Significance
We report the presence of a previously unidentified cholinergic, polymodal chemosensory cell in the mammalian urethra, the potential portal of entry for bacteria and harmful substances into the urogenital system. These cells exhibit structural markers of respiratory chemosensory cells (“brush cells”). They use the classical taste transduction cascade to detect potential hazardous compounds (bitter, umami, uropathogenic bacteria) and release acetylcholine in response. They lie next to sensory nerve fibers that carry acetylcholine receptors, and placing a bitter compound in the urethra enhances activity of the bladder detrusor muscle. Thus, monitoring of urethral content is linked to bladder control via a previously unrecognized cell type.
Abstract
Chemosensory cells in the mucosal surface of the respiratory tract (“brush cells”) use the canonical taste transduction cascade to detect potentially hazardous content and trigger local protective and aversive respiratory reflexes on stimulation. So far, the urogenital tract has been considered to lack this cell type. Here we report the presence of a previously unidentified cholinergic, polymodal chemosensory cell in the mammalian urethra, the potential portal of entry for bacteria and harmful substances into the urogenital system, but not in further centrally located parts of the urinary tract, such as the bladder, ureter, and renal pelvis. Urethral brush cells express bitter and umami taste receptors and downstream components of the taste transduction cascade; respond to stimulation with bitter (denatonium), umami (monosodium glutamate), and uropathogenic Escherichia coli; and release acetylcholine to communicate with other cells. They are approached by sensory nerve fibers expressing nicotinic acetylcholine receptors, and intraurethral application of denatonium reflexively increases activity of the bladder detrusor muscle in anesthetized rats. We propose a concept of urinary bladder control involving a previously unidentified cholinergic chemosensory cell monitoring the chemical composition of the urethral luminal microenvironment for potential hazardous content.
Mucosal surfaces of the mammalian respiratory and gastrointestinal tract contain solitary epithelial cells with characteristic microvilli at their tip, from which the name “brush cells” derives (1–3). In the respiratory tract, these brush cells serve as sentinels, using the canonical taste transduction cascade to monitor the mucosal lining fluid for potential harmful substances, such as “bitter” bacterial products, and evoking reflexes aimed at combating further ingression of such compounds, such as closure of ducts leading into adjacent compartments (vomeronasal organ) or respiratory reflexes (4–7).
In line with such a sentinel function, brush cell abundance decreases with increasing distance to the opening to the outside world, being numerous in the nose and nearly absent in the intrapulmonary airways. Physiologically, the urinary tract allows passage in only one direction to release urine, but ascending infection by uropathogenic bacteria is not uncommon and is a major risk factor in fatal kidney disease and male infertility (8, 9). We hypothesized that the urogenital tract also may be equipped with chemosensory sentinel cells that monitor the lumen for potential hazardous content.
Results
Brush Cells Are Positioned at the Portal of Entry into the Urinary Tract.
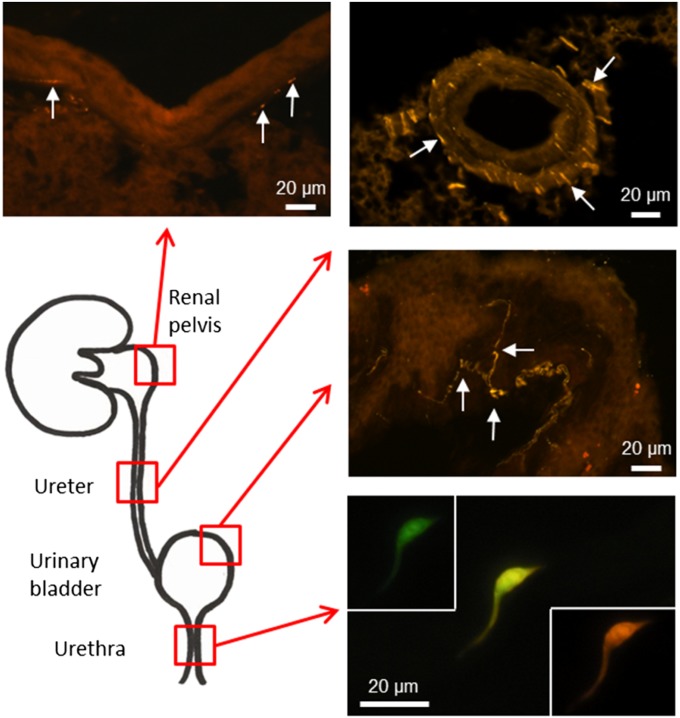
The majority of chemosensory brush cells of the mouse airways use acetylcholine as a signaling molecule and can be readily identified in mice expressing GFP driven by the promoter of the acetylcholine-synthesizing enzyme choline acetyltransferase (ChAT-eGFP mice) (4, 6). On screening of tissue sections and whole-mount preparations of the urogenital tract of two independently generated ChAT-eGFP mouse strains (10, 11), we observed solitary ChAT-eGFP+ cells in the epithelium of the urethra, but not in the epithelial linings of the renal pelvis, ureter, urinary bladder, and pelvic segment of the vas deferens (Fig. 1 and Fig. S1). The total numbers of such urethral cells did not differ significantly between male and female animals (mean ± SD, 379 ± 99 and 551 ± 253, respectively, P = 0.26, t test; n = 6 for each sex). In male prostate, coagulating, and seminal glands and in both male and female paraurethral glands, these cells are located in the excretory ducts close to the opening into the urethra, but not in the glandular bodies themselves (Fig. S2). These cells vary in shape from the typical flask-like structure of tracheal brush cells, with a broad base at the basement membrane and an elongated tip reaching the lumen, to more complex morphologies with slender foot processes reaching the basal lamina directly or in an oblique course (Figs. 2 and 3). Horizontally oriented cell bodies with unclear connections to the luminal surface are seen as well (Fig. 3 A and B).
Fig. 1.
Cholinergic eGFP-expressing epithelial cells are restricted to the urethra in the urinary tract. Visualization of GFP was enhanced by application of a chicken anti-eGFP antibody, followed by Cy3-conjugated anti-chicken Ig. GFP and Cy3 channels are shown separately and also in the merged image for the slender epithelia cell in the urethra. In all other parts of the urinary system, cholinergic nerve fibers (arrows) were visualized by this procedure, but no ChAT-eGFP–expressing epithelial cells were seen.
