help provoke the stimulation of a spectrum of different immune responses, including the Th2-biased responses that drive allergic airway inflammation, or Th1-biased responses that protect against some microorganisms and provide immunity to tumors [1, 4].
It is not yet clear how these diverse regulatory functions are induced in different disease contexts, but a capacity for NKT cells to rapidly release different cytokine profiles under different environmental conditions is likely to be a central feature. This, in turn, may be influenced by the maturation or activation status of the NKT cells, their physical location, and the existence of phenotypically distinct NKT cell subsets. It would therefore be useful to be able to characterize cytokine expression by NKT cells directly in vivo to help define their function. However, as cytokine activities are often localized and short-lived, faithful analysis of their in vivo expression is often difficult. Typically, cytokine expression has been extrapolated from in vitro restimulation studies on isolated cells or from the analysis of cytokine knockout and transgenic overexpressing mice. However, in vitro analyses are potentially devoid of critical signals present in tissues and are often reliant on potent restimulation, thereby only providing readout of the “potential” of cells to secrete cytokines. In addition, analyses in knockout mice are often confounded by compensatory mechanisms induced in the absence of a given cytokine, and unusual effects of elevated dose may affect analysis of overexpression in transgenic animals.
Recent studies have exploited the use of transgenic animals in which cytokine reporters have been introduced without deleting the cytokine itself. An example is analysis of IL-4, the canonical marker for Th2 cells, in 4get reporter mice, in which a bicistronic construct encoding EGFP linked via an IRES to IL-4 was inserted at the Il4 locus, allowing IL-4 and GFP expression from the same allele [5]. These mice have provided useful information about cytokine production in conventional T cells, including defining the regulatory steps involved in IL-4-biased Th2 responses. However, in NKT cells, reporter expression did not accurately reflect IL-4 protein expression [6]. Rather, as the GFP reporters were very efficiently translated via the IRES sequence, GFP expression reflected the presence of mRNA transcripts but did not correlate with translation of IL-4 protein. Interestingly, as all NKT cells in the periphery were reporter-positive in 4get mice, this system provided strong evidence that NKT cells are poised for potent IL-4 production by virtue of retaining significant levels of preformed Il4 mRNA. Despite these insights, 4get mice remain unsuitable for directly examining IL-4 protein production by NKT cells in vivo.
Here, we report on the use of two IL-4-substituting reporter strains to examine IL-4 production by NKT cells. In G4 mice [7], the first exon and 178 nucleotides of the first intron of the Il4 gene have been replaced with a sequence encoding GFP. In KN2 mice [8], the first two exons of IL-4 have been replaced by a sequence for huCD2. With the use of heterozygous animals, we were able to analyze expression from the transgenic Il4 locus in an IL-4-sufficient environment. As was expected for a useful surrogate of IL-4 protein expression, mature NKT cells in the periphery did not express the reporter unless triggered to do so by administration of the CD1d-binding glycolipid ligand α-GalCer.
Interestingly, in the thymus (and to a lesser extent, in the spleen), both strains did show some constitutive reporter expression in the absence of administration of any ligands. This IL-4 production was confirmed at the protein level and was attributed to NKT cells early in the maturation process. The kinetics of IL-4 production by immature NKT cells were specific to different genetic backgrounds, with production prolonged in Th2-prone BALB/c animals relative to C57BL/6 animals, pointing to a developmental program under different levels of genetic control. This innate triggering of IL-4 production would therefore appear to be part of the developmental process of NKT cells; a program that may, in turn, influence development and conditioning of surrounding cells in the thymus.
MATERIALS AND METHODS
Mice
Breeding pairs of the inbred strains BALB/c and C57BL/6 were obtained from the Animal Resource Centre (Canning Vale, Western Australia). The IL-4 reporter G4 strains (referred to as BALB/c IL-4G4/G4 and C57 IL-4G4/G4 in the text) [7] and STAT6−/− mice were provided by Prof. W. E. Paul (NIH, Bethesda, MD, USA). The BALB/c IL-4G4/G4 mice were crossed to the STAT6−/− mice as described [9]. Also used were the IL-4 reporter KN2 strain (referred to as BALB/c IL-4KN2/KN2) [8], CD1d−/− mice [10], IL-4Rα−/− mice [11], and BALB.B6-Cmv1r mice congenic for C57BL/6 NK1.1 [12]. All experimental protocols were given Ethics Committee approval and performed according to institutional guidelines.
In vivo stimulation of NKT cells
The NKT cell ligand α-GalCer was manufactured as described [13], solubilized at 1 mg/ml in 150 mM NaCl, 0.5% Tween 20, and diluted in PBS for i.v. injection into the tail vein (200 ng/mouse).
Analysis of NKT cells by flow cytometry
All staining steps were performed on ice. Nonspecific FcR-mediated antibody binding was blocked with anti-CD16/32 antibody (24G2; prepared in-house). Cells were then incubated for 30 min with CD1d Tetr and then a further 10 min with specific mAb. Antibodies used included: anti-CD4-Pacific Blue (clone GK1.5), anti-CD3-PE-Cy7 (clone 145-2C11), and biotin-conjugated anti-NK1.1 (clone PK136) from eBioscience (San Diego, CA, USA). Streptavidin allophycocyanin-Alexa Fluor 750 (Invitrogen, Carlsbad, CA, USA) was used as a secondary reagent. Anti-CD44 (clone IM7) was labeled in-house with Alexa Fluor 647 (Invitrogen). Also used were anti-CD4-allophycocyanin (clone GK1.5), anti-CD45/B220-PerCP (clone RA-6B2), anti-βTCR-allophycocyanin (clone H57-597), anti-CD4-Pacific Blue (clone RM 4-5), anti-NK1.1-PE-Cy7 (clone PK136), and anti-CD44-FITC (clone IM7), all from BD PharMingen (San Diego, CA, USA). Experiments were analyzed using a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) with PI (BD PharMingen) as a viability dye or a BD LSRII SORP flow cytometer with DAPI (Invitrogen) as a viability dye.
