Significance
Although the neurons and molecules that mediate the sensing of light, odors, tastants, temperature, and pressure have been elucidated, how humidity is sensed in most animals remains unclear. We used the experimental amenability of the model nematode Caenorhabditis elegans to discover a mechanism for sensing humidity. This worm pairs mechanical and thermal information associated with humidity levels via parallel sensory pathways. This strategy, first proposed more than 100 years ago by Thunberg, is notable for not requiring specialized sensory apparatus (or even hair). Because these neurons and their molecular sensors have equivalents represented in diverse animals, the humidity sensation mechanism that we describe here could be used by many animals, including humans.
Keywords: mechanosensation, thermosensation
Abstract
All terrestrial animals must find a proper level of moisture to ensure their health and survival. The cellular-molecular basis for sensing humidity is unknown in most animals, however. We used the model nematode Caenorhabditis elegans to uncover a mechanism for sensing humidity. We found that whereas C. elegans showed no obvious preference for humidity levels under standard culture conditions, worms displayed a strong preference after pairing starvation with different humidity levels, orienting to gradients as shallow as 0.03% relative humidity per millimeter. Cell-specific ablation and rescue experiments demonstrate that orientation to humidity in C. elegans requires the obligatory combination of distinct mechanosensitive and thermosensitive pathways. The mechanosensitive pathway requires a conserved DEG/ENaC/ASIC mechanoreceptor complex in the FLP neuron pair. Because humidity levels influence the hydration of the worm’s cuticle, our results suggest that FLP may convey humidity information by reporting the degree that subcuticular dendritic sensory branches of FLP neurons are stretched by hydration. The thermosensitive pathway requires cGMP-gated channels in the AFD neuron pair. Because humidity levels affect evaporative cooling, AFD may convey humidity information by reporting thermal flux. Thus, humidity sensation arises as a metamodality in C. elegans that requires the integration of parallel mechanosensory and thermosensory pathways. This hygrosensation strategy, first proposed by Thunberg more than 100 y ago, may be conserved because the underlying pathways have cellular and molecular equivalents across a wide range of species, including insects and humans.
Moisture is essential for life. As such, many animals have adapted different behavioral mechanisms to migrate toward their preferred moisture level (hygrotaxis) (1–6). For instance, Drosophila avoid high humidity that impedes flight, whereas green frogs orient toward high humidity to maintain hydration (5, 6). Animals also sense moisture levels to determine important information about their environment; for example, moths detect humidity levels around flowers to deduce which ones might be damaged and contain less nectar (7). These behaviors are often critical to keep an animal within its niche and regulate essential processes such as growth and reproduction. Thus, it is surprising that the molecular basis for how different humidity levels are detected and encoded by the nervous system (hygrosensation) remains unknown in most animals.
The search for humidity receptors has achieved the most progress in insects. For instance, distinct sets of hygrosensitive neurons have been found in dome-shaped organs on the antenna of the giant cockroach (8). One set activates with moist air, and the other set responds to dry air. Similar moist and dry receptive neurons have been detected in the branched arista subsegment of the antennae in adult Drosophila (9). Removal of the arista or deletion of any one of three TRP channels expressed in the arista prevents hygrotaxis (5, 9). These TRP channels represent tantalizing candidates for moisture receptors because different TRP channels were required for activity of moist or dry neuronal responses (9). Whether these TRP channels contribute to hygrosensation in other animals remains to be seen, however.
Humidity also can be detected by animals that lack branched organs or hair that changes shape with hydration. In 1905, Thunberg (10) proposed that humidity may be perceived in humans as the synthesis of mechanical distension associated with changes in skin hydration, along with temperature signals from the rate of evaporative cooling. This old idea might apply to other animals as well; for instance, the hygrosensitive organs in cockroach and Drosophila also house thermosensitive neurons (8, 11). Whether paired thermosensitive neurons are required for hygrosensation in insects or, for that matter, whether any animal (including humans) senses humidity via this mechanism, remains unknown.
To gain information about the neuromolecular basis for hygrosensation, we studied how the free-living nematode Caenorhabditis elegans responds to humidity gradients. This model has been used to successfully elucidate neuronal mechanisms and molecules critical for diverse sensory pathways (12–14). We expected C. elegans to be sensitive to humidity because its small volume (∼3.8 × 106 μm3) and hydrostatic skeleton make it vulnerable to desiccation and overhydration, which are often lethal to this tiny (∼1 mm) worm (15). Although C. elegans does not feature an arista-like appendage, its completely described nervous system of 302 neurons conveniently limits the search for candidate hygroreceptive neurons. Here we report that C. elegans appears to use a strategy for hygrosensation first predicted by Thunberg (10) that combines dual mechanosensory and thermosensory pathways.
