Significance
The diversity of living things generally peaks in the tropics and declines toward the poles. This “latitudinal gradient” is Earth’s most prevalent biogeographic pattern, but biologists do not agree about its cause. Here, we use geographic and evolutionary data for over 12,500 species of woody flowering plants to test the “tropical conservatism hypothesis,” which attributes the phenomenal diversity of tropical environments to their large extent over the past 55 million years (My) and the evolutionary conservatism of environmental tolerances. As predicted, we find that transitions between tropical and temperate environments are quite rare over the evolutionary history and that most temperate lineages originated as Earth cooled over the past 34 My. Thus, the correlation between diversity and climate reflects plants’ evolutionary history.
Keywords: biogeography, out of the tropics, paleoclimate, evolutionary speed, environmental niche
Abstract
Plant diversity, like that of most other taxonomic groups, peaks in the tropics, where climatic conditions are warm and wet, and it declines toward the temperate and polar zones as conditions become colder and drier, with more seasonally variable temperatures. Climate and evolutionary history are often considered competing explanations for the latitudinal gradient, but they are linked by the evolutionarily conserved environmental adaptations of species and the history of Earth’s climate system. The tropical conservatism hypothesis (TCH) invokes niche conservatism, climatic limitations on establishment and survival, and paleoclimatic history to explain the latitudinal diversity gradient. Here, we use latitudinal distributions for over 12,500 woody angiosperm species, a fossil-calibrated supertree, and null modeling to test predictions of the TCH. Regional assemblages in the northern and southern temperate zones are less phylogenetically diverse than expected based on their species richness, because temperate taxa are clustered into relatively few clades. Moreover, lineages with temperate affinities are generally younger and nested within older, more tropical lineages. As predicted by the TCH, the vast majority of temperate lineages have arisen since global cooling began at the Eocene-Oligocene boundary (34 Mya). By linking physiological tolerances of species to evolutionary and biogeographic processes, phylogenetic niche conservatism may provide a theoretical framework for a generalized explanation for Earth’s predominant pattern of biodiversity.
The latitudinal gradient in species richness is one of the most consistent patterns in biogeography, but there is little consensus about the relative importance of the processes that generate it (1–3). Climatic conditions vary strongly with latitude, and analyses based on current climatic conditions provide ample explanatory power, at least in a statistical sense, especially in plants (4, 5). Plant diversity generally peaks where climatic conditions are warm, wet, and more seasonally stable, and declines as conditions become colder and drier, with more seasonally variable temperatures (4, 6–8). However, it has been difficult to link these correlative approaches directly with the ecological, evolutionary, and biogeographical processes that generate and maintain biodiversity, namely, diversification (speciation − extinction), dispersal, and local coexistence (9–13). Explaining such broad-scale patterns of diversity thus requires that we consider how climatic variation relates to the ecological processes that structure communities (e.g., physiological tolerances, species interactions) in an explicitly biogeographical and evolutionary context (11, 13–16). Here, we use latitudinal distributions for over 12,500 woody angiosperm species in the New World (17) and a fossil-calibrated supertree (18–21) resolved to the family level to test whether the latitudinal biodiversity gradient shows evidence of historically contingent evolutionary processes. Specifically, we test several predictions of the tropical conservatism hypothesis (TCH).
The TCH (13) links environmental tolerances, diversification, dispersal, and evolutionary history based on two assumptions, one historical and one evolutionary. Historically, tropical (or “megathermal”) environments were much more extensive during the Paleocene and Eocene (65 to 34 Mya) when many currently extant angiosperm lineages were diversifying (22). Evolutionarily, the TCH proposes that due to environmental niche conservatism (23), dispersal from the tropics into the temperate zones is limited by the ability of organisms from historically tropical lineages to adapt to colder, drier climates with more seasonally variable temperatures (23). Based on this line of reasoning, high current tropical diversity results from a combination of (i) differential net diversification rates of tropical lineages due to larger cumulative area of tropical environments, (ii) greater time for diversification in tropical environments, and (iii) limited dispersal of tropical lineages into the temperate environments.
If the TCH is correct, evolutionary transitions between tropical and temperate environments should be relatively rare, because environmental tolerances are conserved. As a result, temperate taxa should represent a phylogenetically clustered subset of the overall species pool and temperate lineages should be nested within tropical clades (24). Moreover, because angiosperms have been evolving for 140–200 million years (My) (19) and temperate and boreal environments have expanded at the expense of tropical environments only since cooling began in the Oligocene (34 Mya), most of these more temperate clades should have originated or diversified relatively recently.
Earlier studies of evolutionary diversity gradient hypotheses (24, 25) were hampered by limited data, but the tremendous growth and synthesis of biogeographical, paleontological, paleoclimatic, and phylogenetic data have reinvigorated evolutionary approaches to the latitudinal gradient (11). Recent studies have found support for the TCH in frogs (26), mammals (27), butterflies (28, 29), and vertebrates (30). In contrast, a recent review of 111 phylogenies representing multiple taxonomic groups, including angiosperms, tested multiple alternative evolutionary hypotheses explaining the latitudinal gradient and found only limited support for the TCH (31). In particular, the TCH was contrasted with the diversification rate hypothesis (DRH) and the “out of the tropics model” (OTM). However, these hypotheses are not strict alternatives; instead, they form a nested hierarchy, with more complex hypotheses adding assumptions to the simpler hypotheses.