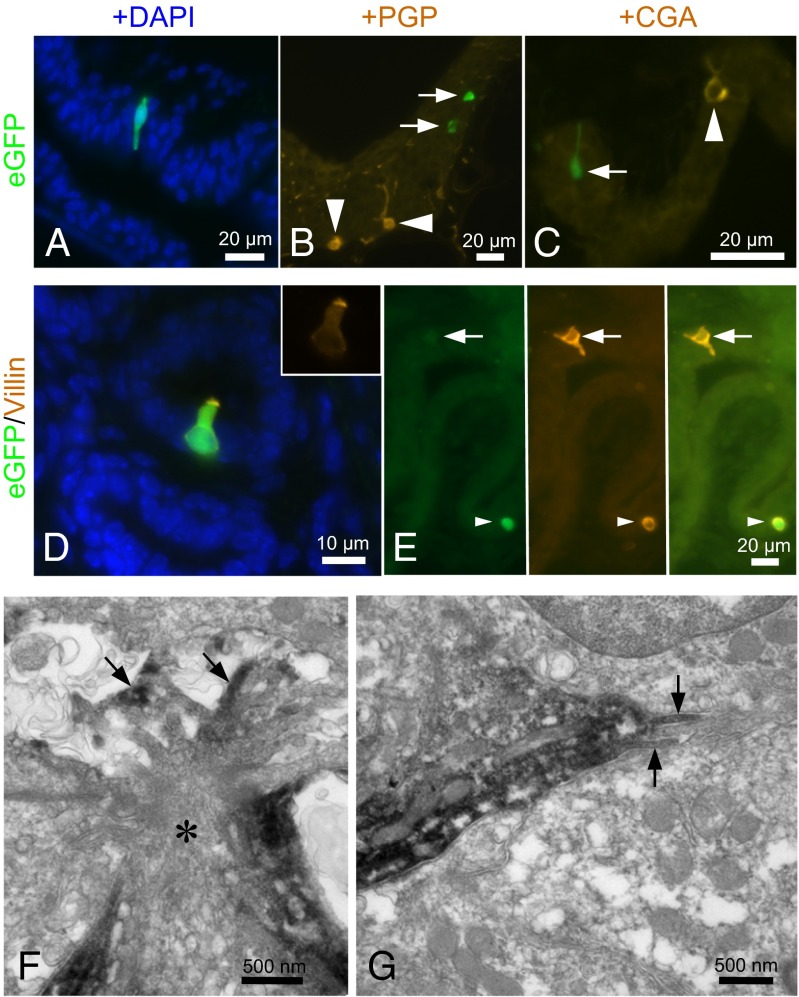
Fig. 2.
A cholinergic epithelial cell of the urethra is a brush cell, not a neuroendocrine cell. (A) Male penile urethra. A slender ChAT-eGFP cell extends through the epithelial layer. Nuclei are counterstained with DAPI. (B) Female urethra. ChAT-eGFP cells (arrows) are distinct from protein gene product 9.5 (PGP)-immunoreactive neuroendocrine cells (arrowheads). (C) Male urethra. The ChAT-eGFP cell (arrow) does not express the neuroendocrine cell marker chromogranin A (CGA). The arrowhead indicates a CGA+ neuroendocrine cell. (D) Male urethra, colliculus seminalis. Merged image showing eGFP (green), villin immunoreactivity (orange), and cell nuclei (DAPI; blue). The flask-shaped ChAT-eGFP cell is immunoreactive for the brush cell marker villin. (Inset) Villin only, showing the concentration of this microvillous protein at the apical cell pole. (E) Male urethra. Another villin+ brush cell is not ChAT+ (arrows). Arrowheads indicate double-positive cells. (F) Female urethra, pre-embedding ultrastructural eGFP immunolabeling. The apical region of the eGFP-immunoreactive cell (asterisk) exhibits characteristic brush cell features, with numerous filaments, tubulovesicular structures, and microvilli (arrows) projecting into the lumen. In this particular case, the microvilli extend into an intercellular cavity in the upper layer of the epithelium that had no visible connection to the urethral lumen. (G) Same cell as depicted in F exhibiting lateral microvilli (arrows), another brush cell feature.
Fig. 3.
Cholinergic urethral brush cells express components of the canonical taste transduction cascade. (A) Male urethra. ChAT-eGFP (green) and TRPM5 (orange) are expressed by the same cell; nuclei are labeled in blue with DAPI. (B) Female urethra. An obliquely oriented ChAT-eGFP cell is immunolabeled for PLCβ2. In this section plane, no connection of this cell to the urethral lumen is visible. (C) Male urethra, diverticular region. α-Gustducin (αGust)+ ChAT-eGFP cell extending a slender process toward the lumen. (D) Schematic drawing of the canonical transduction cascade known from taste buds (based upon information published in ref. 49) depicting the position and role of α-gustducin, PLCβ2, and TRPM5 in this process. DAG, diacylglycerol; ER, endoplasmic reticulum; IP3, inositol trisphosphate; TasR, taste receptor.
Double-labeling procedures revealed that these cells are distinct from the previously known urethral neuroendocrine cell population (12, 13) expressing protein gene product 9.5, serotonin, and chromogranin A (Fig. 2 B and C and Table S1). In contrast to these neuroendocrine cells, nearly all (>90%) urethral ChAT-eGFP+ cells express villin (Fig. 2D and Table S2) and at the ultrastructural level exhibit a tuft of apical microvilli and additional basolateral microvilli (Fig. 2 F and G), the defining features of respiratory and gastrointestinal brush cells (14). A significant additional population of urethral villin-positive cells (≥50%) lacked ChAT-eGFP fluorescence (Fig. 2E and Table S2), indicative of the presence of distinct populations of cholinergic and noncholinergic urethral brush cells.
Cholinergic Urethral Brush Cells Use the Canonical Taste Transduction Cascade to Detect Bitter Substances.