Results
C. elegans Senses Humidity.
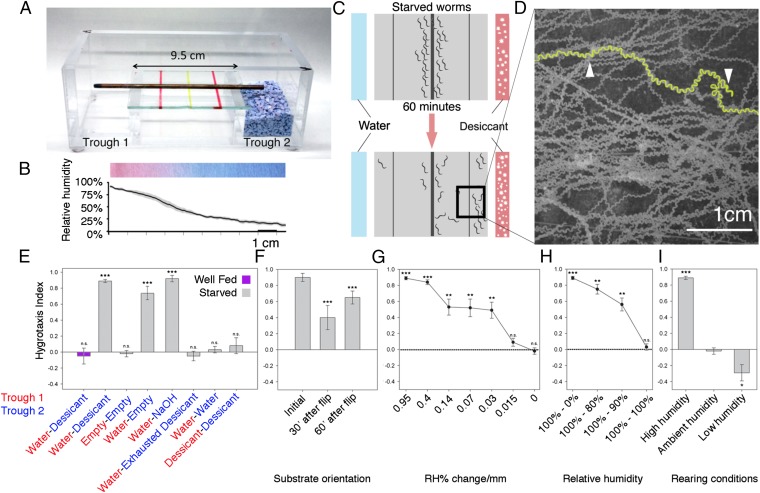
To determine whether C. elegans could migrate to a preferred level of atmospheric moisture, we developed a hygrotaxis assay with a controlled humidity gradient. In brief, worms were assayed in a plastic container resembling a horizontal electrophoresis gel box with an elevated central area bordered by two troughs (Fig. 1A and Fig. S1). Distilled water or desiccant (fresh Drierite) was poured into the troughs at opposite ends. Worms (n = 20–200) were then transferred to the middle of a thin agarose pad on a glass pane placed between the two troughs. Finally, the chamber was sealed with plastic wrap to establish the humidity gradient and start the hygrotaxis assay. In separate experiments, the development and stability of the humidity gradient were quantified with dried paper strips infused with the colorimetric indicator cobalt (II) chloride (Fig. 1B). A humidity gradient ranging from 10% to 95% relative humidity (RH), with 0.89% RH per millimeter, formed within 5 min and was maintained for at least 90 min (Fig. 1B). Hygrotaxis performance was quantified with a behavioral index, Ihtx, with a value of +1 signifying migration toward the dry side, −1 signifying migration toward the humid side, and 0 reflecting no preference.
Fig. 1.
C. elegans can sense humidity. (A) Troughs contain water or desiccant to generate a humidity gradient within a chamber sealed by plastic wrap. (B) Quantification of the humidity gradient with colorimetric indicator cobalt (II) chloride. Gray indicates SEM. n = 4. (C) Worms migrate freely over 60 min. (D) Montage of 300 superimposed photos of worms taken over 10 min in part of the hygrotaxis chamber. Trajectories appear as contiguous images of individual worms. Representative trajectory is in yellow; reorienting turns are indicated by arrows. (E) Well-fed worms (purple bar) show no preference for high or low humidity. In contrast, starved worms (gray bars) migrate toward the dry side, as indicated by a positive hygrotaxis index. Starved worms assayed in no-gradient conditions show no preference. Starved worms perform hygrotaxis with a gradient generated with a different desiccant (NaOH), but not with exhausted Drierite. (F) Hygrotaxis depends on the humidity gradient in air and not the substrate, because worms quickly reorient toward the dry side when substrate is flipped by 180°. (G) Hygrosensation sensitivity. The humidity gradient was controlled through the use of different concentrations of aqueous NaOH in each trough. (H) Humidity sensitivity at higher base humidity levels. (I) The preference for humidity is plastic. Starvation in humid or dry conditions influences the preference for dry and humid sides of the gradient, respectively. Asterisks denote the difference from 0 for all panels. *P < 0.05; **P < 0.01; ***P < 0.001.
Initially, we used well-fed adult worms (following convention), and found that they showed no humidity preference, with an average Ihtx value indistinguishable from 0 (Fig. 1E). However, we discovered that we could obtain significantly more robust results with starved animals (see the discussion on hygrotaxis plasticity below), and thus we restricted our experiments to starved worms unless noted otherwise.
Most worms migrated to the dry side, as indicated by a significantly positive index of hygrotaxis performance (Ihtx = 0.89 ± 0.02 SEM; P < 0.001) (Fig. 1E). Time series video analyses revealed that most individuals took highly directed paths down the humidity gradient by gradually adjusting their bearing toward the dry side, indicative of the klinotaxic weathervane strategy, interspersed with sharp turns for reorientation, suggesting the klinokinetic pirouette strategy (Fig. 1D and Movie S1). These combined strategies would allow C. elegans to move efficiently to its preferred humidity level (16, 17). In contrast, control worms assayed in the absence of a humidity gradient (with both troughs empty) showed no preference (Fig. 1E).