Lymphocyte isolation from thymus, spleen, and liver
Thymus or spleen tissue was teased through gauze to give single-cell suspensions, with RBCs removed from splenocyte preparations with RBC lysing buffer (Qiagen, Germantown, MD, USA). Livers were perfused with PBS, incubated for 30 min at 37°C in 2.4 mg/ml collagenase type II (Gibco, Auckland, New Zealand), and 0.2 mg/ml DNase I (Roche, Mannheim, Germany) and layered over a Lymphoprep gradient to enrich for lymphocytes (Axis-Shield PoC AS, Oslo, Norway).
Analysis of thymic suspensions in vitro
Thymic suspensions were cultured at 2 × 108 cells/ml overnight in the absence of additional stimulation to assess cytokine release into supernatant using the Bioplex system (BioRad, Auckland, New Zealand). Some cultures were initially depleted of NKT cells by staining with a PE-labeled, α-GalCer-loaded mouse CD1d Tetr (ProImmune, Oxford, UK), followed by depletion with anti-PE-coated magnetic beads (Miltenyi Biotec, North Ryde, Australia).
Statistical analyses
Nonparametric tests were used to assess statistical significance. For comparing the means of two groups, the Mann Whitney test was used. For comparing more than two groups, the Kruskal Wallis one-way ANOVA was used with Dunn's multiple comparison test. For comparisons of two variables, a two-way ANOVA with Bonferroni's post-test was used. All statistical analyses were done with Prism 5.0 software (GraphPad Software, La Jolla, CA, USA), with P values of <0.05 considered significant (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001).
RESULTS
Reporter expression by NKT cells in G4 mice following administration of α-GalCer
We first examined reporter expression from the Il4 locus by NKT cells in G4 mice. The IL-4-substituting knockin strategy in these mice ensures that expression of GFP protein is a faithful surrogate for IL-4 protein expression; GFP mRNA is more stable than IL-4 mRNA, and GFP protein has a relatively long half-life [14], so its fluorescence can be used as a highly sensitive reporter even in low levels of IL-4 production [9]. Initial studies were conducted in heterozygous animals, permitting analysis of expression from the transgenic Il4 locus in an IL-4-sufficient environment. Animals on BALB/c (BALB/c IL-4+/G4) and C57BL/6 (C57 IL-4+/G4) backgrounds were used. A general gating strategy involving fluorescent α-GalCer-loaded CD1d Tetr to detect NKT cells is provided in Supplemental Fig. 1.
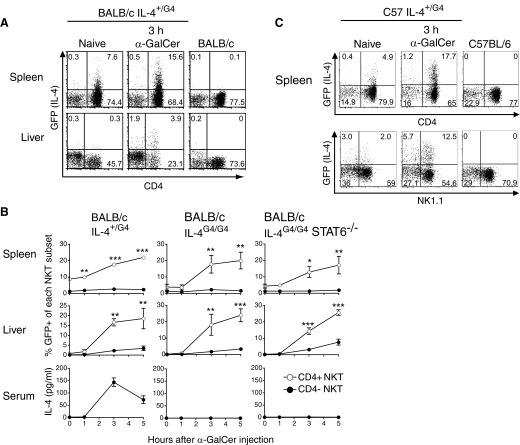
The expression of IL-4 was assessed in G4 mice directly after in vivo stimulation with the specific NKT ligand α-GalCer. After i.v. α-GalCer, IL-4 reporter expression was seen in NKT cells of the spleens and livers of BALB/c IL-4+/G4 mice, which increased significantly over a 5-h time period (Fig. 1A and B). Expression of the IL-4 reporter in spleen and liver correlated with induction of secretion of IL-4 protein into the serum, although IL-4 protein levels peaked at 3 h after injection, whereas GFP levels were still sustained at 5 h, reflecting the longer half-life of the reporter protein [14]. Significantly, the induced expression from the Il4 locus was largely confined to CD4+ NKT cells in both tissues, confirming a bias in IL-4 production with NKT cell phenotype reported in earlier in vitro restimulation studies [15–18]. Intracellular cytokine staining of splenic NKT cells in WT animals confirmed the preferential production of IL-4 by the CD4+ subset in response to administration of α-GalCer (Supplemental Fig. 2). Of note, protocols involving incubation at 37°C, even in the presence of brefeldin A and monensin, failed to show significant differences in IL-4 production between CD4+ and CD4− subsets, suggesting that IL-4 is degraded rapidly at this temperature, highlighting the potential for misleading results in studies using this form of in vitro manipulation.
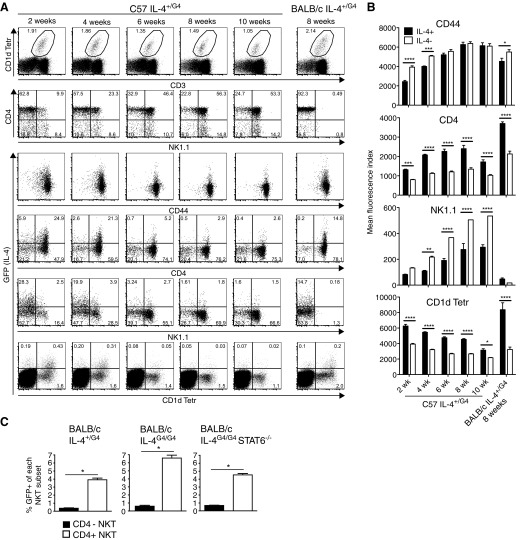
Figure 1. i.v. administration of α-GalCer induces IL-4 reporter expression in NKT cell in G4 mice.
(A) Representative dot plots showing expression of the IL-4 reporter on gated NKT cells (CD1d Tetr-binding cells, as shown in Supplemental Fig. 1) from spleen and liver of heterozygous G4 mice on a BALB/c background administered 200 ng α-GalCer, relative to nontransgenic and untreated control animals. (B) Mean percentages of IL-4 reporter-positive cells (±sem) within the CD4+ and CD4− NKT cell subsets of the indicated tissues over time after α-GalCer administration (n=4–6 animals/group). Significant differences between the subsets were determined by two-way ANOVA with Bonferroni post-test. Tissues were also examined from similarly treated homozygous G4 mice and mice homozygous for the G4 reporter and STAT6 deficiency. A graph showing levels of IL-4 protein in the serum is also presented. (C) Representative dot plots from a similar analysis in G4 mice on a C57BL/6 background.