Respiratory chemosensory brush cells express elements of the canonical taste transduction cascade, including the taste-specific G protein α-gustducin, phospholipase Cβ2 (PLCβ2), and the transient potential receptor cation channel melanostatin 5 (TRPM5) (5, 6, 15–17). These proteins are also expressed in urethras from ChAT-eGFP and WT mice (Fig. 3 A–D). ChAT-eGFP and TRPM5 immunolabeling matched nearly 1:1 (Fig. 3A and Table S3), and the majority of ChAT-eGFP+ cells exhibited PLCβ2 immunoreactivity (Fig. 3B), although an additional PLCβ2+/ChAT-eGFP− cell population exists (Table S3 and Fig. S3 A and B). Clear α-gustducin immunolabeling was observed in approximately one-third of ChAT-eGFP+ cells (Fig. 3C, Table S3, and Fig. S3A). These incomplete colocalization patterns argue for the presence of subpopulations of urethral brush cells, including cholinergic/TRPM5+ and noncholinergic cells, mostly TRPM5− cells.
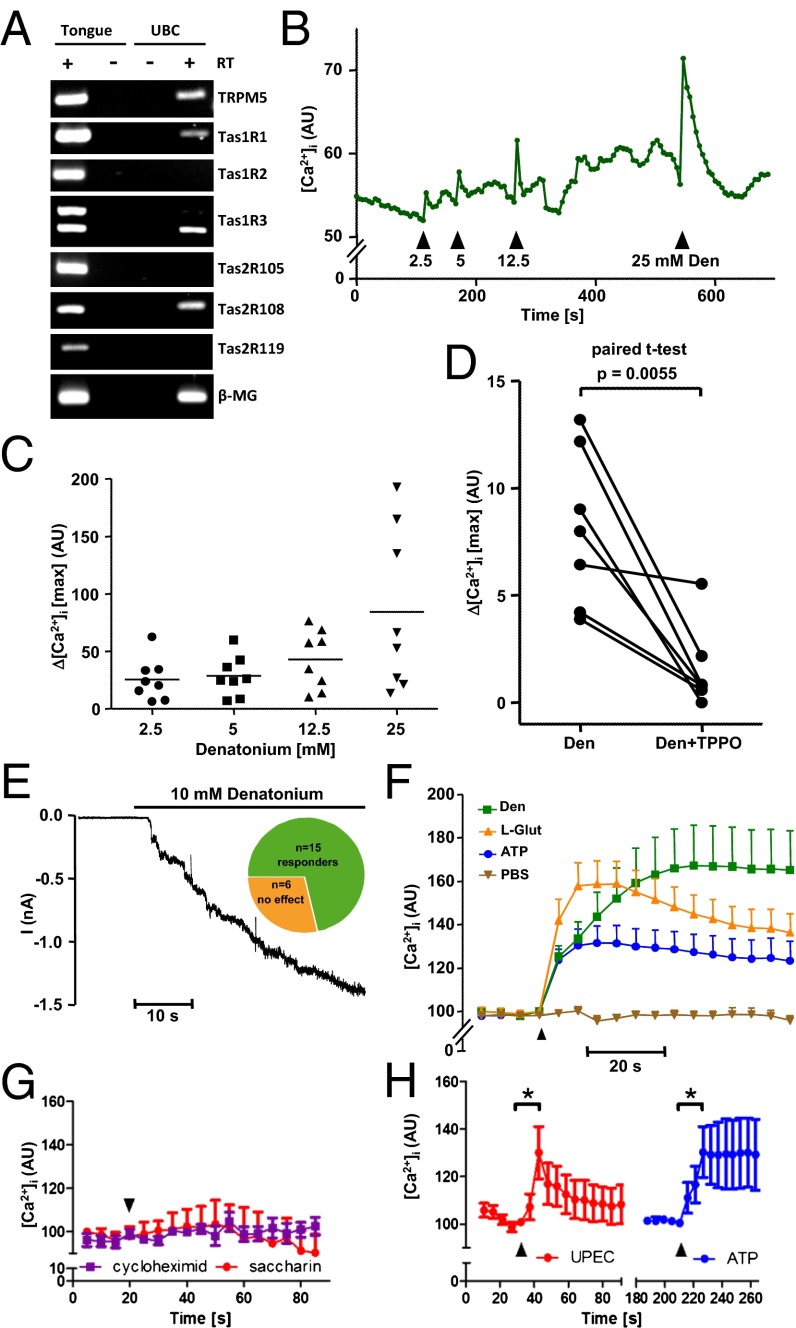
Accordingly, TRPM5 antibody, different from that used for immunolabeling and directed against an extracellular domain, served to isolate the presumptive cholinergic chemosensory cells for RT-PCR analysis. This analysis revealed mRNA expression of the bitter taste receptor Tas2R108 (for which denatonium is a ligand), but not of Tas2R105 (for which cycloheximide is a ligand) or of Tas2R119 (Fig. 4A). Cellular responses in intracellular calcium concentration ([Ca2+]i) to unspecific (e.g., ATP) and gustatory stimuli were recorded from dissociated cells identified by ChAT-eGFP fluorescence and from cells isolated from WT and ChAT-eGFP mice via the TRPM5 antibody conjugated to magnetic beads. Consistent with detection of Tas2R108 mRNA, denatonium caused a dose-dependent (2.5–25 mM) increase in [Ca2+]i in the majority of cells tested (45/49; 92%) (Fig. 4 B and C). Although TPRM5 itself is a monovalent-specific cation channel activated by Ca2+ rather than a Ca2+ channel itself (18), its inhibition by triphenylphosphine oxide (TPPO) is known to significantly reduce the tastant-induced rise in [Ca2+]i in mouse taste bud cells (19); we found that this action applies to denatonium responses in urethral cells as well (Fig. 4D). In whole-cell patch-clamp recordings, denatonium caused activation of a delayed and long-lasting inward current in 15 of 21 cells (Fig. 4E). In addition, immediate and highly reversible currents were triggered by short, repetitive pulses of denatonium (Fig. S4).
Fig. 4.