Migration to the dry side could not be explained by a number of other possibilities. For instance, the desiccant Drierite did not appear to emanate an attractive odor, because worms distributed randomly when assayed with exhausted desiccant (Fig. 1E). Hygrotaxis also was robust when sodium hydroxide pellets were used as an alternative desiccant (Fig. 1E). Worms also moved away from water and toward an empty side without desiccant (Fig. 1E).
We next wondered whether migration to the dry side could be explained by an unintentional attractive or repellent gradient on the substrate. This did not appear to be the case, because most worms that migrated to the dry side migrated back across the 9.5-cm pad within 30–60 min after the substrate was reoriented by 180° (Fig. 1F and Movie S1).
To determine the sensitivity of C. elegans to humidity, we assessed hygrotaxis performance in a range of controlled moisture gradients. Humidity levels were set by taking advantage of the fact that the amount of water released from a solution of aqueous sodium hydroxide is directly related to its concentration (18). For each assay, one side was set to the room humidity at the outset of the assay (range, 50–65% RH), while the other side was set to a lower RH. Worms displayed hygrotaxis, with approximately three of four worms crawling to the dry side even when the gradient was as shallow as 0.03% RH per millimeter (Fig. 1G). To further examine the humidity levels that C. elegans may detect, we also assayed worms in humidity gradients at higher base values (80–100% and 90–100% RH), and found that hygrotaxis remained robust to these gradients (Fig. 1H). Taken together, our results demonstrate that C. elegans detects humidity with exquisite sensitivity.
C. elegans Humidity Preference Is Plastic.
Initially, we were puzzled as to why C. elegans migrated to the dry side of the assay chamber, where it may be in danger of desiccation. Thus, we considered factors that might influence moisture preference in our assay. One factor is the humidity in which the worms are raised. We found that standard rearing conditions for C. elegans (raised on moist agar seeded with bacteria for food within a sealed container) ensured that they were constantly exposed to >95% RH (n = 10).
A second factor that dictates the aversiveness or attractiveness of sensory cues in C. elegans and other animals is the associated feeding state (12, 19). Indeed, our robust hygrotaxis results were achieved only with worms taken from “starved” culture plates (Materials and Methods). We hypothesized that worms starved under these humid conditions may associate the starved state with humidity and thus favor dry conditions, and, conversely, worms starved in dry conditions may favor humid conditions. To test this idea, we starved worms under low-humidity conditions (∼15% RH) for 18 h. These worms showed a modest but significant preference for the humid side of the gradient, as evidenced by an average Ihtx of −0.27 ± 0.12 SEM (Fig. 1I). In contrast, when we starved worms in humid conditions, we found that they developed a preference for the dry side that was most robust after 18 h off food (Fig. 1I and Fig. S2, time course of hygrotaxis plasticity). Taken together, these results strongly suggest that the preferred humidity level of C. elegans is plastic, although not necessarily via associative learning.
Sensory Pathways Dispensable for Hygrosensation.
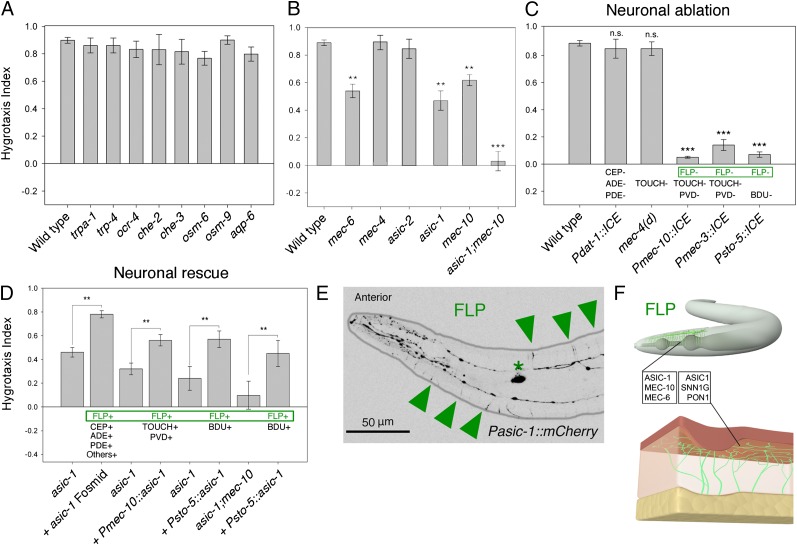
Previous studies of the molecular basis for hygrosensation identified three invertebrate-specific TRP channels as candidate hygroreceptive molecules in adult Drosophila: water-witch, nanchung, and inactive (9). We found that deletion of the C. elegans orthologs of these genes (trpa-1, trp-4, and ocr-4), however, caused no deficit in hygrotaxis performance in the steep humidity gradient (0.89% RH per millimeter) (Fig. 2A).
