Significance
CO2 fixation is the most important biosynthesis process on Earth, enabling autotrophic organisms to synthesize their entire biomass from inorganic carbon at the expense of energy generated by photo- or chemotrophic processes. In the present study we demonstrate an autotrophy pathway that represents the most energy-efficient mechanism for fixing inorganic carbon in the presence of oxygen. This novel variant of the hydroxypropionate/hydroxybutyrate cycle appears to be common in a ubiquitous and abundant group of microorganisms that can thrive in nutrient-limited environments. This discovery offers a biochemical explanation for the remarkable ecological success of the ammonia-oxidizing archaea in extremely nutrient-limited environments typical of most of the open ocean.
Keywords: Nitrosopumilus maritimus, autotrophy
Abstract
Archaea of the phylum Thaumarchaeota are among the most abundant prokaryotes on Earth and are widely distributed in marine, terrestrial, and geothermal environments. All studied Thaumarchaeota couple the oxidation of ammonia at extremely low concentrations with carbon fixation. As the predominant nitrifiers in the ocean and in various soils, ammonia-oxidizing archaea contribute significantly to the global nitrogen and carbon cycles. Here we provide biochemical evidence that thaumarchaeal ammonia oxidizers assimilate inorganic carbon via a modified version of the autotrophic hydroxypropionate/hydroxybutyrate cycle of Crenarchaeota that is far more energy efficient than any other aerobic autotrophic pathway. The identified genes of this cycle were found in the genomes of all sequenced representatives of the phylum Thaumarchaeota, indicating the environmental significance of this efficient CO2-fixation pathway. Comparative phylogenetic analysis of proteins of this pathway suggests that the hydroxypropionate/hydroxybutyrate cycle emerged independently in Crenarchaeota and Thaumarchaeota, thus supporting the hypothesis of an early evolutionary separation of both archaeal phyla. We conclude that high efficiency of anabolism exemplified by this autotrophic cycle perfectly suits the lifestyle of ammonia-oxidizing archaea, which thrive at a constantly low energy supply, thus offering a biochemical explanation for their ecological success in nutrient-limited environments.
All characterized representatives of the recently proposed phylum Thaumarchaeota (1) are chemolithotrophs that oxidize ammonia aerobically to nitrite via a yet unknown pathway (2). Initial experiments on the first cultured representative of this phylum, the marine archaeon Nitrosopumilus maritimus (3) showed many cellular, genomic, and physiological features that reflect an oligophilic lifestyle, being adapted to very low energy supply (3, 4). Indeed, kinetic studies on N. maritimus established its extremely high affinity for ammonia/ammonium (hereafter referred to simply as “ammonia”) (5). This affinity enables archaeal nitrifiers to grow at extremely low ammonia concentrations (in the low nanomolar range) and thus outcompete their bacterial counterparts in oligotrophic environments such as the open ocean (6–8). Consequently, ammonia-oxidizing archaea (AOA) almost certainly exert primary control over nitrification as well as contributing significantly to primary production in the ocean and in soils (9–13).
Autotrophic CO2 fixation is the central anabolic process in thaumarchaeal ammonia oxidizers. However, the energy available from the oxidation of the nanomolar concentrations of ammonia typical of the marine environment is very low. Thus, we hypothesized that highly efficient anabolic pathways would provide the thaumarchaea an ecological advantage relative to the ammonia-oxidizing bacteria (AOB) that are more competitive at higher ammonia concentrations. Among the six autotrophic CO2-fixation pathways described to date, three use oxygen-tolerant enzymes and therefore are regarded as aerobic, i.e., the widespread Calvin–Benson cycle, the 3-hydroxypropionate bicycle in Chloroflexi, and the hydroxypropionate/ hydroxybutyrate (HP/HB) cycle in aerobic Crenarchaeota (14–18). All these cycles have significant energy demands and vary only slightly in the amount of ATP required for the synthesis of cellular precursor metabolites from CO2, suggesting that they are distinguished by other features (14, 15, 18).