Urethral cholinergic brush cells are polymodal chemosensors. (A) RT-PCR, agarose gel. Urethral brush cells (UBCs) were isolated by magnetic beads coated with TRPM5 antibody. Tongue, positive control; β2-microglobulin (β-MG), housekeeping gene; +/− RT, aliquots processed with/without reverse transcription. (B and C) Dose-dependent increase in [Ca2+]i in dissociated UBC in response to the Tas2R108 agonist denatonium. All drugs were added under continuous flow in the chamber, so that indicated concentrations were reached initially and then washed out. (B) Confocal laser scanning recording of changes in Calcium Orange fluorescence in a single cholinergic (eGFP+) brush cell. The y axis depicts arbitrary units (AU) correlating to [Ca2+]i. (C) The y axis depicts maximum [Ca2+]i increases in eight cholinergic cells in response to increasing denatonium concentrations. (D) Same technique as in B and C. Shown are maximum [Ca2+]i increases evoked by 25 mM denatonium before and after application of the TRPM5 inhibitor TPPO (0.25 mM) in seven cholinergic brush cells. (E) Whole-cell patch-clamp recording, with membrane potential clamped to −60 mV. Increased inward current was observed in 15 of 21 cells (responders). (F–H) Same technique as in B–D. (F) Superimposed calcium increases in 32 polymodal cells that responded to all three stimuli (0.5 mM ATP, 25 mM denatonium, and 25 mM l-glutamate). (G) In contrast, dissociated cells did not respond to the Tas2R105 agonist cycloheximide (Cyc; 0.1 mM) or to the artificial sweetener saccharin (Sac; 5 mM). (H) Heat-inactivated uropathogenic E. coli (UPEC; 2–5 × 107 cfu) induced [Ca2+]i increase to the same extent as ATP. Graphs depict mean and SEM. *P < 0.05, paired t test.
Cholinergic Urethral Brush Cells Are Polymodal Chemosensors.
Tas1R family members (Tas1R1–3) participate in sweet and umami (free L-amino acids) perception. Isolated urethral cholinergic chemosensory cells expressed mRNAs coding for Tas1R1 and Tas1R3, whereas Tas1R2 expression was not detected (Fig. 4A). Tas1R1–Tas1R3 coexpression yields an umami receptor (20). Accordingly, urethral brush cells responded with increases in [Ca2+]i to monosodium glutamate (umami, 25 mM; 90%; 38/42 cells) in addition to denatonium and ATP (0.5 mM; 91%; 43/47 cells). The majority of cells (86%; 36/42) responded to both the bitter receptor agonist denatonium and glutamate, exhibiting properties of a polymodal (bitter/umami) chemosensor (Fig. 4F).
Notably, not all bitter substances triggered responses in urethral brush cells. In line with the lack of detectable Tas2R105 mRNA in urethral brush cells (Fig. 4A), cycloheximide, a Tas2R105 agonist, did not evoke changes in [Ca2+]i at a concentration of 0.1 mM (Fig. 4G), which is known to stimulate a [Ca2+]i increase in Tas2R105-transfected cells (21). In addition to synthetic bitter and umami stimuli, heat-inactivated uropathogenic Escherichia coli (strain CFT073; 2–5 × 107 cfu) also triggered a rise in [Ca2+]i (Fig. 4H). Tas1R2–Tas1R3 coexpression yields a sweet receptor (22). Consistent with the absence of detectable Tas1R2 mRNA in urethral brush cells (Fig. 4A), the artificial sweetener saccharin (5 mM) did not trigger a [Ca2+]i response in urethral brush cells (Fig. 4G).
Urethral Brush Cells Use Acetylcholine for Stimulus-Induced Paracrine Signaling.
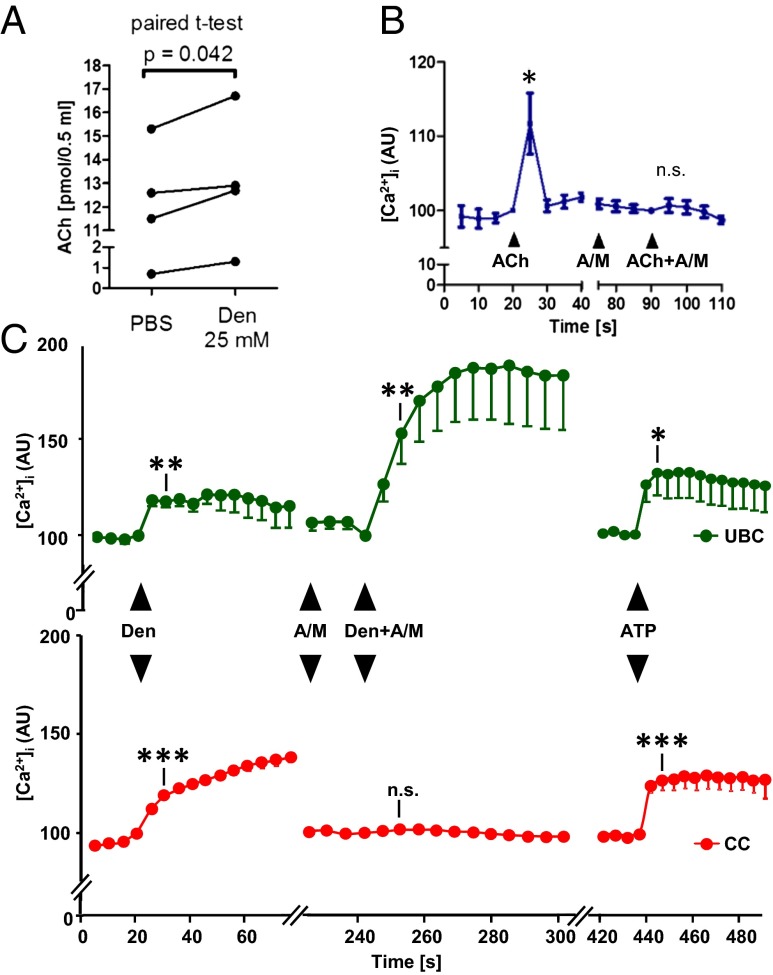
The concept of brush cells serving as chemosensory sentinels monitoring the chemical composition of mucosal surface fluids and initiating local or reflexive responses implies stimulus-induced signaling to neighboring cells. In view of these cells’ ChAT expression, we tested for acetylcholine as a potential messenger released by urethral brush cells. Acetylcholine content in the supernatant (0.5 mL) of isolated urethral cells was increased by an average of 0.875 pmol (from a baseline of 10.0 pmol) in response to stimulation with denatonium (25 mM, 5 min) (Fig. 5A). In our experiments, we noted a rise in [Ca2+]i in response to denatonium in non–GFP-expressing cells when situated in the vicinity of ChAT-eGFP+ cells, but not in isolated cells resting on coverslips devoid of ChAT-eGFP cells (Fig. S5). Such cells express functional acetylcholine receptors; administration of 25 µM acetylcholine together with the esterase inhibitor physostigmine (5 µM) evoked a rise in [Ca2+]i in eGFP− cells that was sensitive to a cholinergic blocker mixture (2 µM atropine and 20 µM mecamylamine) (Fig. 5B). In the presence of the same cholinergic blockers, denatonium still evoked a rise in [Ca2+]i, even at a slightly enhanced level, in ChAT-eGFP cells, but no longer in eGFP− cells, demonstrating stimulus-evoked cholinergic signaling between chemosensory cells and surrounding cells (Fig. 5C).