Fig. 2.
Humidity sensation requires a conserved DEG/ENaC/ASIC mechanosensory gene set in neuron pair FLP. (A) Mutation in many genes critical for diverse sensory modalities fails to perturb hygrotaxis. (B) Deletion of mechanosensitive genes mec-6, asic-1, and mec-10 disrupts hygrotaxis. (C) Cell-specific ablation shows the requirement for mechanosensitive neuron pair FLP in hygrotaxis. Ablated neurons are noted under the bars. (D) Cell-specific rescue shows the sufficiency of FLP neurons for asic-1 in hygrotaxis. Rescued neurons are noted under the bars. (E) Photomicrograph of Pasic-1::mCherry expression in FLP neuron with soma (asterisk) and branched dendrites (arrowheads) beneath the cuticle. (F) Cartoon of FLP in worm and analogous mammalian multidendritic neuron in skin with orthologous mechanosensory components. Asterisks denote the difference from WT in B and C and the difference from paired controls in D. *P < 0.05; **P < 0.01; ***P < 0.001.
Might humidity sensation require an established sensory pathway? To test this idea, we next assayed a range of mutants defective in different subsets of sensory neurons or sensory modalities. We first reasoned that sensory cilia might be important for hygrosensation. Worms lacking functional cilia are severely compromised in many sensory behaviors, including olfactory chemotaxis, taste chemotaxis, and avoidance of hyperosmolarity (13); however, we were surprised to find that mutants with defective cilia (e.g., che-2, che-3 and osm-6) were able to perform hygrotaxis as efficiently as WT worms (Fig. 2A). We also found many additional mutants with defects in specific sensory transduction molecules in ciliated neurons that also were normal for hygrotaxis. Of note, these included osm-9 [TRPV ortholog required to detect hyperosmolarity and nose touch (13)], trp-4 [TRPN ortholog required to detect substrate texture (14)], and aqp-6 [the only aquaporin channel selectively expressed in sensory neurons (20)] (Fig. 2A). Taken together, these negative results demonstrate that hygrosensation is distinct from many previously studied sensory modalities in C. elegans, including olfaction, taste, and osmosensation.
Hygrosensation Requires an ASIC-1/MEC-10/MEC-6 Putative Mechanosensitive Complex in Branched FLP Neurons.
Thunberg (10) hypothesized that hygrosensation may require a mechanosensory pathway. The TRP class and DEG/ENaC/ASIC class of ion channels are the two largest classes of known mechanoreceptors in animals (21). We first tested the possibility that TRP channels other than the foregoing might be involved in hygrosensation, but found that deletion of any 1 of the 15 predicted TRP channel genes had no effect on hygrotaxis. We next tested a role for DEG/ENaC/ASIC-class ion channels by investigating the hygrotaxis ability of worms lacking the peroxidase MEC-6, a critical subunit for DEG/ENaC/ASIC-class ion channels (14). The mec-6 mutant worms exhibited significantly impaired hygrotaxis (Fig. 2B). This finding led us to then test deletion strains for all available DEG/ENaC/ASIC genes with reported expression in neurons. Most DEG/ENaC/ASIC mutants displayed normal hygrotaxis; for instance, the well-studied mec-4 mutant, which is defective in the six so-called “touch” neurons, performed hygrotaxis normally (Fig. 2B). The mec-10 and asic-1 single mutants displayed significantly diminished hygrotaxis, however (Fig. 2B). Deletion of the only other ASIC ortholog, asic-2, had no effect on hygrotaxis (Fig. 2B).
Because DEG/ENaC/ASIC mechanosensitive ion channels are known to function as homotrimers or heterotrimers (22), we asked whether the residual hygrotaxis ability observed for the mec-10 and asic-1 single mutants might be explained by partial compensation by the other gene. Consistent with this idea, deletion of both mec-10 and asic-1 abolished hygrotaxis (Fig. 2B). The asic-1 and mec-10 single- and double-mutant strains performed normal olfactory chemotaxis, however, strongly suggesting that these genes have critical roles in hygrosensation rather than in general sensation or movement (Fig. S3). In addition, we found that transformation of the asic-1(null) mutant with a fosmid containing the asic-1 gene and its regulatory elements rescued hygrotaxis performance (Fig. 2D). These results demonstrate that the mec-10 and asic-1 genes are partially redundant and together are essential for hygrosensation.