The DRH is the least restrictive hypothesis, proposing that the tropics are more diverse simply because the net diversification rate (speciation − extinction) is higher there, whether due to the larger extent of tropical habitats over evolutionary time (32), their climatic stability on multiple time scales (33), or faster rates of molecular evolution and/or coevolution at higher temperatures (1, 34, 35). The OTM builds upon the assumption of differential diversification rates in the tropics and adds differential dispersal from the tropics to the temperate zone, leading to a pattern of most temperate clades having tropical ancestors (32). In turn, the TCH assumes both differential diversification and differential dispersal, but it further assumes that niche conservatism will limit dispersal out of the tropics to a few clades that develop the necessary innovations (36). In addition, the TCH makes assumptions about the timing of dispersal out of the tropics, based on Earth’s paleoclimatic history. Here, we focus primarily on the specific assumptions of the TCH (environmental niche conservatism and the timing of tropical–temperate transitions), although we also examine aspects of the OTM (i.e., differential dispersal, tropical nestedness of temperate clades) in the process.
With specific reference to plants, several recent studies support components of the TCH as a generalized explanation for the latitudinal gradient in angiosperms. On the broadest scale, the diversity of trees across 11 regional forested biomes is correlated with the time-integrated area of that biome since the Eocene (55 Mya) (37), but not with current biome area, which supports the time-for-speciation and cumulative area components of the TCH but does not address the phylogenetic composition of the different biomes. Conversely, a study of over 11,000 Southern Hemisphere plant species demonstrates that shifts from one biome to another are evolutionarily quite rare (38), which implies environmental niche conservatism, but it does not address biogeographic patterns of diversity among biomes. Finally, a global compilation of angiosperm family distributions shows that among arborescent families, the average age of families declines from the tropics into the temperate and boreal zones, as predicted by the TCH (10). However, although temperate families are a nested subset of tropical families (10), and plant physiology and ecology are reasonably conserved at the family level (39–42), the phylogenetic conservatism of latitudinal distributions has never been tested directly.
Here, we use data compiled by Weiser et al. (17) to test whether the TCH can help explain the latitudinal gradient in woody angiosperms (species with persistent, perennial stem tissue, including trees, shrubs, lianas, and hemiepiphytes) in the New World. When growth form information about species was lacking, we included taxa as woody if their genus or family was characteristically woody. These data, drawn from a large synthesis of herbarium records, field guides, and published vegetation surveys, describe the latitudinal distribution based on the northernmost and southernmost records of 12,521 species from 169 families, with range limits spanning from 54.8° S to 74° N latitude. Based on a recent estimate of roughly 150,000 species of seed plants in the New World (43), and assuming that 95% are angiosperms and that ∼40–60% of angiosperms are woody (36), we estimate that our data represent ca. 15–22% of the total diversity of woody angiosperm species in the New World. We focus on the angiosperms because they represent a monophyletic lineage that has undergone substantial diversification since the Cretaceous. Focusing on woody angiosperms also limits variation in life history characteristics that may influence both rates of diversification and biogeographic patterns, which often differ between herbaceous and woody taxa (3, 10, 44). Although our dataset is both taxonomically and geographical broad, it should be noted that species locations are undoubtedly undersampled in the tropics, especially relative to the northern temperate zone. Also, because of our focus on woody species (which are better sampled geographically in the tropics), we are missing or grossly undersampling clades that are dominated by herbaceous taxa. We take these unavoidable limitations into account when interpreting our results.
Based on these distributional data, we calculated a “tropicality index” (TI) for each species as the proportion of its latitudinal range that falls within the tropics minus the proportion of the latitudinal range that falls within temperate areas, which produces a continuous measure from −1 (temperate only) to 0 (one-half temperate, one-half tropical) to 1 (tropical only). Because range sizes and range boundaries of woody plants are closely associated with environmental factors (45, 46), we use TI as a proxy measure of environmental habitat affinity, rather than geographic distribution per se. Although this purely latitudinal definition of “tropical” is very coarse and undoubtedly misclassifies the habitat affinity for some taxa (e.g., tropical montane species), we lacked the comprehensive geographical range information that would be necessary to describe the environmental tolerances of so many species in greater detail, especially for tropical taxa. For convenience, we refer to species or lineages as “temperate” if more than three-fourths of the latitudinal range is in the temperate zone (i.e., −1 ≤ TI ≤ −0.5) and as “semitemperate” if between one-half and three-fourths of the latitudinal range is in the temperate zone (0 ≤ TI < −0.5), with similar definitions for tropical lineages and taxa.
By combining this distributional information with a phylogeny that is well resolved to the family level and reasonably dated based on fossil calibrations (47, 48), we test the key predictions of the TCH:
-
i
) Temperate zone regional assemblages should represent a phylogenetically nonrandom subset of New World plant diversity; temperate species should not simply be drawn at random from across the phylogeny, and characteristically temperate lineages should be nested within older, more tropical clades.
-
ii
) The “tropicality” of lineages should be highly conserved, reflecting the evolutionary inertia of environmental tolerances, and transitions out of the tropics (or out of the temperate) should be relatively rare.
-
iii
) Finally, most of the temperate lineages should have arisen only since the Eocene-Oligocene boundary, when the Earth began to cool and tropical environments contracted relative to the temperate and boreal zones.