Fig. 5.
Urethral brush cells release acetylcholine. (A) Acetylcholine (Ach) content in the supernatant (0.5 mL) of isolated urethral cells after a 5-min exposure to PBS (control) or denatonium. Isolates from one urethra are treated as paired data. (B) Calcium recordings from eight eGFP− urethral cells located on coverslips that did not contain eGFP+ cells. In the continuous presence of the acetylcholine esterase inhibitor physostigmine (5 µM), acetylcholine (25 µM) evokes an increase in [Ca2+]i that is sensitive to a mixture (A+M) of muscarinic (2 µM atropine) and nicotinic (20 µM mecamylamine) acetylcholine receptor blockers. (C) Parallel [Ca2+]i recordings from eGFP+ (n = 13, Upper) and eGFP− (n = 74; Lower) isolated urethral cells located in their vicinity (same field of view during confocal laser scanning recording) on the coverslip. Both cell types respond to denatonium (Den; 25 mM) with a [Ca2+]i increase. This responsiveness is lost in eGFP− cells, but persists (even at enhanced levels; P = 0.04, paired t test) in eGFP+ cells after pretreatment with cholinergic blockers (A+M; A), demonstrating denatonium-evoked cholinergic signaling from eGFP+ to eGFP− cells. Response to the nongustatory stimulus ATP demonstrates the viability of cells at the end of the experiment. n.s., nonsignificant. *P < 0.05; **P < 0.01; ***P < 0.001, paired t test compared with value immediately before substance application.
Sensory Nerve Fibers Approach Urethral Brush Cells and Elicit Reflex Bladder Activation on Urethral Bitter Substance Application.
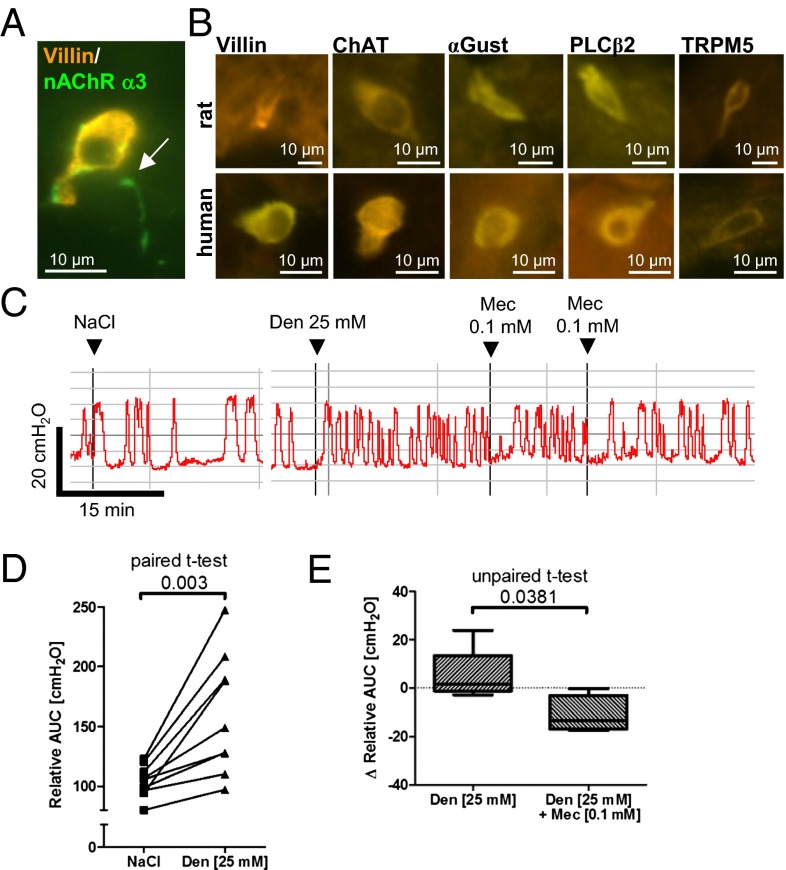
Cholinergic chemosensory cells of the respiratory tract are approached by cholinoceptive sensory nerve fibers that initiate protective respiratory reflexes (4–6). The dominant nicotinic acetylcholine receptor (nAChR) subtype of viscerosensory neurons contains the α3 subunit, and such neurons project to the murine lower urinary tract (23). Using a GFP reporter mouse strain for this promoter (24), we identified a dense nerve fiber network immediately underneath and partially penetrating into the urethral epithelium, coming into contact with villin-positive brush cells (Fig. 6A). Because urethral sensory nerve fibers are known to be linked to the micturition reflex and to coordinate muscle constriction of the bladder and urethra (25), we assessed for reflex coupling between urethral bitter sensing and detrusor activity. The mouse urethra is too small to allow simultaneous cystometric recording of intravesical pressure and urethral manipulation, and thus rat is the model of choice for this purpose (26).
Fig. 6.