The dual requirement of the mec-10 and asic-1 genes for hygrotaxis suggests candidate neurons for hygrosensation. MEC-10 is expressed only in the six touch neurons and two additional pairs of mechanosensitive multidendritic neurons, FLP and PVD (14, 23). We found evidence of ASIC-1 expression in FLP neurons, but not in PVD neurons, because an asic-1 promoter drove expression of the fluorophore mCherry in this neuron pair (Fig. 2E). We also found Pasic-1::mCherry expression in other neurons, including the mechanosensitive dopaminergic sensory neurons previously reported to express asic-1 (24).
To test the requirement of these different sensory neurons in hygrotaxis, we used strains with different subsets of neurons that had been destroyed via expression of a human cell death caspase (ICE) or via a gain-of-function mutation in mec-4 (14, 25). We first found that ablation of the touch and multidendritic neurons (via Pmec-10::ICE or Pmec-3::ICE) abolished hygrotaxis (Fig. 2C). We next found that ablation of the touch neurons [via mec-4(d)] or mechanosensitive dopamine neurons (CEP, ADE, and PDE via Pdat-1::ICE) had no effect on hygrotaxis (Fig. 2C). These results suggest that one or both of the multidendritic neuron classes are required for hygrosensation. To test the FLP neurons, we ablated them via expression of Psto-5::ICE and found that this strain failed to perform hygrotaxis (Fig. 2C). The abnormal hygrotaxis of Pmec-10::ICE and Psto-5::ICE strains could not be explained by a general deficit in movement or orientation, because these strains taxed to an attractive odor as quickly and robustly as WT worms (Fig. S3); thus, we conclude that FLP is a major neuron class required for hygrosensation.
However, is FLP sufficient for hygrosensation? We then assessed whether asic-1(null) mutant worms with asic-1 rescued in FLP using the Psto-5 promoter could perform hygrotaxis. These asic-1 (FLP+) transgenic worms displayed significant improvement in hygrotaxis performance while their untransformed sisters performed similarly to the asic-1(null) mutant (Fig. 2D). Rescue of asic-1 expression in FLP neurons of the asic-1;mec-10 double mutant similarly rescued hygrotaxis ability (Fig. 2D). These results point to the multidendritic FLP neurons as using the ASIC-1, MEC-10, and MEC-6 mechanosensitive proteins (perhaps as a single complex) to transduce humidity.
Hygrosensation Also Requires the TAX-4 cGMP-Gated Channel in Thermosensitive AFD Neurons.
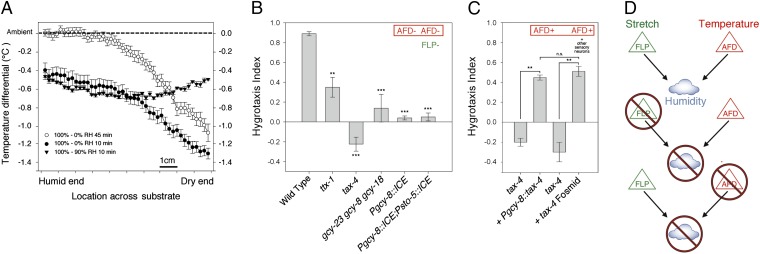
Thunberg (10) also proposed that mechanical cues on the skin would need to be integrated with thermal cues to disambiguate other gentle mechanical stimuli from humidity stimuli. Are there significant thermal cues present for the worms in our assay conditions? A thermal photo of the steep humidity gradient showed a mild temperature gradient of 0.09 °C cm−1 restricted to the dry half of the assay chamber (Fig. 3A). A more subtle thermal gradient also was present with the shallow humidity gradient with a higher baseline humidity of 90% RH (Fig. 3A). Thermal gradients concomitantly accompany humid gradients, because RH influences the rate of evaporative cooling of water from a damp surface. We hypothesized that C. elegans may use this subtle temperature gradient on the semimoist substrate and/or through its body as water evaporates through its semipermeable cuticle to perform hygrotaxis.
Fig. 3.