Analysis of reporter expression in G4 animals on a C57BL/6 background also showed preferential Il4 locus activation by CD4+ NKT cells after α-GalCer administration (Fig. 1C). In C57BL/6 animals, it is possible to further subdivide NKT cells on the basis of NK1.1 expression, with many mature NKT cells in the spleen having progressed through a final stage of maturation characterized by up-regulation of this marker. However, Il4 locus activation was not correlated with NK1.1 marker expression, with NK1.1+ and NK1.1− cells expressing the reporter after α-GalCer administration.
The expression of IL-4 by conventional CD4+ T cells is commonly regarded as requiring an initial external source of IL-4 protein and regulation via a positive-feedback loop involving STAT6 signaling [19–22], although recently, the requirement for an initial source of IL-4 protein has been challenged [9, 23, 24]. In contrast, NKT cells manufacture IL-4 in the absence of IL-4 protein or STAT6 signaling, as established using in vitro studies [17, 19, 25, 26] and in vivo studies with anti-CD3 or α-GalCer injection [11, 27, 28]. Analysis of reporter expression in IL-4G4/G4 mice, which are IL-4−/−, and in animals from a cross between BALB/c IL-4G4/G4 and STAT6−/− mice (IL-4G4/G4 STAT6−/− mice) confirmed that Il4 locus activation was independent of an original source IL-4 and STAT-6 signaling (Fig. 1B), providing further evidence that reporter expression in NKT cells of G4 mice is a faithful surrogate for IL-4 protein expression.
A notable feature of reporter expression in splenic NKT cells on the BALB/c and C57BL/6 backgrounds was evidence of Il4 locus activation in the absence of α-GalCer administration, albeit limited to low-level fluorescence in a small proportion of cells (Fig. 1). We therefore chose to examine this innate activity in more detail to determine where and when such cytokine production occurred.
Innate IL-4 production by immature NKT cells in the thymus
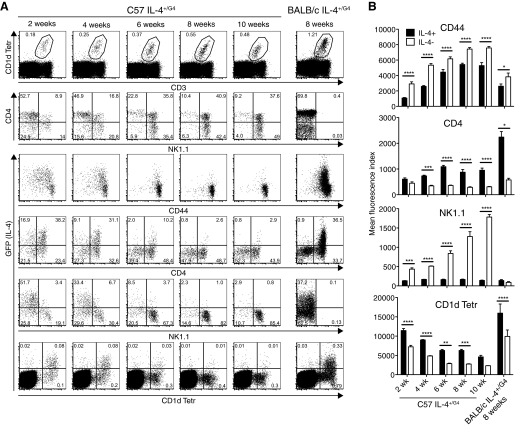
As was observed in the spleen, a proportion of thymic NKT cells expressed GFP in heterozygous G4 animals without any in vivo or in vitro manipulation (Fig. 2A). In C57 IL-4+/G4 mice, it was necessary to examine animals below 10 weeks of age to see substantial numbers of cells expressing the IL-4 reporter. In contrast, a distinct GFP+ population could be observed in 8-week-old BALB/c IL-4+/G4 mice, highlighting differences in genetic control of this cytokine between the two strains. Analysis in C57 IL-4+/G4 mice, which express NK1.1, made it possible to correlate Il4 locus activation with defined stages of NKT cell maturation based on expression of CD4, CD44, and NK1.1 [29–33]. Reporter expression was strongly associated with cells that had low levels of NK1.1 and expressed lower levels of CD44 than their IL-4 reporter-negative counterparts (Fig. 2B). They also expressed higher levels of CD4. This NK1.1lo CD44lo CD4+ phenotype corresponds to NKT cells in Stage 1 of the maturation process but could also encompass NKT cells in Stage 0 of the maturation process (NK1.1lo CD24+ CD44lo CD4+). However, the latter cells are rare (<0.001% of total thymocytes) and therefore, could not account for all of the reporter-positive cells observed.
Figure 2. Immature thymic NKT cells pass through a stage of innate IL-4 secretion.
(A) Flow cytometry was used to analyze GFP as a surrogate for IL-4 expression in thymocytes from naïve heterozygous G4 mice on C57BL/6 and BALB/c backgrounds. Dot plots show gating of NKT cells and analysis of GFP, CD4, CD44, and NK1.1 expression. (B) Mean fluorescence intensity of binding of CD4, CD44, and NK1.1 antibodies and CD1d Tetr on GFP+ (IL-4+) and GFP− (IL-4−) NKT cells; bars represent mean ± sem from four to six mice/age group analyzed (two-way ANOVA with Bonferroni post-test).
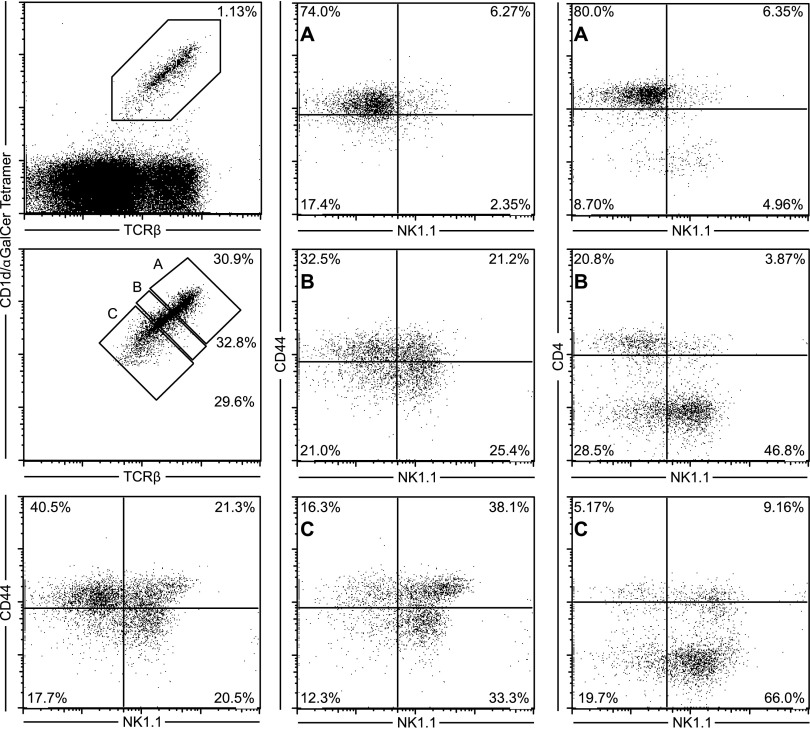
In C57 IL-4+/G4 mice, a reasonably uniform level of binding of the fluorescent CD1d Tetr was observed among similarly aged mice, but overall, fluorescence intensity decreased with age in the groups assessed (Fig. 2A and B). Increased binding of CD1d Tetr is likely to reflect a higher level of TCR expression, which is also a known feature of immature NKT cells [16, 34]. Indeed, a reduction in fluorescence intensity of staining with CD1d Tetr was seen as NKT cells progressed through the defined stages of NKT maturation in these mice (Supplemental Fig. 3). It was notable, therefore, that the IL-4 reporter-positive NKT cells in each age group exhibited higher TCR levels than their reporter-negative counterparts, suggesting that it was the less-mature cells in each age group that had the capacity for innate Il4 locus activation. In BALB/c IL-4+/G4 mice, the binding of CD1d Tetr was less uniform, with intermediate and high fluorescence intensity observed. Again, expression of the IL-4 reporter was associated with higher levels of CD1d Tetr binding and hence, higher TCR expression. Reporter expression was also largely confined to cells that expressed CD4 in this strain. Whereas animals on a BALB/c background do not express the NK1.1 marker used to help define maturation stage, in a BALB/c strain that was congenic for NK1.1 [12], we independently showed that the CD4+ CD1d Tetrhi phenotype correlated with reduced NK1.1 expression typical of Stage 1 of maturation (Fig. 3).
