Recognizing a person we know seems like an unremarkable occurrence within the stream of everyday events. However, the ease with which most of us recognize a familiar face belies the daunting complexity of the underlying computational challenges—challenges that become apparent in the not so rare cases of face-blindness and in attempts to develop computational models that mimic human face recognition performance. Neuroscience has shown, since the discovery of face-selective cells in the early 1970s, that face recognition is a highly evolved ability in primates that relies on very specialized hardware: we now know that millions of face cells exist in the primate brain, that they are specialized for the processing of particular facial dimensions depending on which area they are located in, and that this spatial segregation of function is maintained within a network of highly interconnected face areas. Even some of the algorithms by which face cells detect and analyze faces are becoming clear. How this highly sophisticated face-processing system comes about, and how individual cells acquire their selectivity, is still the matter of much debate, yet it is clear that experience with the visual world, and with faces in particular, must play an important role. In PNAS, McMahon et al. (1) now provide an entirely new perspective on the question of plasticity in face recognition. With a new technical approach that allows for the activity of face cells to be monitored over days, weeks, months, and, in rare cases, even a year, they determined the selectivity of face cells in one part of the brain and found it to be remarkably stable over these long periods of time. This finding comes as a great surprise to a field that has gotten used to the notion of plasticity, and might indicate a hitherto unrealized form of functional specialization within the primate face-recognition system.
Face-selective neurons were first discovered in the rhesus monkey, the species studied by McMahon et al., in a part of the brain, the inferotemporal cortex (2), known at the time to be essential for object recognition. Many studies in subsequent years found face cells throughout the entire expanse of inferotemporal cortex and the immediately dorsally located portions of the superior temporal sulcus (3), both of which we will refer to as IT, and began characterizing what facial feature a given cell was selective for (e.g., the presence of eyes) (3). This research documented specialization for face processing at the single-cell level and a remarkable diversity and range of selectivities for facial information. However, only with the advent of a large-scale imaging technique, functional MRI (fMRI), could the larger picture of the organization of face cells be appreciated: face selective areas, each a few millimeters in diameter, were found within IT (4, 5) (Fig. 1A), a pattern not dissimilar to the one that had been found in the human brain (6). Single-unit recordings then showed that these fMRI-identified face areas contain very high fractions of face-selective cells (7, 8). With each area harboring hundreds of thousands of face cells, and with 12 such areas residing in the two hemispheres of the temporal lobes, the primate brain invests large amounts of hardware to the task of face recognition. This hardware is organized into a fixed number of face areas with stereotypical locations across individuals, interconnected with strong and selective links to form an integrated face-processing network (9). This form of organization appears to exist to support functional specialization of face cells at different locations (8, 10). For example, cells in one region are tuned to a specific head orientation or a specific gaze direction, whereas cells in other regions abstract from these view-specific and other fleeting aspects of how a face is seen at a given moment to extract the intrinsic properties that differentiate one individual face from another. Such divisions of labor between face areas have been found in fMRI studies of the human brain as well (11). Thus, the primate brain not only invests a large quantity of neural hardware to face recognition, but this hardware is also precisely organized and finely tuned. The study of McMahon et al. now adds a new dimension to our understanding of the system’s fine-tuning.