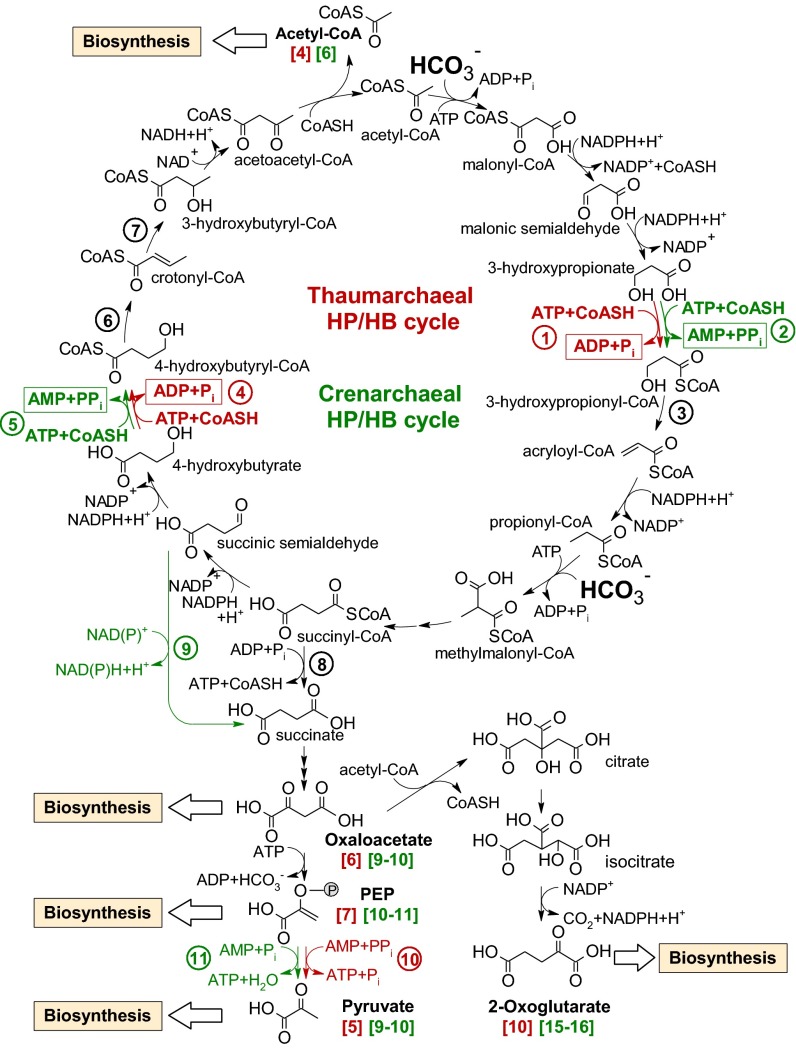
Genomic studies of the Thaumarchaeota suggested a functioning HP/HB cycle (4, 13, 19–22), which was first identified in the thermophilic crenarchaeon Metallosphaera sedula (19) and was extended to other aerobic Crenarchaeota (14, 23). Briefly, one acetyl-CoA and two bicarbonate molecules are converted via 3-hydroxypropionate to succinyl-CoA. Succinyl-CoA, in turn, is converted via 4-hydroxybutyrate to two molecules of acetyl-CoA, one of which serves as carbon precursor (Fig. 1). Assignment of a functional HP/HB cycle in Thaumarchaeota has been based solely on the detection of genes coding for characteristic enzymes of this cycle (acetyl-CoA/propionyl-CoA carboxylase, methylmalonyl-CoA mutase, 4-hydroxybutyryl-CoA dehydratase) in the absence of genes diagnostic for the other autotrophic pathways (4, 19, 20). However, certain genes encoding homologs for various enzymes specific to the cycle could not be identified in the genomes of Thaumarchaeota, and experimental evidence for its operation was missing. In addition, M. sedula and N. maritimus showed differences in stable carbon isotope fractionation relative to the inorganic carbon source. Although N. maritimus exhibited a fractionation of ca. −20‰ (24), the M. sedula biomass showed only a minor depletion of approximately −2‰ (25). Although the different discrimination effects may be attributed to the growth conditions of the two organisms (28 °C at pH 7.5 and 65 °C at pH 2, respectively), differences in carbon-fixing carboxylases cannot be excluded, thus underscoring the need for a biochemical characterization of the autotrophic CO2 fixation pathway in N. maritimus.