Humidity sensation requires cGMP-gated channel signaling in thermosensitive neuron pair AFD. (A) Quantification of temperature gradients accompanying humidity gradients at two time points. Temperatures are reported relative to the temperature adjacent to the hygrotaxis chamber (22.1 °C). Bars indicate SEM. n = 4. (B) Mutations and genetic ablations that disrupt the function of thermosensory neuron pair AFD abolish hygrotaxis. (C) Rescue of the cGMP-gated channel gene tax-4 in AFD neurons partially rescues hygrotaxis. (D) Thunberg model for hygrosensation in C. elegans. Normal hygrosensation requires the information from mechanosensory (via FLP) and thermosensory (via AFD) pathways. Deletion of either pathway results in defective hygrosensation. Asterisks denote the difference from WT in B and the difference from paired controls in D. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether thermal cues also may provide hygrosensitive information for C. elegans, we asked whether the thermosensation mutant ttx-1 could perform hygrotaxis. This mutant, which lacks a transcription factor essential for differentiation of the major thermosensitive neuron pair AFD, is the most profoundly defective thermotaxis mutant (12, 26). The ttx-1 mutant worms exhibited defective hygrotaxis (Fig. 3B). Likewise, a mutant defective in three guanylyl cyclase genes (gcy) that together are specifically required for AFD sensory transduction failed to perform hygrotaxis (Fig. 3B). To independently test the role of AFD neurons in hygrosensation, we genetically ablated them by expressing ICE with the AFD-specific promoter Pgcy-8. This AFD-ablated strain failed to perform hygrotaxis, strongly suggesting that AFD neurons have an essential role in hygrosensation (Fig. 3B).
Thermosensory information is transduced in the AFD neurons via a conserved cGMP-gated cation channel encoded by the tax-4 gene (27). We found that the tax-4 mutant performed abnormal hygrotaxis, demonstrating either no significant preference or only a mild preference for the humid side (Fig. 3B). The tax-4 gene is widely expressed in various sensory neurons (27). To determine whether tax-4 is required in AFD neurons for hygrotaxis, we expressed it in a tax-4 mutant background in AFD, which partially rescued hygrotaxis (Fig. 3C). To test whether tax-4 expression in additional neurons and with alternative isoforms might yield more complete rescue, we attempted rescue with a fosmid containing its endogenous regulatory elements and splicing information. This strain displayed the same level of rescue, however (Fig. 3C). We conclude that the TAX-4 channel may function primarily in AFD neurons to transduce the thermal information critical for hygrosensation.
Discussion
Through the use of a novel assay, we discovered that C. elegans is exquisitely sensitive to humidity, demonstrating robust orientation in shallow humidity gradients across a range of humidity conditions. Wheras C. elegans has been shown to sense a variety of sensory modalities (e.g., olfaction, taste, thermal, osmotic), our study now adds the additional sensory modality of hygrosensation to this clever worm’s behavioral repertoire. This orientation is subject to behavioral plasticity, with dependence on the humidity of rearing conditions as well as the feeding state (Fig. 1I and Fig. S2). This characteristic is consistent with other C. elegans sensory modalities, including chemotaxis and thermotaxis (12, 13, 19). In contrast to previous examples of sensory plasticity, however, we found that C. elegans would orient to humidity gradients only when cultured in a starved condition. This unexpected finding raises the possibility that C. elegans might show evidence of sensing additional sensory modalities only after a sensory stimulus is paired with aversive culture conditions. Further work is needed to test whether hygrotaxis plasticity involves associative learning and/or additional forms of plasticity.
Our ablation and cell-specific rescue experiments indicate that the multidendritic FLP neurons convey the mechanosensitive component of humidity levels in C. elegans (Fig. 2 C and D). Although hygroreceptive responses have been found in the antennae of insects, this is the first description of a hygrosensitive neuron in nonspecialized tissue. Thus, our findings can provide a basis for understanding humidity sensation in animals without antennae, or even hair.
The unique branched morphology of the mechanosensitive FLP neurons suggests a hygrosensation mechanism. Unlike most neurons in C. elegans, which display only a single unbranched dendrite, FLP is distinct, with its dendrite forming a “candelabra” of quaternary branching. The large receptive field of these dendrites (anterior third of the worm) and their position just microns below the epidermis (28) (Fig. 2 E and F) make them well suited for monitoring the spatially diffuse mechanical force imposed by skin hydration. This proposal may help explain how the worm distinguishes hydration-induced stretch from other mechanical stimuli. The subtle increase in cuticle stretch by hydration may selectively activate FLP without affecting nearby mechanosensory neurons that do not have branched dendrites. These neurons include the touch neurons, which have rod-shaped sensory dendrites aligned along the length of the animal, as well as anterior mechanosensory neurons, which detect vibrations or harsh prodding at the tip of the head with tiny cilia (14, 28).