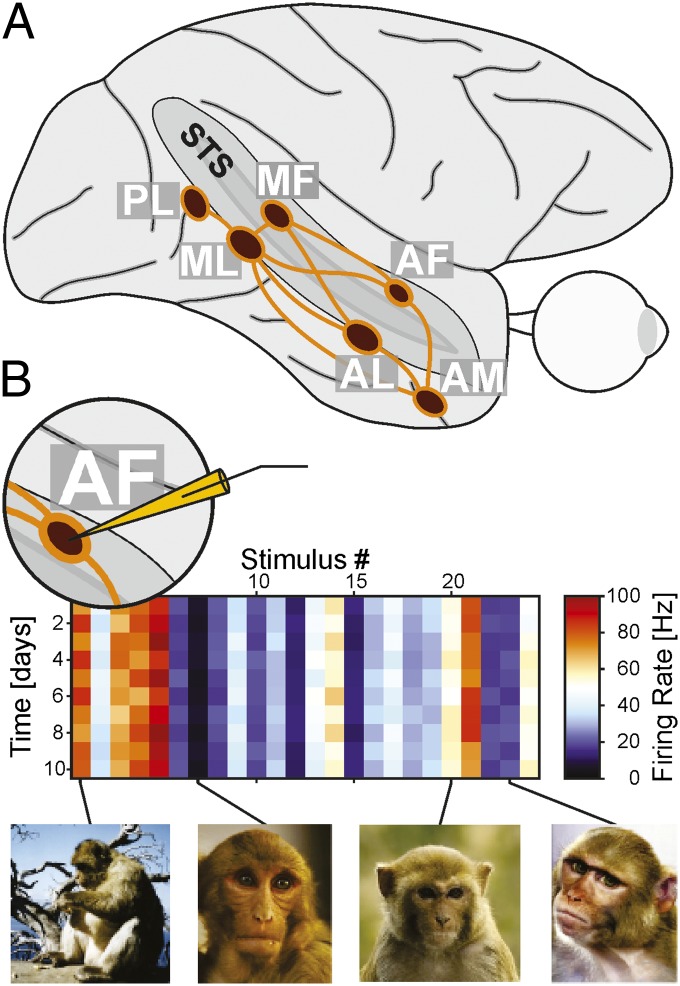
Fig. 1.
(A) Side view of schematic of rhesus monkey eye and brain with six face-selective areas (dark red) and known connections (9) between them. The superior temporal sulcus (STSS) is opened to make face areas inside visible. Face areas are named based on their anatomical location. (B) Schematic of a hypothetical long-term recording from face area AF (Inset, Left), based on data from ref. 1. Firing rate (color coded) is shown as a function of stimulus number (horizontal axis, with four example stimuli below) and recording session (top to bottom). This hypothetical cell showed selectivity across different stimuli (preferring some faces or face-body combinations over others), with little variation across days.
McMahon et al. ask a new question about the system: how stable is the selectivity of a given neuron to a complex stimulus like a face over the course of long periods of time, weeks, months, even a year, while animals are exposed to many new visual and social experiences? If face cells exist to signal pieces of information that are of utmost importance to a socially living primate, one would expect these signals to be highly reliable and, possibly, very stable over time. As important as the question is, with typical durations of experiments measuring stimulus selectivity lasting on the order of an hour, the question could not be answered until recently, when a recording technology was introduced that allows for highly stable long-term recordings (12). McMahon et al. first locate face-selective areas with fMRI and then targeted one face area for long-term recordings with bundles of insulated recording wires. These wires could be independently positioned, but once activity from a neuron was isolated well, the corresponding wire would be left in place for long periods of time. Based on recordings from more than 100 cells, McMahon et al. find that face cells, typically, not exceptionally, would keep their highly specific selectivity profile over weeks and months (Fig. 1B). Given how many levels of circuitry are necessary to establish face selectivity, from the eyes on through multiple stages of processing, each potentially subject to plastic changes, achieving long-term stability of face selectivity at the top of the processing hierarchy is an astounding finding. It is particularly surprising to a field that is not prepared for it.
Over the last two decades, it was discoveries about the visual system’s plasticity that dominated the thinking about high-level object recognition and face recognition in particular (13). Early life experience with faces of one species, it was found, shaped later abilities to recognize faces from that species and from other species (14), and when animals learned to discriminate between complex object shapes early in life, entirely new areas for these shapes developed (15). The synaptic connections between cells in the visual cortex, required to establish stimulus selectivity, were observed to be in constant turnover (16). Neurons were found to acquire selectivity to multiple physically unrelated object shapes when animals were trained to associate them (17), and similar cross-stimulus dependencies were automatically acquired, even when no active task required their association, simply based on stimulus statistics (18). The selectivity of an IT neuron could be significantly changed within an hour, when, with a clever trick, object shape was changed during a saccadic eye movement (19). Even face cells had been found to change their responses as novel face stimuli became familiar (20). It thus appears that face and high-level object recognition systems should be set up for life-long learning and
McMahon et al. now provide an entirely new perspective on the question of plasticity in face recognition.