Figure 3. Strong binding of CD1d Tetr is associated with an immature NKT cell phenotype in a BALB/c strain congenic for NK1.1.
Increased levels of CD1d Tetr binding are correlated with an immature CD4+CD44hiNK1.1− phenotype in thymic NKT cells from naïve BALB.B6-Cmv1r mice. The gating strategy used to identify NKT cells is shown together with representative FACS plots of CD4, CD44, and NK1.1 expression on (A) CD1d Tetrhi, (B) CD1d Tetrint, and (C) CD1d Tetrlo cells. Results are representative of five different mice.
We also analyzed thymic NKT cells in KN2 mice, another IL-4 reporter strain on a BALB/c background, in which huCD2 serves as the reporter sequence [8]. These mice also exhibited innate expression from the Il4 locus in thymic NKT cells, again most strongly in cells with a CD4+ CD1d Tetrhi CD44lo phenotype typical of Stage 1 of maturation (Fig. 4).
Figure 4. Analysis of huCD2 as a surrogate for IL-4 expression in KN2 mice.
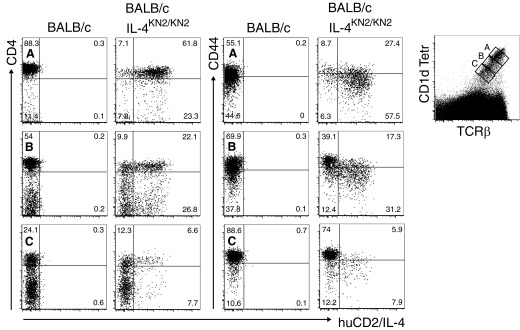
Expression of the reporter huCD2 was examined in thymic NKT cells from naïve homozygous KN2 mice compared with BALB/c controls. The gating strategy used to identify NKT cells is shown together with representative FACS plots of CD4 and CD44 on (A) CD1d Tetrhi, (B) CD1d Tetrint, and (C) CD1d Tetrlo cells. Dot plots are representative of three mice/group.
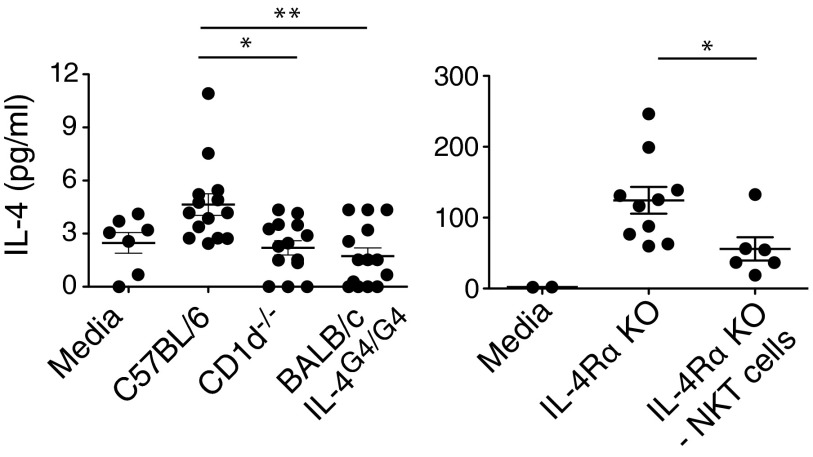
To examine whether IL-4 protein was indeed manufactured and secreted in the thymus, cytokine bead array methodology was used to assess IL-4 protein levels in suspensions of thymic tissue cultured overnight without any external stimulus (Fig. 5). Low levels of IL-4 protein were reproducibly detected in cultures from C57BL/6 mice, whereas negligible levels were seen in age-matched, CD1d−/− mice on the same genetic background (which do not harbor NKT cells). Analysis was also conducted in animals deficient in IL-4R expression that could not use any IL-4 protein produced, resulting in an accumulation of the cytokine. Significantly, the higher levels of IL-4 detected in these cultures could be ablated significantly by depleting NKT cells from the suspensions. Together with the previous results from the reporter mice, we conclude that IL-4 protein is produced and secreted by NKT cells spanning Stage 1 of the maturation process in the thymus.
Figure 5. IL-4 protein is detected in thymic cultures.
Mean levels of IL-4 protein in cell suspensions prepared from thymic tissue from mice from the indicated mouse strains cultured for 24 h; NKT cells were removed from some cultures, as indicated, using PE-labeled CD1d Tetr and anti-PE-coated magnetic beads. KO, Knockout.