Results
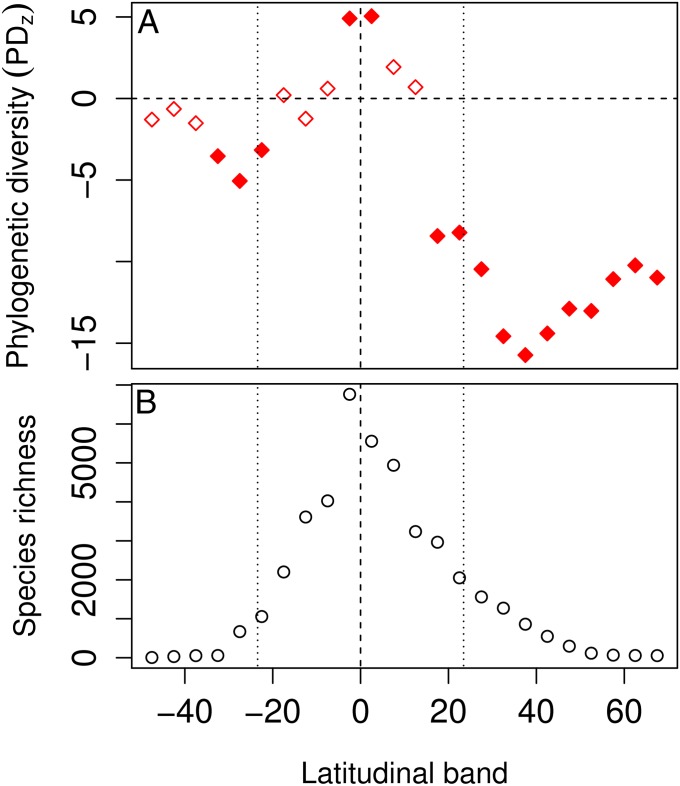
As predicted by the TCH, the woody angiosperm floras of the temperate zones were less phylogenetically diverse than expected (Fig. 1). After standardization for differences in species richness, temperate latitudes in both the Southern and Northern Hemispheres exhibited a lower than expected phylogenetic diversity value (PDz). In the Northern Hemisphere, clustering actually begins south of tropical boundary, and in both hemispheres, the PDz rises from the warm temperate zone toward the poles, even as richness continues to decline. Interestingly, latitudinal bands directly on the equator were actually more phylogenetically diverse than expected, even though they accounted for well over one-half of the species in the database.
Fig. 1.
Latitudinal gradients in standardized PDzs, which are adjusted for latitudinal differences in species richness (A) and species richness (B). Data are for all species overlapping each 5° latitudinal band. Filled symbols in A are significantly phylogenetically clustered (PDz < −1.96) or overdispersed (PDz > 1.96) based on randomizations (Materials and Methods). Vertical lines highlight the equator (dashed line) and the tropical boundaries (dotted line).
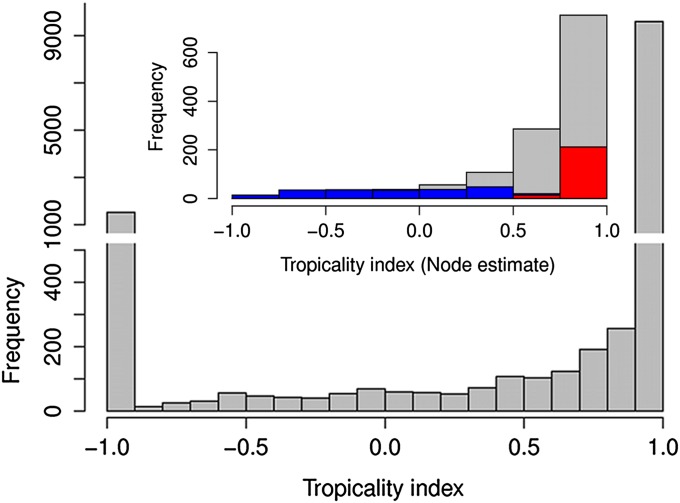
The distribution of species’ TIs was highly skewed and bimodal, with 10,271 tropical species (0.5 < TI ≤ 1) and another 1,646 temperate species (−1 ≤ TI ≤ −0.5; Fig. 2). Of the remaining 604 species, 350 were semitropical (0 < TI ≤ 0.5) and 254 were semitemperate (−0.5< TI ≤ 0). The distribution of tropicality values estimated for 1,329 ancestral nodes exhibited a similar negative skew, with a vast majority of tropical lineages (Fig. 2, Inset). Interestingly, almost all temperate (100%) and semitemperate (92%) nodes and 52% of semitropical nodes exhibited tropicality values that were more temperate than expected, based on a null model randomizing geographical distributions across the tips of the tree (Fig. 2, blue bars). Despite the dominance of tropical taxa in the dataset, 20% of tropical nodes were even more tropical than would be expected if geographical distributions were distributed at random over the phylogeny.
Fig. 2.
Distribution of TIs for 12,521 species (gray) and their 1,033 estimates for ancestral nodes (Inset). For the nodes, colored fractions of the bar represent nodes that were significantly more tropical (red) or temperate (blue) than expected based on 999 randomizations of tropicality values across the tips of the phylogeny.