thus their components for change. However, McMahon et al. find stability across the vast majority of neurons they studied. Their finding might thus serve as another example of a biological system maintaining its complex organization as the constituent components are exchanged. How are we to reconcile the stability of the single face cell, a cell that likely acquired at least part of its selectivity through learning early in life, with the evident malleability of object and face-recognition systems?
McMahon et al. suggest that stability of facial encoding might in fact be a property unique to the one face area they studied and not to all. This idea of a new kind of functional division between face areas, while not directly supported by data from comparative measurements across face areas, is a plausible possibility and compatible with the spatial separation of the area McMahon et al. studied from those regions where plastic effects have been found before. Given the technical advance the paper by McMahon et al. presents, directly testing this idea is certainly within reach for these authors. Future cross-area comparisons will likely investigate the role of behavioral relevance of stimuli and explicitly manipulate stimulus statistics. One general lesson to be learned from the study of McMahon et al. is that yet new dimensions of functional organization remain to be discovered in the face-processing system. Our picture of the intricacy and beauty of this system has just gained a new facet.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See companion article on page 8251.
References
- 1.McMahon DBT, Jones AP, Bondar IV, Leopold DA. Face-selective neurons maintain consistent visual responses across months. Proc Natl Acad Sci USA. 2014;111:8251–8256. doi: 10.1073/pnas.1318331111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross CG. Genealogy of the “grandmother cell”. Neuroscientist. 2002;8(5):512–518. doi: 10.1177/107385802237175. [DOI] [PubMed] [Google Scholar]
- 3.Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philos Trans R Soc Lond B Biol Sci. 1992;335(1273):23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 4.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6(9):989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsk MA, et al. Neural representations of faces and body parts in macaque and human cortex: A comparative FMRI study. J Neurophysiol. 2009;101(5):2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311(5761):670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330(6005):845–851. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320(5881):1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Souza WC, Eifuku S, Tamura R, Nishijo H, Ono T. Differential characteristics of face neuron responses within the anterior superior temporal sulcus of macaques. J Neurophysiol. 2005;94(2):1252–1266. doi: 10.1152/jn.00949.2004. [DOI] [PubMed] [Google Scholar]
- 11.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 12.Bondar IV, Leopold DA, Richmond BJ, Victor JD, Logothetis NK. Long-term stability of visual pattern selective responses of monkey temporal lobe neurons. PLoS ONE. 2009;4(12):e8222. doi: 10.1371/journal.pone.0008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier I, Nelson CA. The development of face expertise. Curr Opin Neurobiol. 2001;11(2):219–224. doi: 10.1016/s0959-4388(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 14.Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci USA. 2008;105(1):394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srihasam K, Mandeville JB, Morocz IA, Sullivan KJ, Livingstone MS. Behavioral and anatomical consequences of early versus late symbol training in macaques. Neuron. 2012;73(3):608–619. doi: 10.1016/j.neuron.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49(6):877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354(6349):152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- 18.Meyer T, Olson CR. Statistical learning of visual transitions in monkey inferotemporal cortex. Proc Natl Acad Sci USA. 2011;108(48):19401–19406. doi: 10.1073/pnas.1112895108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, DiCarlo JJ. Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science. 2008;321(5895):1502–1507. doi: 10.1126/science.1160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolls ET, Baylis GC, Hasselmo ME, Nalwa V. The effect of learning on the face selective responses of neurons in the cortex in the superior temporal sulcus of the monkey. Exp Brain Res. 1989;76(1):153–164. doi: 10.1007/BF00253632. [DOI] [PubMed] [Google Scholar]