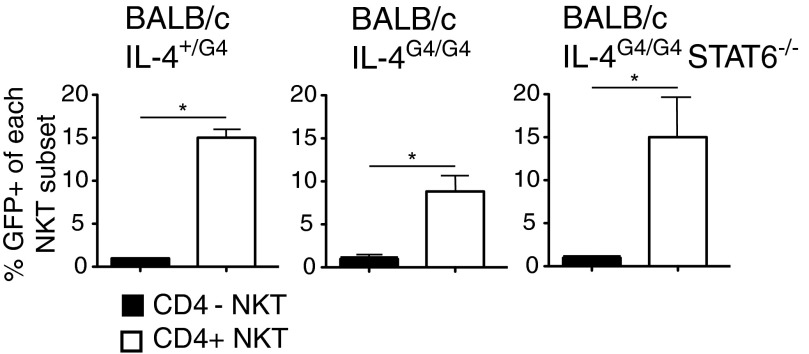
Finally, we examined whether innate IL-4 production by immature thymic NKT cells was independent of an initial source of IL-4 protein and STAT6 signaling, as was seen for α-GalCer-induced IL-4 production in mature cells. Analysis of IL-4 reporter expression in naïve homozygous BALB/c IL-4G4/G4 mice and IL-4G4/G4 STAT6−/− mice showed that the innate expression of the IL-4 reporter in thymic NKT cells could be induced without the presence of these molecules (Fig. 6).
Figure 6. Innate expression from the Il4 locus in NKT cells is independent of an initial source IL-4 and STAT6 signaling.
Analysis of IL-4 reporter expression in thymic NKT cells from groups (n=4–6) of naïve heterozygous G4 mice (BALB/c IL-4+/G4; IL-4-sufficient), homozygous G4 mice (BALB/c IL-4G4/G4; IL-4−/−), and mice homozygous for the G4 reporter and STAT6 deficiency (BALB/c IL-4G4/G4 STAT6−/−). Mean percentages of GFP reporter+ cells in CD4+ and CD4− NKT cells are presented ±sem.
Genetic background influences innate expression of IL-4 by thymic NKT cells
Given the differences observed in Il4 locus activation with age on the BALB/c and C57BL/6 backgrounds, we explored expression of the IL-4 reporter over a longer timespan in G4 mice on both genetic backgrounds (Fig. 7). Total numbers of IL-4 reporter-positive NKT cells peaked at 4 weeks of age in animals on a C57BL/6 background, at which point, they represented ∼40% of all NKT cells. Numbers then declined to undetectable levels by 10 weeks of age. In contrast, numbers of reporter-positive NKT cells peaked between 7 and 15 weeks of age on a BALB/c background, reaching 20–30% of all NKT cells, and then declined, although there were still measurable levels at 28 weeks of age. Given that we have shown that innate Il4 locus activation occurs at a defined maturation stage, the different kinetics of IL-4 activity in NKT cells with age suggest that the implementation of this developmentally programmed process is under different levels of genetic regulation in the two strains.
Figure 7. Proportion of thymic NKT cells exhibiting innate expression from the Il4 locus is determined by age and genetic background.
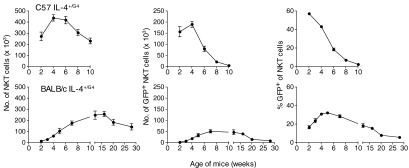
Numbers of IL-4 reporter-positive thymic NKT cells (IL-4+ NKT cells) were determined in different age groups of naïve heterozygous G4 mice on C57BL/6 and BALB/c backgrounds. Right panels show mean percentage of GFP reporter+ of NKT cells/mouse. All graphs show mean values ± sem/group (n=4–6).
Innate expression of IL-4 by NKT cells in the spleen
As noted above, innate Il4 locus activation was also observed in a small population of NKT cells from the spleens of naïve G4 mice (Fig. 8A). Whereas the overall majority of NKT cells in the spleens expressed high levels of CD44 (Fig. 8B), reflecting a predominance of cells that had transitioned through the maturation step from Stage 1 (CD44lo NK1.1− cells) to Stage 2 (CD44hi NK1.1− cells) [29], in young C57 IL-4+/G4 mice (up to 4 weeks old), cohorts of cells expressing lower levels of CD44 were present, perhaps reflecting cells undergoing an earlier maturation step in the periphery; expression of the IL-4 reporter was associated with the lower expression of CD44 in these young animals. At all ages and on C57BL/6 and BALB/c genetic backgrounds, the IL-4 reporter-positive NKT cells expressed higher levels of CD4 and TCR than their reporter-negative counterparts, whereas on the C57BL/6 background, they also expressed lower, but not negligible, levels of NK1.1 (Fig. 8B). The innate Il4 locus activation in the spleen was therefore associated with immature NKT cells that were mostly at Stage 2 of the maturation process (CD4+ CD44hi NK1.1−), with some cells transitioning to Stage 3 associated with up-regulation of NK1.1. Analysis in IL-4G4/G4 and IL-4G4/G4 STAT6−/− mice showed that the Il4 locus activation in splenic NKT cells was also independent of an original source IL-4 and STAT6 (Fig. 8C). The accumulated data indicate a similar process of innate Il4 locus activation to NKT cells in the thymus, although generally observed at a slightly later phase of the maturation process.
Figure 8. Splenic NKT cells exhibit innate expression of IL-4.
(A) Representative dot plots of analysis of IL-4 reporter expression in NKT cells from the spleen of naïve heterozygous G4 mice on C57BL/6 and BALB/c backgrounds. (B) Mean fluorescence intensity of binding of CD4, CD44, and NK1.1 antibodies and CD1d Tetr on GFP+ (IL-4+) and GFP− (IL-4−) NKT cells; bars represent mean ± sem from four to six mice/age group analyzed (two-way ANOVA with Bonferroni post-test). (C) Expression of the IL-4 reporter in splenic NKT cells from groups (n=4–6) of naïve, heterozygous G4 mice, homozygous G4 mice, and mice homozygous for G4 and STAT6 deficiency. Mean percentages of GFP reporter+ cells in CD4+ and CD4− NKT cells are presented ±sem.