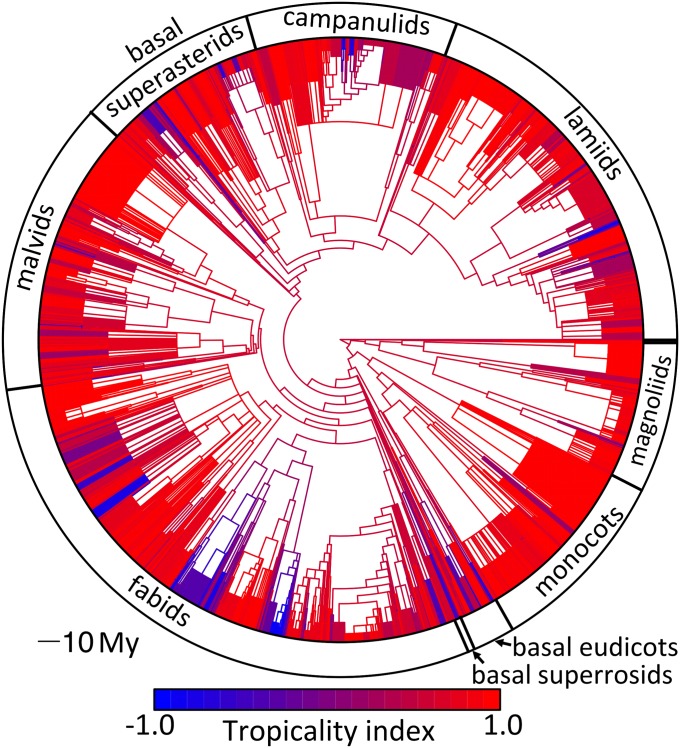
The temperate and semitemperate species (TI < 0) were clearly clustered together and nested within more ancient tropical lineages (Fig. 3). Most prominently, temperate species are strongly clustered in several fabid lineages (Fagales and Rosales) and lineages in the basal superasterids (Caryophyllales, Ericales, and Cornales), as well as in a few less speciose campanuliid, lamiid, and basal eudicot lineages (Table S1). At the same time, temperate species were broadly distributed across the phylogeny. At least one temperate or semitemperate species occurred in 103 of the 169 families in the dataset, but only 66 families contained more than two. In contrast, tropical or semitropical taxa occurred in all but seven families. Although temperate and semitemperate species (TI < 1, n = 1,883) made up only 15% of the species, they made up more than one-half of 23 families. Thus, tropicality appears to exhibit a high degree of phylogenetic structure, as predicted by the TCH.
Fig. 3.
Phylogenetic tree of all 12,521 species represented in the analysis. Edges are colored according to the estimated tropicality value of the ancestral node ranging from red (TI = 1) to blue (TI = −1). The outer circle shows the boundaries of different large clades. (Scale bar: 10 My.)
Evolutionary transitions in tropicality were strongly biased toward the tropical and temperate extremes (Fig. 4; χ2 test: χ2 = 6,923, df = 9, P > 10−15), which further supports the assumption of niche conservatism. Transitions out of the tropics were estimated to be quite rare; the descendants of tropical ancestors retained tropical affinities in 94% of the divergences. Temperate lineages displayed similar conservatism, with temperate ancestors producing similarly temperate descendants 90% of the time (Fig. 4). Conversely, semitropical and semitemperate lineages tended to diverge, producing either more tropical or more temperate descendants. However, despite the relative rarity of evolutionary transitions away from the tropics, 44% of temperate lineages exhibit tropical or semitropical ancestry, in support of the OTM, simply because of the enormous number of tropical lineages.
Fig. 4.
Diagram of ancestral–descendent transitions among different latitudinal zones. Circles are proportional to the log of the number of lineages (nodes + tips) in each zone, and the pie sectors represent the fractional ancestry of descendent lineages in that zone (i.e., the fraction of transitions to that zone from each zone). The size of the arrows and the percentages represent the fraction of transitions from that zone to each zone. Thin dashed arrows represent only 1–4% of transitions out of the respective zones. Zones are defined by ranges of tropicality values: tropical (red): 0.5 ≤ TI ≤ 1.0, semitropical (dark red): 0 ≤ TI < 0.5, semitemperate (dark blue): −0.5 ≤ TI < 0, temperate (blue): −1 ≤ TI < −0.5.
Temperate and semitemperate ancestors (TI < 0) are not only far more prevalent than expected at random (Fig. 2, Inset) but largely confined to the past 34 My, as predicted by the TCH (Fig. 5, gray box at right). Even though most nodes are relatively recent, temperate and semitemperate nodes are significantly younger than tropical and semitropical nodes overall (Wilcoxon rank sum test: W = 71,291, P = 0.002). Interestingly, several of the more ancient temperate lineages originate either within or quite close to earlier cool periods documented in the paleoclimatic record (49).
Fig. 5.
Ancestral estimates for the TI as a function of the crown age (My) of the lineage. Partitions at the top delineate geological epochs [lower (Klow) and upper (Kup) Cretaceous, Paleocene (Pε), Eocene (EO), Oligocene (OG), Miocene (MI), and Pliocene (PO)], whereas the gray bands represent cool periods in the paleoclimate record (49).
Discussion
Our results strongly support the core predictions of the TCH: temperate assemblages represent phylogenetically clustered subsets of woody angiosperm diversity nested within tropical clades, and transitions to temperate habitats are evolutionarily rare, phylogenetically conserved, and concentrated in the past 34 My. The strength of the pattern we find is especially striking because tropical species are almost certainly undersampled in our dataset relative to the temperate species. Adding more species isolated to the tropical environments (TI = 1), and mostly within tropical lineages, would only strengthen the conclusions drawn here. Thus, the TCH is likely an important part of any explanation for the latitudinal species richness gradient in woody angiosperms in the New World.