Sensory nerve fibers approach urethral brush cells and elicit reflex bladder activation on application of a bitter substance. (A) A sensory nerve fiber expressing eGFP under the control of the nicotinic receptor α3-subunit (arrow) establishes contact with a villin-immunoreactive brush cell in the mouse urethra. (B) Immunolabeling. Solitary epithelial cells with immunoreactivity to brush cell markers, taste transduction cascade components, and ChAT in rat and human urethral epithelium. (C–E) Cystometric recordings from urethane-anesthesized rats. The bladder was continuously filled with saline (0.04 mL/min), causing a rise in intravesical pressure and initiating detrusor contraction and micturition. (C) Original recording of a single experiment showing that urethral instillation of denatonium increases detrusor activity compared with saline application, which is diminished by the nicotinic blocker mecamylamine (Mec). (D and E) Detrusor activity quantified as AUC. (D) Denatonium increases detrusor activity. AUC was determined over a period of 18–36 min in each condition, in nine experiments. (E) First, recordings were made after stimulation with a single dose of denatonium for a minimum of 18 min, with AUC/min as the baseline value. AUC/min decreased after application of mecamylamine, but increased over time when no additional compound was applied.
We first validated by immunohistochemistry the occurrence of solitary villin-, α-gustducin–, PLCβ2-, TRPM5-, and ChAT-immunoreactive cells in the urethral mucosa of rats (Fig. 6B). Notably, such cells were also detected in two human urethral specimens obtained during prostatectomy (Fig. 6B), demonstrating that their occurrence is not restricted to rodents. Continuous filling of the bladder with saline solution (0.04 mL/min) through a catheter inserted into the bladder dome simulated natural bladder filling in fast motion and caused cycles of rising intravesical pressure and micturition in urethane-anesthetized rats. Urethral instillation of saline solution (0.9%; 50 µL) through the external urethral orifice augmented pressure rises only slightly, but a single dose of denatonium (25 mM; 50 µL) significantly increased detrusor activity far beyond the first micturition, causing washout or a drastic dilution of urethral content (Fig. 6 C and D). Lower denatonium concentrations (2.5–12.5 mM; n = 2 each) had no obvious effect, resembling the effectiveness of denatonium on [Ca2+]i rises in isolated urethral chemosensory cells. Intraurethral administration of the general nicotinic receptor blocker mecamylamine (10−4 M) significantly reduced the denatonium-induced increase in detrusor activity, although the activity did not completely return to baseline (Fig. 6 C and E). When administered before denatonium, mecamylamine also did not completely block the denatonium-induced rise in detrusor activity (n = 2).
Discussion
Up to now, solitary chemosensory or brush cells have been identified in the respiratory and gastrointestinal tracts, but not in any other mammalian organ system. Likely owing due to their anatomic restriction to the portal of entry into the urogenital tract (i.e., the urethra and glandular ducts opening into it), these chemosensory cells have escaped detection in previous searches for urogenital brush cells that focused on more centrally located organs, including the kidney, uterus, and prostate gland, before the sentinel function of solitary chemosensory cells had been proposed (14). Coexpression patterns of various components of the taste transduction cascade and ChAT-eGFP suggest the presence of more than one chemosensory cell type in the urethra, including a cholinergic type using PLCβ2 and TRPM5, proteins essential for oropharyngeal bitter and umami perception (27), for downstream signaling. Consistent with our [Ca2+]i measurements, this signaling pathway involves Gβγ stimulation of PLCβ2 with subsequent release of Ca2+ from intracellular stores (28).
The role of the taste-specific Gα protein α-gustducin (29), which is expected to activate a phosphodiesterase, is less clear (28). In taste buds, α-gustducin is inconsistently coexpressed with T1R1–3, particularly in the posterior tongue (30), and its genetic ablation diminishes, but does not abrogate, bitter and umami gustation (31, 32). Similarly, the vast majority (≥90%) of cholinergic urethral brush cells respond to bitter (denatonium) and umami (L-glutamate), but only approximately one-third coexpress α-gustducin.
A striking feature of cholinergic/TRPM5+ urethral chemosensory cells is the coexpression of taste receptors of the Tas1R and Tas2R families and the resulting polymodal response to both bitter and umami stimuli. Similarly, solitary nasal chemosensory cells coexpress Tas1R3 with Tas2R5 and Tas2R8 (33); however, although the responses of these cells to bitter stimuli are well documented (34), whether they also respond to umami remains to be established. This situation is in contrast to oropharyngeal gustation, where Tas1R and Tas2R receptors are not coexpressed in taste buds (33, 35), and 83% of receptor cells in murine vallate papillae respond to only a single taste quality, which provides a basis for taste coding in taste buds (36).
In oropharyngeal gustation, bitter represents an aversive stimulus and umami represents a rewarding stimulus, raising the question as to the possible functional meaning of sensing of both qualities by a single cell. In contrast, on other mucosal surfaces, such as the urethral lining, these qualities represent potentially harmful (aversive) content. Bacteria produce and secrete bitter receptor-activating substances (5, 37, 38). In biofilms, such substances from the Gram-negative bacterium Pseudomonas aeruginosa, one of the predominant causative microorganisms in catheter-associated urinary tract infection (39), can reach concentrations as high as 600 µM (40). On the other hand, glutamate metabolism is positively linked to the pathogenic potential of Proteus mirabilis in the urinary tract (41), and free amino acids (i.e., umami) facilitate bacterial growth in urine (42). Thus, the bitter/umami polymodality of chemosensory cells may serve to broaden the spectrum for recognition of potential hazardous material in the urethral lumen. Most importantly, these chemosensory cells responded to heat-inactivated uropathogenic E. coli, the primary cause of urinary tract infection (43).
Acetylcholine, a secretory product of these cells, may alter sensitivity of this process in an autocrine manner, as demonstrated by the enhanced tastant response after application of a muscarinic/nicotinic receptor blocker mixture. In the more complex lingual taste buds, autocrine cholinergic signaling enhances taste signaling via muscarinic receptors (44). Our cell culture experiments revealed that the amount of acetylcholine released on bitter stimulation is also sufficient to induce paracrine effects. In situ, these cholinergic cells, like those in the trachea (6), are directly approached by sensory nerve fibers expressing the nicotinic acetylcholine receptor α3 subunit, and luminal denatonium evoked reflex activation of the detrusor muscle at a concentration that stimulated urethral chemosensory cells. This reflex activation was sensitive to local application of a nicotinic receptor blocker, demonstrating the involvement of cholinergic, nicotinic transmission from chemosensory cells to sensory nerve fibers. Nonetheless, the denatonium-induced increase in detrusor activity was not entirely abrogated, possibly owing to (i) additional involvement of excitatory muscarinic acetylcholine receptors that are also expressed by urinary tract afferent neurons (45); (ii) additional involvement of a cotransmitter, such as ATP, which transmits information from taste cells to afferent fibers in taste buds (46); or (iii) insufficient access of intraluminally applied mecamylamine to the basolaterally located communication site between chemosensory cells and nerve fibers, as lower urinary tract epithelia form an extraordinary tight barrier (47).