Fig. 1.
Reactions of the crenarchaeal and thaumarchaeal variants of the HP/HB cycle (modified from ref. 19). The reactions determining energy efficiency of the crenarchaeal (M. sedula) cycle are shown in green, and those for the N. maritimus variant are shown in red. Reactions common to both are shown in black. Note that although the two pathways have similar reactions and intermediates, they are significantly different in energy efficiency (Table 4) and evolved independently in Crenarchaeota and Thaumarchaeota (see text). The numbers in square brackets represent moles of high-energy anhydride bonds of ATP required to form 1 mol of the corresponding central precursor metabolites (see also Table 4). Enzymes as numbered in circles are 1, 3-hydroxypropionyl-CoA synthetase (ADP-forming); 2, 3-hydroxypropionyl-CoA synthetase (AMP-forming); 3, 3-hydroxypropionyl-CoA dehydratase; 4, 4-hydroxybutyryl-CoA synthetase (ADP-forming); 5, 4-hydroxybutyryl-CoA synthetase (AMP-forming); 6, 4-hydroxybutyryl-CoA dehydratase; 7, crotonyl-CoA hydratase; 8, succinyl-CoA synthetase (ADP-forming); 9, succinic semialdehyde dehydrogenase; 10, pyruvate-phosphate dikinase; and 11, pyruvate-water dikinase.
Here, using N. maritimus as a model organism, we demonstrate that Thaumarchaeota possess a modified version of the HP/HB cycle that is distinct from the cycle operating in Crenarchaeota (Fig. 1). Hence, our study shows that the HP/HB cycle apparently evolved twice in Archaea. Importantly, the identified mechanism for CO2 fixation is far more energy efficient than any other aerobic autotrophic pathway. Thus, this study provides a plausible biochemical explanation for the ability of AOA to thrive under extremely low-nutrient concentrations.
Results
Activities of HP/HB Cycle Enzymes in Cell Extracts of N. maritimus.
Direct evidence for the operation of the HP/HB cycle in Thaumarchaeota was provided by biochemical analysis of cell extracts generated from 12–15 L batch cultures of N. maritimus. Cells were grown autotrophically to a density of 3 × 107 cells/mL (∼0.6 mg/L dry mass) with a generation time of 35 h, which requires a specific carbon-fixation rate of 27 nmol⋅min−1⋅mg−1 protein. If two molecules of CO2 are fixed in one turn of the autotrophic CO2 fixation cycle, the minimal in vivo specific activity of this enzyme system is 13.5 nmol⋅min−1⋅mg−1 protein.
In accordance with results of genomic studies, the activities of the key enzyme of the Calvin–Benson cycle, ribulose 1,5-bisphosphate carboxylase/oxygenase, and of the 3-hydroxypropionate bicycle, mesaconyl-CoA C1–C4 CoA-transferase, could not be detected in cell extracts (SI Appendix, Table S1). In contrast, the key carboxylase of the HP/HB cycle, a biotin-containing acetyl-CoA/propionyl-CoA carboxylase, was active (Table 1). The activity was dependent on the addition of ATP and was completely inhibited by avidin, a specific inhibitor of biotin-dependent enzymes. Moreover, N. maritimus expressed a biotinylated protein, as shown by biotin staining of cell extracts with a size matching the predicted mass of the biotin carboxyl carrier protein of this carboxylase (Nmar_0274, 18.5 kDa) (SI Appendix, Fig. S1). The observed low activity probably is caused by the low stability of the enzyme complex. For example, in vitro Escherichia coli acetyl-CoA carboxylase is one of the least stable of the characterized enzymes (26), and its activity can hardly be detected in cell extracts.
Table 1.