Our observation that temperate affinities occur mostly in younger lineages is qualitatively consistent with a recent global study of family richness patterns documenting younger average family ages in the temperate zone (10; see also SI Text and Figs. S1 and S2). However, the link between family ages, per se, and the TCH is obscured by the fact that the stem-group ages of most families predate the Oligocene cooling. By using the TI and estimating ancestral states, we were able to avoid the limitations of focusing solely on a single arbitrary taxonomic level and current biogeographic distributions. Although many temperate species are clustered within a few clades, most of the temperate nodes are at the level of genera within families. Thus, many temperate families also contain tropical taxa, and, in turn, they are nested within more tropical lineages.
The strong support we find for the TCH in angiosperms conflicts with a recent survey of 111 phylogenies representing vertebrates, invertebrates, and angiosperms (31), which, in contrast to other recent studies (e.g., ref. 30), finds that transitions from the tropics to the temperate zones are relatively common, in support of the of the OTM rather than the TCH. However, this conclusion may be biased in two ways. First, Jansson et al. (31) assess whether transitions are common by quantifying the fraction of temperate lineages with tropical ancestors, which ignores the fact that even if transition to the temperate by tropical lineages is exceedingly rare, as we find here, many temperate lineages will still have tropical ancestry simply because there are so many more tropical lineages. Second, by limiting their sample to clades that contain both temperate and tropical taxa, these authors may, in fact, overestimate the frequency of transitions by ignoring the many tropical lineages that remain in the tropics, and thus contain no temperate taxa.
In contrast, our analysis may underestimate the frequency of tropical-to-temperate transitions due to several limitations of our dataset. First, because our data are confined to the New World, we miss transitions that occurred in the Old World for cosmopolitan lineages. Second, because we examine only woody taxa, we miss lineages that transition to the temperate by adopting an herbaceous habit, which is a common adaptation to freezing temperatures (24, 36). Third, because our purely geographic designation of the tropics is binary, we may classify as tropical some lineages that, in fact, are adapted to temperate-like environments. At the same time, our unavoidable undersampling of tropical taxa may lead us to overestimate the frequency of transitions from the tropics to the temperate. These empirical constraints can only be resolved through the integrative development of global, taxonomically comprehensive distributional, phylogenetic, climatic, and ecophysiological data resources.
Keeping these caveats in mind, several subtler aspects of our results warrant further comment. First, temperate assemblages in the Northern Hemisphere appear to be more phylogenetically distinct than in the Southern Hemisphere. The difference may be due to the lower level of sampling in the Southern Hemisphere, as evidenced by the fact that our dataset includes just 179 southern temperate zone species, compared with 1,704 northern temperate zone species. However, the PDz measure controls for differences in species richness. Thus, this result likely reflects the fact that biomes dominated by temperate-adapted woody vegetation are both of much smaller extent and more geographically isolated in the Southern Hemisphere than in Northern Hemisphere, providing both more area and more time for diversification in the northern temperate zone (37, 50, 51).
Second, it is also interesting that the two latitudinal bands adjacent to the equator (which contained 7,368 of the 12,521 species) were also phylogenetically overdispersed. A similar pattern of regional phylogenetic overdispersion observed among palms (Arecaceae) worldwide (including equatorial South America) has been ascribed to contact zones between biogeographic realms (52). These observations suggest that the recent recognition of a Panamanian zoogeographic zone that is distinct from the traditional Neotropical biogeographic zone (53) may apply to plants as well. On a more regional scale, the east-west rainfall gradient of equatorial South America and the development of complex topography with the Andean uplift during the Cretaceous to Oligocene (54) may contribute to phylogenetic overdispersion through the turnover of lineages along environmental gradients [phylogenetic β-diversity (55, 56)].
Third, the increase in phylogenetic diversity from the warm temperate zone to the poles may be related to patterns of latitudinal extent. In our data, latitudinal extents increase with latitude, especially in temperate North America (17). Among North American tree species, this increase in range size with latitude, known as “Rapoport’s rule” (57), is consistent with the hypothesis that a latitudinal gradient in climatic variability selects for species with broader climatic tolerances at high latitudes (45). As a result, high-latitude temperate assemblages tend to represent biogeographic subsets of those found at lower latitudes. Thus, although the transition from the tropics to the temperate zone involves the loss of larger, more deeply rooted tropical clades, reductions of species richness at higher latitudes occur mostly through the loss of small-ranged taxa toward the tips of the phylogeny.
Finally, several of the temperate lineages originating long before the Oligocene may have been associated with earlier cool periods (Fig. 5), which suggests that the TCH may explain earlier colonizations of the temperate zone as well. Paleoclimate proxies suggest generally warm temperatures before the Oligocene, with a greatly reduced temperature gradient from the equator to the poles (58, 59). However, the late Cretaceous was punctuated with several shorter cooling episodes, some lasting several My (49, 60, 61), and the first angiosperm-dominated deciduous forest environments appeared during the middle to late Cretaceous, often at very high latitudes (22, 62, 63). Thus, the same dynamics of temperate adaptation and niche conservatism could apply during these shorter Cretaceous cool periods as well. Clearly, the phylogenetic and paleoclimate age estimates we use here are subject to considerable uncertainty; however, as the temporal and spatial resolutions of paleoclimate reconstructions and the fossil calibrations of molecular phylogenies improve, the evolutionary details of paleobiogeographic patterns should come into clearer focus.