The FLP neuron pair in C. elegans is most analogous in morphology and position to subcutaneous sensory neurons with branched dendrites found in fly larvae, mice, and humans (28–31) (Fig. 2F). Likewise, the ASIC-1, MEC-10, and MEC-6 proteins have clear orthologs in insects and in higher animals, including humans (SNN1G, PON1, and ACCN2), that form a mechanosensitive complex in branched subcutaneous sensory neurons (although PON1 may be secreted) (14, 32–34). Thus, our findings suggest the possibly of a conserved humidity-sensing strategy in which humidity levels may be encoded by the extent to which the dendritic field is stretched by skin hydration. If mechanosensory channels composed of MEC-10 and/or ASIC-1 subunits are found in the branched dendrites, then this stretching via hydration may depolarize FLP after an influx of cations. Thus, as with previous findings of FLP activating aversive reorienting turns from mechanical stimuli to the head, humidity-induced stretch of the FLP neurons may mediate reorienting turns as the worm crawls up the humidity gradient. Conversely, the worm would tend to reorient less frequently when crawling down the humidity gradient, in effect extending “runs” toward the dry side (yellow track in Fig. 1D). This suggests that FLP represents humid air as an aversive stimulus like mechanical stimuli to the head. The presence of orthologous DEG/ENaC mechanosensitive proteins in fly, mouse, and human epithelial sensory neurons suggests that this may be an evolutionally adaptive hygrosensation strategy.
In addition, we demonstrate an essential role for themosensory input in hygrosensation. Thermal input related to humidity levels appears to originate from the AFD neuron pair via TAX-4 cGMP-gated channel signaling (Fig. 3 B and C). When migrating down a humidity gradient, a worm experiences evaporative cooling from its body and in some cases a temperature drop owing to the evaporation of water from the moist substrate. Because C. elegans integrates temperature changes over time, an adult worm crawling down a humidity gradient of 0.5 °C cm−1 experiences a substrate temperature drop of ∼0.01 °C s−1, in addition to the cooling effects of the vapor-pressure deficient produced by moisture evaporation through the worm’s cuticle. Given that C. elegans displays isothermal tracking by detecting temperature differences of 0.01 °C mm−1 via AFD, humidity gradients probably produce salient temperature cues (12). Alternatively, the FLP neurons may also convey thermal information (although FLP may respond only to noxious heat) (23, 35, 36). The strategy of assessing thermal cues in parallel to mechanical cues may further disambiguate hygrosensory signals from either single modality.
Although many insects have hygrosensitive neurons in organs that also contain mechanosensitive and thermosensitive neurons, no previous study has determined whether selective deletion of either the mechanosensitive or thermosensitive pathways abolishes hygrosensation (Fig. 3D). This requirement of two distinct sensory pathways for a single sensory modality contrasts with all other sensory modalities in C. elegans and validates Thunberg’s 100-y-old hypothesis in which humidity sensation may arise from the integration of combined mechanical and thermosensory stimuli.
Materials and Methods
Nematode Strains and Culture Conditions.
C. elegans was cultured at 20 °C under standard conditions and fed Escherichia coli (OP50) (37). WT worms were Bristol variety N2. Additional strains are described in SI Materials and Methods.
Hygrotaxis Assay.
Worms were assayed in a custom polycarbonate plastic container that resembled a horizontal electrophoresis gel box with an elevated central area bordered by two troughs of 26 cm2 each, separated by 9.5 cm (Fig. 1A). Fixed weights of distilled water and desiccant (fresh Drierite or NaOH) were poured into opposite troughs (Fig. S1). Adult worms (starved for 18 h) were collected from their cultivation plates with a solution of sorbitol (Sigma-Aldrich); 150 mM and 250 mM produced identical results. C. elegans is neither attracted to nor repelled by sorbitol (38). Worms were next rinsed to remove bacteria, and then transferred in a 35-μL sorbitol solution to the middle of a thin agarose pad on a piece of glass in the middle of the chamber. A copper bar placed across the middle allowed the simultaneous assay of different strains in the same humidity gradient. Worms survived the low osmolarity of the agarose pads as long as the pads were dried (at room temperature in the hood for 35–55 min). A sterile cotton swab (Sigma-Aldrich) was used to wick away excess liquid and spread the worms across the center starting line. The container was then tightly sealed with plastic wrap (Fisher) to allow a moisture gradient to form.
Worms on each side of the assay were counted after 60 min to compute a performance index for hygrotaxis, Ihtx, representing the number of worms on the dry side minus the number on the humid side, divided by the total number on both sides (Fig. 1C). With this scheme, perfect hygrotaxis toward the dry side yields an Ihtx of +1, perfect hygrotaxis toward water yields an Ihtx of −1, and no preference yields an Ihtx of 0. n signifies the number of times that a population assay was conducted. Each assay reflects 20–200 individual worms in scoring positions. Transgenic worms were run with their untransformed sisters in the same assay as a control.