DISCUSSION
The G4 and KN2 reporter strains used here provide faithful and sensitive surrogates for IL-4 protein expression, avoiding the confounding effects of in vitro manipulation to assess cytokine induction. This heightened sensitivity revealed an innate phase of IL-4 production occurring during NKT cell development in the thymus, with IL-4 protein manufactured and secreted by cells spanning Stage 1 of the maturation program, characterized by a CD4+ CD44lo NK1.1− phenotype. Whereas we have used the term innate to describe this process, we appreciate that it may be secondary to a specific interaction in the thymus, likely involving TCR engagement with an endogenous ligand; regardless, the IL-4 production appears to be a programmed response occurring at a specific point in the fate map of these cells. It is unlikely that this phenomenon reflects low-level stimulation by environmental antigens, as all of the animals used were housed under the same specific pathogen-free conditions, yet the kinetics of innate IL-4 production were specific to different genetic backgrounds; these observations point to a developmental program under different levels of genetic control in the mouse strains examined. Whereas the signaling events that drive this process have yet to be defined, we observed innate production of IL-4 in immature NKT cells in the absence of an initial external source of IL-4 protein and STAT6 signaling, as has been reported previously for their mature counterparts [17, 25, 26] (and confirmed here in mature cells in response to α-GalCer-treatment).
Evidence of innate expression from the Il4 locus was also observed in NKT cells of the spleens of G4 mice (Fig. 8) and KN2 mice (not shown). This expression was predominantly in cells in Stage 2 of the maturation process, which in G4 animals on a C57BL/6 background, was characterized by a CD4+ CD44hi NK1.1− phenotype. The periphery is populated initially by NK1.1− cells at Stage 2, which then undergo maturation to Stage 3, associated with up-regulation of NK1.1 [29, 33]. However, a significant proportion of fully mature NKT cells is also known to be NK1.1− [35], so it was possible that mature cells were contributing to the IL-4 production observed in the spleen. However, we favor the likelihood that immature cells were responsible, as IL-4 reporter expression was seen in cells that were strongly bound by the CD1d Tetr, indicating high TCR expression, and as numbers of IL-4 reporter-positive cells were higher in younger G4 animals, when more immature cells would be expected [29, 31, 32, 36]. The fact that IL-4 production in the thymus was associated with Stage 1 of the maturation process and in the spleen with Stage 2 could reflect the possibility that these two stages actually represent a continuum associated with a postselection NKT cell-proliferative phase [37], with IL-4 cytokine release a feature throughout. Also, given the longer half-lives of the GFP and huCD2 reporter proteins relative to IL-4 itself, it is possible that the reporter activity seen in the spleen may reflect recent thymic emigrants in which IL-4 had been induced earlier at Stage 1 in the thymus. Overall, the observation that IL-4 production is actually released by immature cells is in keeping with previous in vitro studies that had shown that immature cells have the capacity to produce this cytokine. These previous studies showed that in response to in vitro stimulation, immature cells release cytokines IL-4 and IL-10 but little IFN-γ, whereas by Stage 3, which is associated with reduced proliferation, the NKT cells acquired the capacity to produce abundant IFN-γ but less IL-4 and little if any IL-10 [29, 31, 38].
It has been reported previously that CNS-2, downstream of the Il4 locus, is a constitutively active enhancer in NKT cells, which was also observed in CD44hi memory phenotype CD4+ T cells [39]. Like the innate IL-4 production observed here, this constitutive activity was independent of IL-4 and STAT6 signaling. A correlation with maturation stage of NKT cells was not investigated, but it is interesting to speculate that the CNS-2 enhancer is driving the developmentally programmed activity reported here. However, this possibility is confounded by the fact that no constitutive GFP or huCD2 expression was seen in CD44hi memory phenotype CD4+ T cells in the reporter mice in our study; further work is therefore required to reconcile these observations.
The percentage of NKT cells showing expression from the Il4 locus in the thymus and spleen reduced with age, likely reflecting an age-related accumulation of more mature cells [29, 31, 32, 36]. As noted, the kinetics of innate IL-4 production differed significantly with genetic background, with the percentage of IL-4-producing cells in the thymus reduced to negligible levels by 10 weeks of age in G4 animals on a C57BL/6 background but with distinct populations observed beyond 20 weeks of age on a BALB/c background. It has long been known that animals on these different genetic backgrounds respond very differently to pathogens and allergens, with BALB/c animals commonly regarded as more Th2-prone [40]. As IL-4 production amplifies Th2 responses, it is possible that the continued innate production of IL-4 by thymic NKT cells contributes to the Th2 bias in BALB/c animals. Others have reported that NKT cell-derived IL-4 is important for sustaining “memory-like T cells”, a subset of thymic cells that acquires effector function through a maturation process rather than interaction with a specific antigen [41]. Significantly, these memory-like T cells were found in large numbers in BALB/c, but not C57BL/6 mice, and factors that alter levels of the transcription factor PLZF, such as KLF13 and KLF2, were shown to alter their IL-4-dependent generation [41]. Furthermore, whereas NKT cell lineage development is severely arrested in the complete absence of PLZF [42, 43], the few NKT cells that are present in PLZF−/− mice do not contain the preformed mRNA for IL-4 characteristic of WT NKT cells [42, 43]. The activity of PLZF is therefore required for IL-4 production by NKT cells and is likely to be a crucial player in the innate production that we have observed.
The high fidelity and sensitivity of the IL-4 reporter mice allowed us to address whether mature NKT cell subsets differ with respect to cytokine production in vivo, as such functionally distinct populations may be selectively invoked in different disease processes. In response to in vivo stimulation with α-GalCer, we observed IL-4 production predominantly in CD4+ NKT cell subsets of spleen and liver. Many previous studies of murine NKT cells have failed to show such a clear difference in IL-4 production between the CD4+ and CD4− NKT subsets [38, 44–46], which may be a reflection of the technical difficulty in measuring this cytokine. In fact, our own analysis of IL-4 expression by intracellular staining techniques provided significantly different results and interpretation depending on the incubation temperature and reagents used. The biological significance of CD4 expression by IL-4-producing NKT cells is unclear, although it has been reported that expression of CD4 can enhance the capacity of human NKT cells to respond to stimulation [47, 48]. We observed lower expression of the activation markers CD25 and CD69 on CD4− NKT cells after stimulation (not shown), suggesting a lower capacity for activation in the absence of CD4. The propensity of CD4+ NKT cells to manufacture IL-4 may suggest that these cells feature significantly in some Th2-driven disease states. In this context, a CD4+ NKT population expressing the IL-25R IL-17RB was shown to be involved in IL-25-mediated airway hyper-reactivity; these cells were also shown to exhibit higher IL-4 mRNA levels than their CD4− counterparts [49].