Phylogenetic niche conservatism provides a biological link between the physiological tolerances of species and the evolutionary processes that generate patterns of biodiversity along environmental gradients (10, 11, 13, 23, 25, 39). As such, our analyses indicate that although climate and evolutionary history are often considered competing hypotheses for explaining the latitudinal gradient (1, 2), they are, in fact, complementary (14, 16, 64). For example, Hawkins et al. (10) found that in explaining the latitudinal gradient and angiosperm family richness, more than two-thirds of the explanatory power of climate was confounded with family age, making it impossible to separate the two influences. The correlation between climatic and biodiversity gradients stems from how long-term variation in climate affects macroevolutionary processes. By integrating both evolutionary and ecological processes that generate biodiversity gradients, the niche conservatism perspective may provide a framework for bringing together disparate hypotheses that have all too frequently been considered in isolation. Any generalized explanation for Earth’s predominant pattern of biodiversity will clearly have to be flexible enough to provide this sort of synthesis.
Materials and Methods
Data.
We obtained data on the ranges of 12,521 woody angiosperm species (perennial trees, shrubs, lianas, and hemiepiphytes), representing 169 families, from the Synthesis and Analysis of Local Vegetation Inventories Across Scales (SALVIAS) database (www.salvias.net), which is drawn from an extensive compilation of field guides, regional floras, and online herbarium databases (17). A breakdown of species among the major angiosperm lineages is provided in Table S1.
Our phylogeny was based on the consensus supertree of the Angiosperm Phylogeny Group (65, 66). In particular, we used the angiosperm tree provided by Phylomatic (www.phylodiversity.net, tree R20120892) (48). We then used divergence time estimates based on 560 angiosperm taxa, three genes, and 35 fossil calibration points (19, 47) to assign ages (in My) to 109 nodes within the phylogeny. Whenever the two calibration studies conflicted, we used dates from the more recent study (47). The remaining nodes were assigned an age corresponding to the midpoint between their nearest dated ancestral and descendent nodes using Phylocom (67). Because of the large taxonomic scope of our data and the poor resolution of most intrafamilial phylogenies, we treated all confamilial genera and congeneric species as polytomies unless they were resolved in the original supertree. The coarseness of our phylogenetic resolution and dating procedure places limits on the details of our analysis, but this lack of precision should not bias our ability to detect broad patterns across 144 My of evolution and 12,521 taxa.
As described above, we assigned each species a TI, based on its latitudinal range boundaries, using 23.5°N and S as the tropical boundaries. To compile regional assemblages along the latitudinal gradient, we tallied the species overlapping each 5° latitudinal band from −50° S to 70° N.
Analyses.
To test whether temperate taxa are a phylogenetically restricted subset of New World angiosperms, we calculated Faith’s phylogenetic diversity (PD) (68) for each latitudinal band using the Picante package in R 2.15.2 (69, 70). Because PD depends on species richness, we standardized the PDz by subtracting the mean and dividing by the SD of a distribution of values from 999 randomizations of the species identities across the tips of the phylogenies. If the observed PDz was in the lower 2.5% of the random distribution (α = 0.05, two-tailed test), the assemblage in that latitudinal band was considered phylogenetically clustered, given the species richness of that band and the underlying phylogeny. Likewise, an observed PDz in the upper 2.5% of the distribution signifies phylogenetic overdispersion of the species present in the band.
To estimate ancestral tropicality values, we compared multiple models of character evolution, including white noise (WN), Pagel’s λ-transformed random walk (RW), Brownian motion (BM), and Ornstein–Uhlenbeck processes, using the R package GEIGER (71). A comparison of Akaike’s information criterion for the different models suggested that the RW model provided the best fit to the data with λ = 0.76 (Tables S2 and S3). We then used maximum likelihood methods to reconstruct ancestral tropicality values based on sequential rerooting of the phylogeny [the fastAnc function in the R package Phytools (72)]. Ancestral estimates from the different models (except WN, which ignores the phylogeny) were highly correlated with one another (r = 0.92–0.98; Tables S2 and S3) and with estimates made using Felsenstein’s (73) contrast method, made using Phylocom (74) (Fig. S3). Thus, although we present the RW estimates, our results do not depend upon a particular model of character evolution. The alternative estimates are provided in SI Text. Node estimates (from Felsenstein’s contrast method) were compared with 999 randomizations of species across the tips of the phylogeny to identify lineages that are significantly more tropical or more temperate than expected. For tree manipulation and visualization, we used the APE package (75). All R code used in the analysis is available from the corresponding author (A.J.K.).
Supplementary Material
Acknowledgments
We acknowledge the enormous number of botanists, taxonomists, curators, systematists, data managers, and programmers whose efforts have made this study possible. Comments from Brad Boyle, Nate Swenson, Mike Moore, Michael Donoghue, and two anonymous reviewers, as well as conversations with Richard Field and Brad Hawkins, greatly improved both the study and the manuscript. A.J.K. received support from National Science Foundation (NSF) Research Opportunity Award EF-1214332 supplemental to NSF Grant EF-1065861 (Brian Enquist, Principal Investigator) and a sabbatical supplement from Kenyon College. M.D.W. was supported by NSF Grant EF-1065844 (Mike Kaspari, Principle Investigator).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308932111/-/DCSupplemental.