In conclusion, we propose a concept of urinary bladder control involving a previously unidentified cholinergic chemosensory cell monitoring the chemical composition of the urethral luminal microenvironment for potential hazardous content.
Materials and Methods
Animals.
Two independently generated ChAT(BAC)-eGFP mice were provided by M. Kotlikoff (Cornell University), and H. Monyer (University of Heidelberg) (10, 11). Tg(Chrna3-EGFP)BZ135Gsat mice with eGFP driven by the nAChR α3β4α5 cluster were provided by I. Ibanez-Tallon (MDC Molecular Medicine) (24). C57BL/6 mice were obtained from The Jackson Laboratory. Male Clr:WI Wistar rats were obtained from Charles River Deutschland. All animals were housed under standard laboratory conditions (12 h dark, 12 h light). Mice were killed by inhalation of an overdose of isoflurane (Abbott) and exsanguination. The experiments were approved by the local authorities (Rp Giessen, Germany; reference nos. A9/2011, A11/2011, A60/2012, A61/2012, and 12/2013).
Cell Isolation.
Urethrae were dissected, cut into small pieces, and enzymatically digested in dispase (2 mg/mL; Sigma-Aldrich) for 30 min in HBSS (Invitrogen) and 5 min in trypsin/PBS (1:1, Invitrogen) at 37 °C. After centrifugation (60 × g, 5 min) and mechanical dissociation, cells were resuspended in PBS and separated through a cell strainer (70 µM; BD Bioscience). Chemosensory cells were identified by either eGFP or a rabbit polyclonal TRPM5-antibody (ab72151, 1:125; Abcam) directed against an extracellular domain. Cells were incubated for 1 h at 37 °C with this primary antibody, followed by a 1-h incubation with FITC-conjugated donkey anti-rabbit IgG (1:125; Millipore) at 37 °C. The same procedure, but using Cy5-conjugated donkey anti-rabbit IgG (1:400; Dianova), was also applied to cells isolated from ChAT-eGFP mice. As observed in the immunohistochemistry of tissue sections (Table S3), TRPM5 labeling and eGFP expression matched nearly 1:1. For subsequent RT-PCR analysis (primer sequences given in Table S4), chemosensory cells were isolated by incubating dissociated urethra first with the TRPM5 antibody as described above and then with magnetic beads (Invitrogen) coated with goat anti-rabbit IgG (H+L) (PI65-6100; Invitrogen), followed by harvesting by magnetic cell separation.
Measurement of Intracellular Calcium Concentration.
Isolated cells were loaded with fluorescent calcium indicator Calcium Orange AM (3 µL) in Tyrode III solution (297 µL; 8 mM CaCl2, 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM Hepes, 10 mM glucose, 10 mM pyruvic acid, and 5 mM NaHCO3) according to the manufacturer´s protocol (Invitrogen) and then plated on coverslips for 30–60 min at 37 °C. Intracellular calcium concentration was analyzed with a confocal laser scanning microscope (Zeiss LSM 710; 561-nm wavelength generated by a DPSS 561-10 laser) during continuous superfusion (3 mL/min) with Tyrode solution. Fluorescence intensities at the start of the recording period were set arbitrarily at 100%. Test stimuli and concentrations were adenosine 5′-triphosphate bis(Tris) salt dihydrate (ATP, 0.5 mM; Sigma-Aldrich), cycloheximide (0.1 mM; Sigma-Aldrich), denatonium benzoate (25 mM; Molekula), l-glutamic acid monosodium salt monohydrate (l-glutamate, 25 mM; Sigma-Aldrich), saccharin (5 mM; Fluka), acetylcholine chloride (25 µM; Sigma-Aldrich), and heat-inactivated uropathogenic E. coli [UPEC strain CFT073 (NCBI: AE014075, NC_004431), ∼2–5 × 107 cfu, provided by T. Chakraborty, JLU Giessen]. Inhibitors were TPPO (0.25 mM, Sigma-Aldrich), eserine hemisulfate (10 µM; Sigma-Aldrich), mecamylamine hydrochloride (0.02 mM; Sigma-Aldrich), and atropine sulfate (0.002 mM; RBI). Data are presented as mean ± SEM and were analyzed by the two-tailed paired t test.
Patch-Clamp Rrecordings.
Cells were isolated from ChAT-eGFP mice as described for [Ca2+]i recordings, and eGFP-expressing cells were identified with a fluorescence microscope (Zeiss Axiovert 10). The bath solution consisted of 140 mM NaCl, 4.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl, 10 mM Hepes, and 5 mM d-glucose (pH 7.4). The pipette solution consisted of 10 mM NaCl, 18 mM KCl, 92 mM K-gluconate, 0.5 mM MgCl2, 1 mM EDTA, and 10 mM Hepes (pH 7.2). The liquid junction potential was absorbed with the clamped voltage, producing an effective membrane potential of –60 mV. The bath solution included appropriate amounts of mannitol to compensate for osmotic changes or different concentrations of denatonium (1–25 mM), applied by a pressure-driven perfusion system. Transmembrane currents were amplified (EPC 9; Heka Electronics) and recorded continuously (sampled with 10 kHz, filtered with 3 kHz) with Pulse 8.77 software (Heka Electronics).
Urodynamic Measurement.
Rats were anesthetized by an s.c. injection of urethane (1.2 g/kg) at 1 h before surgery and maintained under anesthesia during the surgical preparation while on a heating pad (37 °C). A catheter (PE 50; Intramedic) was inserted into the bladder dome and connected to a pressure transducer and an infusion pump. Saline solution at room temperature was infused into the bladder at a rate of 0.04 mL/min. After a stabilization phase of 15–30 min, the intravesical bladder pressure was recorded continuously, and 50 µL of test stimuli were delivered into the urethral external orifice via a 0.9 × 25 mm cannula (Braun Vasofix G22) mounted on a 100-µL pipette. For final data analysis, areas under the curve (AUC) of equal time periods before and after stimulation were compared; data are presented as AUC/min. The urodynamic recording sessions took 3–4 h for each animal.