Overall, the release of IL-4 by NKT cells in vivo is defined by many factors, including developmental stage, location, age, and genetic background. The manufacture and secretion of IL-4 as part of the developmental program in the thymus described here may have broad consequences, perhaps influencing the development of surrounding cells and ultimately helping shape the quality of conventional adaptive immune responses in the host. Also, the rate at which IL-4-producing NKT cells arise from the thymus in different strains and with age may ultimately determine the ratio of immature to mature cells in the peripheral organs, which has been proposed as a factor in disease susceptibility [34], such as antigen sensitization in the lung (pronounced when ratio is high in young mice) and increased risk of autoimmune disease (pronounced in older mice). However, the differences in capacity for IL-4 production by mature NKT cell subsets that we observed in the periphery must also be considered in this context, with the ratio of CD4+ to CD4− NKT cells also likely to affect disease susceptibility. Thus, the functional diversification between different NKT cell populations, such as the capacity for cytokine production assessed here, should therefore be considered closely in studies of disease or when NKT cell stimulation is to be considered for immunotherapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the New Zealand Health Research Council and Marjorie Barclay Trust. N.D. was supported by a University of Otago Prestigious Scholarship and I.F.H. by a New Zealand HRC Sir Charles Hercus Fellowship. We thank Prof. William Paul for providing the IL-4 reporter strains used in this study, Drs. Gavin Painter and Colin Hayman for providing the α-GalCer, Julie Fletcher for technical assistance, and the Malaghan Biomedical Research Unit for animal husbandry.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- −/−
- deficient
- α-GalCer
- α-galactosylceramide
- CD1d Tetr
- CD1d tetramer
- CNS-2
- conserved noncoding sequence enhancer 2
- G4
- mouse strain with the first exon and 178 nucleotides of the first intron of the Il4 gene replaced with a sequence encoding GFP
- huCD2
- human CD2
- KLF
- Kruppel-like factor
- KN2
- mouse strain with the first two exons of IL-4 replaced by a sequence for human CD2
- NKT cell
- invariant CD1d-dependent NK-like T cell
- PLZF
- promyelocytic leukemia zinc finger
- RBC
- red blood cell
AUTHORSHIP
N.D., K.J.F., N.v.P., D.A.K., S.J.M., M.L.C., and S.M-H. designed and carried out experiments; A.G.B., R.M.L., G.L.G., and I.F.H. designed experiments; and N.D. and I.F.H. wrote the manuscript.
REFERENCES
- 1. Bendelac A., Savage P. B., Teyton L. (2007) The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 [DOI] [PubMed] [Google Scholar]
- 2. Godfrey D. I., MacDonald H. R., Kronenberg M., Smyth M. J., Van Kaer L. (2004) NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 [DOI] [PubMed] [Google Scholar]
- 3. Zajonc D. M., Kronenberg M. (2007) CD1 mediated T cell recognition of glycolipids. Curr. Opin. Struct. Biol. 17, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godfrey D. I., Kronenberg M. (2004) Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mohrs M., Shinkai K., Mohrs K., Locksley R. M. (2001) Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 [DOI] [PubMed] [Google Scholar]
- 6. Stetson D. B., Mohrs M., Reinhardt R. L., Baron J. L., Wang Z. E., Gapin L., Kronenberg M., Locksley R. M. (2003) Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu-Li J., Pannetier C., Guo L., Lohning M., Gu H., Watson C., Assenmacher M., Radbruch A., Paul W. E. (2001) Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity 14, 1–11 [DOI] [PubMed] [Google Scholar]
- 8. Mohrs K., Wakil A. E., Killeen N., Locksley R. M., Mohrs M. (2005) A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Panhuys N., Tang S. C., Prout M., Camberis M., Scarlett D., Roberts J., Hu-Li J., Paul W. E., Le Gros G. (2008) In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc. Natl. Acad. Sci. USA 105, 12423–12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendiratta S. K., Martin W. D., Hong S., Boesteanu A., Joyce S., Van Kaer L. (1997) CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 [DOI] [PubMed] [Google Scholar]
- 11. Noben-Trauth N., Shultz L. D., Brombacher F., Urban J. F., Jr., Gu H., Paul W. E. (1997) An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 94, 10838–10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scalzo A. A., Lyons P. A., Fitzgerald N. A., Forbes C. A., Shellam G. R. (1995) The BALB.B6-Cmv1r mouse: a strain congenic for Cmv1 and the NK gene complex. Immunogenetics 41, 148–151 [DOI] [PubMed] [Google Scholar]
- 13. Lee A., Farrand K. J., Dickgreber N., Hayman C. M., Jurs S., Hermans I. F., Painter G. F. (2006) Novel synthesis of α-galactosyl-ceramides and confirmation of their powerful NKT cell agonist activity. Carbohydr. Res. 341, 2785–2798 [DOI] [PubMed] [Google Scholar]
- 14. Min B., Prout M., Hu-Li J., Zhu J., Jankovic D., Morgan E. S., Urban J. F., Jr., Dvorak A. M., Finkelman F. D., LeGros G., Paul W. E. (2004) Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 200, 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammond K. J., Pelikan S. B., Crowe N. Y., Randle-Barrett E., Nakayama T., Taniguchi M., Smyth M. J., van Driel I. R., Scollay R., Baxter A. G., Godfrey D. I. (1999) NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29, 3768–3781 [DOI] [PubMed] [Google Scholar]
- 16. Stenstrom M., Skold M., Andersson A., Cardell S. L. (2005) Natural killer T-cell populations in C57BL/6 and NK1.1 congenic BALB.NK mice—a novel thymic subset defined in BALB.NK mice. Immunology 114, 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z. Y., Kusam S., Munugalavadla V., Kapur R., Brutkiewicz R. R., Dent A. L. (2006) Regulation of Th2 cytokine expression in NKT cells: unconventional use of Stat6, GATA-3, and NFAT2. J. Immunol. 176, 880–888 [DOI] [PubMed] [Google Scholar]