References
- 1.Rohde K. Latitudinal gradients in species-diversity: The search for the primary cause. Oikos. 1992;65(3):514–527. [Google Scholar]
- 2.Willig MR, Kaufman DM, Stevens RD. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu Rev Ecol Evol Syst. 2003;34:273–309. [Google Scholar]
- 3.Hillebrand H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163(2):192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- 4.Francis AP, Currie DJ. A globally consistent richness-climate relationship for angiosperms. Am Nat. 2003;161(4):523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- 5.Currie DJ. Energy and large-scale patterns of animal-species and plant-species richness. Am Nat. 1991;137(1):27–49. [Google Scholar]
- 6.Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 2007;104(14):5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien EM, Field R, Whittaker RJ. Climatic gradients in woody plant (tree and shrub) diversity: Water-energy dynamics, residual variation, and topography. Oikos. 2000;89(3):588–600. [Google Scholar]
- 8.Swenson NG, et al. The biogeography and filtering of woody plant functional diversity in North and South America. Glob Ecol Biogeogr. 2012;21(8):798–808. [Google Scholar]
- 9.Currie DJ, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol Lett. 2004;7(12):1121–1134. [Google Scholar]
- 10.Hawkins BA, Rodriguez MA, Weller SG. Global angiosperm family richness revisited: Linking ecology and evolution to climate. J Biogeogr. 2011;38(7):1253–1266. [Google Scholar]
- 11.Mittelbach GG, et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol Lett. 2007;10(4):315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 12.Qian H, Ricklefs RE. Taxon richness and climate in angiosperms: Is there a globally consistent relationship that precludes region effects? Am Nat. 2004;163(5):773–779, discussion 780–785. doi: 10.1086/383097. [DOI] [PubMed] [Google Scholar]
- 13.Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends Ecol Evol. 2004;19(12):639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Kozak KH, Wiens JJ. Phylogeny, ecology, and the origins of climate-richness relationships. Ecology. 2012;93(8) Suppl.:S167–S181. [Google Scholar]
- 15.McGlone MS. When history matters: Scale, time, climate and tree diversity. Global Ecology and Biogeography Letters. 1996;5(6):309–314. [Google Scholar]
- 16.Ricklefs RE. Evolutionary diversification and the origin of the diversity-environment relationship. Ecology. 2006;87(7) Suppl:S3–S13. doi: 10.1890/0012-9658(2006)87[3:edatoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Weiser MD, et al. Latitudinal patterns of range size and species richness of New World woody plants. Glob Ecol Biogeogr. 2007;16(5):679–688. [Google Scholar]
- 18.Stevens PF. Angiosperm Phylogeny Website. St. Louis, MO: Missouri Botanical Garden; 2010. [Google Scholar]
- 19.Wikström N, Savolainen V, Chase MW. Evolution of the angiosperms: Calibrating the family tree. Proc Biol Sci. 2001;268(1482):2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltis PS, Soltis DE. The origin and diversification of angiosperms. Am J Bot. 2004;91(10):1614–1626. doi: 10.3732/ajb.91.10.1614. [DOI] [PubMed] [Google Scholar]
- 21.Davies TJ, et al. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc Natl Acad Sci USA. 2004;101(7):1904–1909. doi: 10.1073/pnas.0308127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane PR, Lidgard S. Angiosperm diversification and paleolatitudinal gradients in cretaceous floristic diversity. Science. 1989;246(4930):675–678. doi: 10.1126/science.246.4930.675. [DOI] [PubMed] [Google Scholar]
- 23.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13(10):1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 24.Judd WS, Sanders RW, Donoghue MJ. Angiosperm family pairs: Preliminary phylogenetic analyses. Harv Pap Bot. 1994;5:1–51. [Google Scholar]
- 25.Latham RE, Ricklefs RE. Continental comparisons of temperate-zone tree species diversity. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities: Historical and Geographic Perspectives. Chicago: Univ of Chicago Press; 1993. pp. 294–314. [Google Scholar]
- 26.Wiens JJ, Sukumaran J, Pyron RA, Brown RM. Evolutionary and biogeographic origins of high tropical diversity in old world frogs (Ranidae) Evolution. 2009;63(5):1217–1231. doi: 10.1111/j.1558-5646.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 27.Buckley LB, et al. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc R Soc Lond B Biol Sci. 2010;277(1691):2131–2138. doi: 10.1098/rspb.2010.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condamine FL, Sperling FAH, Wahlberg N, Rasplus J-Y, Kergoat GJ. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol Lett. 2012;15(3):267–277. doi: 10.1111/j.1461-0248.2011.01737.x. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins BA, DeVries PJ. Tropical niche conservatism and the species richness gradient of North American butterflies. J Biogeogr. 2009;36(9):1698–1711. [Google Scholar]
- 30.Smith BT, Bryson RW, Jr, Houston DD, Klicka J. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecol Lett. 2012;15(11):1318–1325. doi: 10.1111/j.1461-0248.2012.01855.x. [DOI] [PubMed] [Google Scholar]
- 31.Jansson R, Rodríguez-Castañeda G, Harding LE. What can multiple phylogenies say about the latitudinal diversity gradient? A new look at the tropical conservatism, out of the tropics, and diversification rate hypotheses. Evolution. 2013;67(6):1741–1755. doi: 10.1111/evo.12089. [DOI] [PubMed] [Google Scholar]
- 32.Jablonski D, Roy K, Valentine JW. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314(5796):102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- 33.Dynesius M, Jansson R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc Natl Acad Sci USA. 2000;97(16):9115–9120. doi: 10.1073/pnas.97.16.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen AP, Brown JH, Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297(5586):1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- 35.Davies TJ, Barraclough TG, Savolainen V, Chase MW. Environmental causes for plant biodiversity gradients. Philos Trans R Soc Lond B Biol Sci. 2004;359(1450):1645–1656. doi: 10.1098/rstb.2004.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanne AE, et al. Three keys to the radiation of angiosperms into freezing environments. Nature. 2014;506(7486):89–92. doi: 10.1038/nature12872. [DOI] [PubMed] [Google Scholar]