Fluorescence microscopy and immunohistochemistry, pre-embedding immunohistochemistry, and EM, RT-PCR, and acetylcholine measurements were performed as described previously (6, 48), with minor modifications. More details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Bodenbenner and K. Michael for technical assistance. This work was supported by the LOEWE Program of the State of Hesse (Non-neuronal Cholinergic Systems, project A5, to W.K. and T.B.) and the Deutsche Forschungsgemeinschaft (T.G. and V.C.). This study was awarded the Eugen-Rehfisch Prize of the Forum Urodynamicum.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402436111/-/DCSupplemental.
References
- 1.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956;44(4):345–412. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- 2.Luciano L, Reale E, Ruska H. 1968. [On a glycogen containing brusc cell in the rectum of the rat.] Z Zellforsch Mikrosk Anat 91(1):153–158. German.
- 3.Sbarbati A, Osculati F. A new fate for old cells: Brush cells and related elements. J Anat. 2005;206(4):349–358. doi: 10.1111/j.1469-7580.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. Chemoreception regulates chemical access to mouse vomeronasal organ: Role of solitary chemosensory cells. PLoS ONE. 2010;5(7):e11924. doi: 10.1371/journal.pone.0011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tizzano M, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107(7):3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasteva G, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108(23):9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91(21-22):992–996. doi: 10.1016/j.lfs.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Bhushan S, et al. Testicular innate immune defense against bacteria. Mol Cell Endocrinol. 2009;306(1-2):37–44. doi: 10.1016/j.mce.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA. Etiology and pathophysiology of pyelonephritis. Am J Kidney Dis. 1991;17(1):1–9. doi: 10.1016/s0272-6386(12)80242-3. [DOI] [PubMed] [Google Scholar]
- 10.Tallini YN, et al. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics. 2006;27(3):391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- 11.von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27(21):5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vittoria A, Cocca T, La Mura E, Cecio A. Immunocytochemistry of paraneurons in the female urethra of the horse, cattle, sheep, and pig. Anat Rec. 1992;233(1):18–24. doi: 10.1002/ar.1092330104. [DOI] [PubMed] [Google Scholar]
- 13.Aumüller G, et al. Regional distribution of neuroendocrine cells in the urogenital duct system of the male rat. Prostate. 2012;72(3):326–337. doi: 10.1002/pros.21437. [DOI] [PubMed] [Google Scholar]
- 14.Höfer D, Drenckhahn D. Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry. 1992;98(4):237–242. doi: 10.1007/BF00271037. [DOI] [PubMed] [Google Scholar]
- 15.Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA. 2003;100(15):8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaske S, et al. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez CA, Margolskee RF, Kinnamon SC, Ogura T. Making sense with TRP channels: Store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 2003;33(5-6):541–549. doi: 10.1016/s0143-4160(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13(13):1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 19.Palmer RK, et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol. 2010;8(6):703–713. doi: 10.1089/adt.2010.0334. [DOI] [PubMed] [Google Scholar]
- 20.Li X, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100(6):703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 22.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106(3):381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 23.Nandigama R, et al. Expression of nicotinic acetylcholine receptor subunit mRNA in mouse bladder afferent neurons. Neuroscience. 2013;229:27–35. doi: 10.1016/j.neuroscience.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 24.Frahm S, et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70(3):522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Jung SY, et al. Urethral afferent nerve activity affects the micturition reflex: Implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162(1):204–212. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- 26.Andersson KE, Soler R, Füllhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30(5):636–646. doi: 10.1002/nau.21108. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 28.Kinnamon SC. Taste receptor signalling—from tongues to lungs. Acta Physiol (Oxf) 2012;204(2):158–168. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste cell-specific G protein closely related to the transducins. Nature. 1992;357(6379):563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 30.Kim MR, et al. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 2003;312(2):500–506. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- 31.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381(6585):796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 32.Glendinning JI, et al. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30(4):299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 33.Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 2008;38(4):505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99(6):2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler E, et al. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 36.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27(40):10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hettinger TP, Formaker BK, Frank ME. Cycloheximide: No ordinary bitter stimulus. Behav Brain Res. 2007;180(1):4–17. doi: 10.1016/j.bbr.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee RJ, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuman EK, Chenoweth CE. Recognition and prevention of healthcare-associated urinary tract infections in the intensive care unit. Crit Care Med. 2010;38(8) Suppl:S373–S379. doi: 10.1097/CCM.0b013e3181e6ce8f. [DOI] [PubMed] [Google Scholar]
- 40.Charlton TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: Application to a model bacterial biofilm. Environ Microbiol. 2000;2(5):530–541. doi: 10.1046/j.1462-2920.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 41.Pearson MM, Yep A, Smith SN, Mobley HL. Transcriptome of Proteus mirabilis in the murine urinary tract: Virulence and nitrogen assimilation gene expression. Infect Immun. 2011;79(7):2619–2631. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aubron C, et al. Changes in urine composition after trauma facilitate bacterial growth. BMC Infect Dis. 2012;12:330. doi: 10.1186/1471-2334-12-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulett GC, et al. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol. 2013;16(1):100–107. doi: 10.1016/j.mib.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Dando R, Roper SD. Acetylcholine is released from taste cells, enhancing taste signalling. J Physiol. 2012;590(Pt 13):3009–3017. doi: 10.1113/jphysiol.2012.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nandigama R, et al. Muscarinic acetylcholine receptor subtypes expressed by mouse bladder afferent neurons. Neuroscience. 2010;168(3):842–850. doi: 10.1016/j.neuroscience.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 47.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297(6):F1477–F1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohr F, Zimmermann M, Klein J. Mice heterozygous for AChE are more sensitive to AChE inhibitors but do not respond to BuChE inhibition. Neuropharmacology. 2013;67:37–45. doi: 10.1016/j.neuropharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav. 2011;105(1):4–13. doi: 10.1016/j.physbeh.2011.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.