- 18. Watarai H., Sekine-Kondo E., Shigeura T., Motomura Y., Yasuda T., Satoh R., Yoshida H., Kubo M., Kawamoto H., Koseki H., Taniguchi M. (2012) Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 10, e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan M. H., Wurster A. L., Smiley S. T., Grusby M. J. (1999) Stat6-dependent and -independent pathways for IL-4 production. J. Immunol. 163, 6536–6540 [PubMed] [Google Scholar]
- 20. Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. (1990) Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 172, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimoda K., van Deursen J., Sangster M. Y., Sarawar S. R., Carson R. T., Tripp R. A., Chu C., Quelle F. W., Nosaka T., Vignali D. A., Doherty P. C., Grosveld G., Paul W. E., Ihle J. N. (1996) Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380, 630–633 [DOI] [PubMed] [Google Scholar]
- 22. Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. (1996) Essential role of Stat6 in IL-4 signalling. Nature 380, 627–630 [DOI] [PubMed] [Google Scholar]
- 23. Finkelman F. D., Morris S. C., Orekhova T., Mori M., Donaldson D., Reiner S. L., Reilly N. L., Schopf L., Urban J. F., Jr. (2000) Stat6 regulation of in vivo IL-4 responses. J. Immunol. 164, 2303–2310 [DOI] [PubMed] [Google Scholar]
- 24. Jankovic D., Kullberg M. C., Noben-Trauth N., Caspar P., Paul W. E., Sher A. (2000) Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J. Immunol. 164, 3047–3055 [DOI] [PubMed] [Google Scholar]
- 25. Chen H., Paul W. E. (1997) Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 159, 2240–2249 [PubMed] [Google Scholar]
- 26. Kaplan M. H., Schindler U., Smiley S. T., Grusby M. J. (1996) Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4, 313–319 [DOI] [PubMed] [Google Scholar]
- 27. Matsuda J. L., Gapin L., Baron J. L., Sidobre S., Stetson D. B., Mohrs M., Locksley R. M., Kronenberg M. (2003) Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl. Acad. Sci. USA 100, 8395–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshimoto T., Paul W. E. (1994) CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179, 1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benlagha K., Kyin T., Beavis A., Teyton L., Bendelac A. (2002) A thymic precursor to the NK T cell lineage. Science 296, 553–555 [DOI] [PubMed] [Google Scholar]
- 30. Benlagha K., Wei D. G., Veiga J., Teyton L., Bendelac A. (2005) Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202, 485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gadue P., Stein P. L. (2002) NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 169, 2397–2406 [DOI] [PubMed] [Google Scholar]
- 32. Gapin L., Matsuda J. L., Surh C. D., Kronenberg M. (2001) NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2, 971–978 [DOI] [PubMed] [Google Scholar]
- 33. Pellicci D. G., Hammond K. J., Uldrich A. P., Baxter A. G., Smyth M. J., Godfrey D. I. (2002) A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1− CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda J. L., Gapin L. (2007) Does the developmental status of Vα14i NKT cells play a role in disease? Int. Rev. Immunol. 26, 5–29 [DOI] [PubMed] [Google Scholar]
- 35. McNab F. W., Pellicci D. G., Field K., Besra G., Smyth M. J., Godfrey D. I., Berzins S. P. (2007) Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J. Immunol. 179, 6630–6637 [DOI] [PubMed] [Google Scholar]
- 36. Berzins S. P., McNab F. W., Jones C. M., Smyth M. J., Godfrey D. I. (2006) Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 176, 4059–4065 [DOI] [PubMed] [Google Scholar]
- 37. Godfrey D. I., Stankovic S., Baxter A. G. (2010) Raising the NKT cell family. Nat. Immunol. 11, 197–206 [DOI] [PubMed] [Google Scholar]
- 38. Coquet J. M., Chakravarti S., Kyparissoudis K., McNab F. W., Pitt L. A., McKenzie B. S., Berzins S. P., Smyth M. J., Godfrey D. I. (2008) Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc. Natl. Acad. Sci. USA 105, 11287–11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka S., Tsukada J., Suzuki W., Hayashi K., Tanigaki K., Tsuji M., Inoue H., Honjo T., Kubo M. (2006) The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity 24, 689–701 [DOI] [PubMed] [Google Scholar]
- 40. Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. (1989) Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee Y. J., Jameson S. C., Hogquist K. A. (2011) Alternative memory in the CD8 T cell lineage. Trends Immunol. 32, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kovalovsky D., Uche O. U., Eladad S., Hobbs R. M., Yi W., Alonzo E., Chua K., Eidson M., Kim H. J., Im J. S., Pandolfi P. P., Sant'Angelo D. B. (2008) The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9, 1055–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Savage A. K., Constantinides M. G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. (2008) The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Askenase P. W., Itakura A., Leite-de-Moraes M. C., Lisbonne M., Roongapinun S., Goldstein D. R., Szczepanik M. (2005) TLR-dependent IL-4 production by invariant Vα14 Jα18+ NKT cells to initiate contact sensitivity in vivo. J. Immunol. 175, 6390–6401 [DOI] [PubMed] [Google Scholar]
- 45. Crowe N. Y., Coquet J. M., Berzins S. P., Kyparissoudis K., Keating R., Pellicci D. G., Hayakawa Y., Godfrey D. I., Smyth M. J. (2005) Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hachem P., Lisbonne M., Michel M. L., Diem S., Roongapinun S., Lefort J., Marchal G., Herbelin A., Askenase P. W., Dy M., Leite-de-Moraes M. C. (2005) α-Galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-γ. Eur. J. Immunol. 35, 2793–2802 [DOI] [PubMed] [Google Scholar]
- 47. Chen X., Wang X., Besra G. S., Gumperz J. E. (2007) Modulation of CD1d-restricted NKT cell responses by CD4. J. Leukoc. Biol. 82, 1455–1465 [DOI] [PubMed] [Google Scholar]
- 48. Thedrez A., de Lalla C., Allain S., Zaccagnino L., Sidobre S., Garavaglia C., Borsellino G., Dellabona P., Bonneville M., Scotet E., Casorati G. (2007) CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood 110, 251–258 [DOI] [PubMed] [Google Scholar]
- 49. Terashima A., Watarai H., Inoue S., Sekine E., Nakagawa R., Hase K., Iwamura C., Nakajima H., Nakayama T., Taniguchi M. (2008) A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.