- 37.Fine PVA, Ree RH. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am Nat. 2006;168(6):796–804. doi: 10.1086/508635. [DOI] [PubMed] [Google Scholar]
- 38.Crisp MD, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458(7239):754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 39.Donoghue MJ. Colloquium paper: A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackerly DD, Reich PB. Convergence and correlations among leaf size and function in seed plants: A comparative test using independent contrasts. Am J Bot. 1999;86(9):1272–1281. [PubMed] [Google Scholar]
- 41.Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am Nat. 2006;168(4):E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 42.Prinzing A, Durka W, Klotz S, Brandl R. The niche of higher plants: Evidence for phylogenetic conservatism. Proc Biol Sci. 2001;268(1483):2383–2389. doi: 10.1098/rspb.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govaerts R. How many species of seed plants are there? Taxon. 2001;50(4):1085–1090. [Google Scholar]
- 44.Smith SA, Donoghue MJ. Rates of molecular evolution are linked to life history in flowering plants. Science. 2008;322(5898):86–89. doi: 10.1126/science.1163197. [DOI] [PubMed] [Google Scholar]
- 45.Morin X, Lechowicz MJ. Geographical and ecological patterns of range size in North American trees. Ecography. 2011;34(5):738–750. [Google Scholar]
- 46.Pither J. Climate tolerance and interspecific variation in geographic range size. Proc Biol Sci. 2003;270(1514):475–481. doi: 10.1098/rspb.2002.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. Am J Bot. 2010;97(8):1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- 48.Webb CO, Donoghue MJ. Phylomatic: Tree assembly for applied phylogenetics. Mol Ecol Notes. 2005;5(1):181–183. [Google Scholar]
- 49.Royer DL. CO2-forced climate thresholds during the Phanerozoic. Geochim Cosmochim Acta. 2006;70(23):5665–5675. [Google Scholar]
- 50.Beerling DJ, Woodward FI. Vegetation and the Terrestrial Carbon Cycle: Modeling the First 400 Million Years. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 51.Ziegler AM, et al. Tracing the tropics across land and sea: Permian to present. Lethaia. 2003;36(3):227–254. [Google Scholar]
- 52.Kissling WD, et al. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proc Natl Acad Sci USA. 2012;109(19):7379–7384. doi: 10.1073/pnas.1120467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holt BG, et al. An update of Wallace’s zoogeographic regions of the world. Science. 2013;339(6115):74–78. doi: 10.1126/science.1228282. [DOI] [PubMed] [Google Scholar]
- 54.Antonelli A, Nylander JAA, Persson C, Sanmartín I. Tracing the impact of the Andean uplift on Neotropical plant evolution. Proc Natl Acad Sci USA. 2009;106(24):9749–9754. doi: 10.1073/pnas.0811421106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine PVA, Kembel SW. Phylogenetic community structure and phylogenetic turnover across space and edaphic gradients in western Amazonian tree communities. Ecography. 2011;34(4):552–565. [Google Scholar]
- 56.Graham CH, Fine PVA. Phylogenetic beta diversity: Linking ecological and evolutionary processes across space in time. Ecol Lett. 2008;11(12):1265–1277. doi: 10.1111/j.1461-0248.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- 57.Stevens GC. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am Nat. 1989;133(2):240–256. [Google Scholar]
- 58.Bijl PK, et al. Early Palaeogene temperature evolution of the southwest Pacific Ocean. Nature. 2009;461(7265):776–779. doi: 10.1038/nature08399. [DOI] [PubMed] [Google Scholar]
- 59.Greenwood DR, Wing SL. Eocene continental climates and latitudinal temperature gradients. Geology. 1995;23(11):1044–1048. [Google Scholar]
- 60.Royer DL, Berner RA, Montanez IP, Tabor NJ, Beerling DJ. CO2 as a primary driver of Phanerozoic climate. GSA Today. 2004;14(3):4–10. [Google Scholar]
- 61.Spicer RA, Parrish JT. Late Cretaceous early Tertiary paleoclimates of Northern high-latitudes—A quantitative view. J Geol Soc London. 1990;147(2):329–341. [Google Scholar]
- 62.Graham A. The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. Am J Bot. 2011;98(3):336–351. doi: 10.3732/ajb.1000353. [DOI] [PubMed] [Google Scholar]
- 63.Wolfe JA. Late Cretaceous-Cenozoic history of deciduousness and the terminal Cretaceous event. Paleobiology. 1987;13(2):215–226. [Google Scholar]
- 64.Jetz W, Fine PVA. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 2012;10(3):e1001292. doi: 10.1371/journal.pbio.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremer B, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009;161(2):105–121. [Google Scholar]
- 66.Bremer B, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc. 2003;141(4):399–436. [Google Scholar]
- 67.Ackerly DD. Taxon sampling, correlated evolution, and independent contrasts. Evolution. 2000;54(5):1480–1492. doi: 10.1111/j.0014-3820.2000.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 68.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61(1):1–10. [Google Scholar]
- 69.Team RDC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 70.Kembel SW, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26(11):1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 71.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. GEIGER: Investigating evolutionary radiations. Bioinformatics. 2008;24(1):129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 72.Revell LJ. Phytools: An R package for phylogenetic comparative biology (and other things) Methods in Ecology and Evolution. 2012;3(2):217–223. [Google Scholar]
- 73.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125(1):1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- 74.Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24(18):2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- 75.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.