Supplementary Material
Acknowledgments
We thank M. Ailion, H. Kim, and S. Iyer for input on the manuscript; M. Reyes, A. Serrano, and S. Truong for help with experiments; A. Crisp and S. Iyer for help with imaging; and V. Maricq, M. Chalfie, and I. Mori for reagents. Worms were obtained from the Caenorhabditis Genetic Center, which is funded by the National Institutes of Health, and the National BioResource Project in Japan. This work was supported by a National Science Foundation Graduate Fellowship (to J.R.) and National Institutes of Health, National Institute of Neurological Disorders and Stroke Grant R01 NS075541 (to J.T.P.-S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322512111/-/DCSupplemental.
References
- 1.Pienkoedki RL. Behavior of the adult alfalfa weevil in diapause. Ann Entomol Soc Am. 1976;69(2):155–157. [Google Scholar]
- 2.Thomson RM. The reactions of mosquitoes to temperature and humidity. Bull Entomol Res. 1938;29:125–140. [Google Scholar]
- 3.Heimburger HV. Reactions of earthworms to temperature and atmospheric humidity. Ecology. 1924;5(3):276–282. [Google Scholar]
- 4.Gunn DL, Kennedy JS. Apparatus for investigating the reactions of land arthropods to humidity. J Exp Biol. 1936;13:450–459. [Google Scholar]
- 5.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci USA. 1996;93(12):6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazar G, Brandle K. Hygrotactic orientation of frogs in the laboratory. Amphib-Reptil. 1994;15(3):285–295. [Google Scholar]
- 7.von Arx M, Goyreta JN, Davidowitzb G, Ragusoa RA. Floral humidity as a reliable sensory cue for profitability assessment by nectar-foraging hawkmoths. Proc Natl Acad Sci USA. 2012;109(24):9471–9476. doi: 10.1073/pnas.1121624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokohari F, Tateda H. Moist and dry hygroreceptors for relative humidity of the cockroach, Periplaneta americana L. J Comp Physiol. 1976;106(2):137–152. [Google Scholar]
- 9.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450(7167):294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 10.Thunberg T. 1905. in Handbuch der Physiologie des Menschen (Braunschweig F. Vieweg, Germany), III, p 708.
- 11.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144(4):614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasakura H, Kuhara A, Mori I. Worm thermotaxis: A model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol. 2007;17(6):712–719. doi: 10.1016/j.conb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman MB. 2006. Mechanosensation. WormBook, doi/10.1895/wormbook.1.62.1.
- 15.Jung J, Nakajima M, Kojima M, Ooe K, Fukuda T. Microchip device for measurement of body volume of C. elegans as bioindicator application. J. Micro-Nano Mechatronics. 2012;7(1-3):3–11. [Google Scholar]
- 16.Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19(21):9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009;29(17):5370–5380. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madge DS. The control of relative humidity with aqueous solutions of sodium hydroxide. Entomol Exp Appl. 1961;4(2):143–147. [Google Scholar]
- 19.Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 20.Huang CG, Lamitina T, Agre P, Strange K. Functional analysis of the aquaporin gene family in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2006;292(5):C1867–C1873. doi: 10.1152/ajpcell.00514.2006. [DOI] [PubMed] [Google Scholar]
- 21.Árnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 22.Jasti J, Furukawa H, Gonzales E-B, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449(7160):316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 23.Chatzigeorgiou M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;13(7):861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO. 2008;27(24):3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24(2):347–361. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 26.Satterlee JS, et al. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31(6):943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu H, Mori I, Rhee J, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17(4):707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 28.White J-G, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 29.Geffeney SL, Goodman MB. How we feel: Ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron. 2012;74(4):609–619. doi: 10.1016/j.neuron.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott SA. Sensory Neurons: Diversity, Development, and Plasticity. New York: Oxford Univ Press; 1992. [Google Scholar]
- 31.Hall DH, Treinin M. How does morphology relate to function in sensory arbors? Trends Neurosci. 2011;34(9):443–451. doi: 10.1016/j.tins.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duggan A, García-Añoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277(7):5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 33.Deakin S, et al. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high-affinity, saturable, desorption mechanism. J Biol Chem. 2002;277(6):4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 34.Calavia MG, et al. Differential localization of acid-sensing ion channels 1 and 2 in human cutaneus pacinian corpuscles. Cell Mol Neurobiol. 2010;30(6):841–848. doi: 10.1007/s10571-010-9511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi A, Byrne Rodgers J, Kotera I, Ryu WS. Behavioral response of Caenorhabditis elegans to localized thermal stimuli. BMC Neurosci. 2013;14(1):66. doi: 10.1186/1471-2202-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Schulze E, Baumeister R. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS ONE. 2012;7(3):e32360. doi: 10.1371/journal.pone.0032360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward S. Chemotaxis by the nematode Caenorhabditis elegans: Identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA. 1973;70(3):817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]