Specific activities of the enzymes of the HP/HB cycle in cell extracts of N. maritimus
| Enzyme | Specific activity, nmol⋅min−1⋅mg−1 protein | Genes in N. maritimus |
| Acetyl-CoA carboxylase | 1.2 ± 0.1 | Nmar_0272/0273/0274 |
| Malonyl-CoA reductase (NADPH)* | 62 ± 6 | ? |
| Malonic semialdehyde reductase (NADPH) | 290 ± 40 | ? |
| 3-Hydroxypropionyl-CoA synthetase (ADP-forming) | 25 ± 5 | Nmar_1309 |
| 3-Hydroxypropionyl-CoA dehydratase | 480 ± 140 | Nmar_1308 |
| Acryloyl-CoA reductase | ≥1.6† | ? |
| Propionyl-CoA carboxylase | 0.5 ± 0.1 | Nmar_0272/0273/0274 |
| Methylmalonyl-CoA epimerase | ND | Nmar_0953 |
| Methylmalonyl-CoA mutase | ND | Nmar_0954/0958 |
| Succinyl-CoA reductase (NADPH)* | 18 ± 2 | ? |
| Succinic semialdehyde reductase (NADPH)* | 400 ± 30 | ? |
| 4-Hydroxybutyryl-CoA synthetase (ADP-forming) | 20 ± 5 | Nmar_0206 |
| 4-Hydroxybutyryl-CoA dehydratase | ≥1.5† | Nmar_0207 |
| Crotonyl-CoA hydratase [(S)-3-hydroxybutyryl-CoA forming] | 16,500 ± 1,400 | Nmar_1308 |
| (S)-3-Hydroxybutyryl-CoA dehydrogenase (NAD+)‡ | 160 ± 20 | Nmar_1028 |
| Acetoacetyl-CoA β-ketothiolase | ≥1.5† | Nmar_0841 and/or Nmar_1631 |
ND, not determined; ?, gene cannot be identified unambiguously based on bioinformatic analysis. The data are mean values and deviations of several independent enzyme assays.
No activity with NADH was detected.
Estimated from the rate of 3-hydroxypropionate conversion to propionyl-CoA (for acryloyl-CoA reductase) or from the rate of 4-hydroxybutyrate conversion to acetyl-CoA (for 4-hydroxybutyryl-CoA dehydratase and acetoacetyl-CoA β-ketothiolase).
No activity with NADP+ was detected.
N. maritimus cell extracts also catalyzed the postulated conversion of 3-hydroxypropionate into propionyl-CoA as well as the conversion of 4-hydroxybutyrate into two molecules of acetyl-CoA (SI Appendix, Figs. S2 and S3). The other enzyme activities necessary for the functioning of the HP/HB cycle were detected also (Table 1). The conversion of 4-hydroxybutyrate into two molecules of acetyl-CoA could be measured under oxic conditions (ambient air), showing that the key enzyme required for this conversion, 4-hydroxybutyryl-CoA dehydratase, is oxygen tolerant.
Acetyl-CoA is the product of the proposed HP/HB cycle and is used for biosynthesis (Fig. 1). However, in N. maritimus, acetyl-CoA cannot be reductively carboxylated to pyruvate as it is in anaerobes (SI Appendix, Table S1), so another assimilation mechanism is required. A similar situation occurs in M. sedula, where, by an additional half turn of the cycle, acetyl-CoA plus another two bicarbonate molecules are converted into succinyl-CoA. Succinyl-CoA then is converted to malate or oxaloacetate, which can be decarboxylated to pyruvate or phosphoenolpyruvate (27). Hence the precursor metabolites, primarily pyruvate, phosphoenolpyruvate, oxaloacetate, 2-oxoglutarate, and a few others, are generated from succinyl-CoA (Fig. 1). Accordingly, N. maritimus possesses an ADP-forming succinyl-CoA synthetase (Fig. 1 and SI Appendix, Table S1) operating in the reverse direction to drain carbon precursor metabolites off the HP/HB cycle. Phosphoenolpyruvate carboxykinase (Nmar_0392) is the only enzyme encoded in the genome of N. maritimus capable of catalyzing oxaloacetate conversion to phosphoenolpyruvate and thus connecting pools of C4 and C3 compounds.
Characterization of Thaumarchaeal 4-hydroxybutyryl-CoA Dehydratase.
The apparent oxygen insensitivity of 4-hydroxybutyryl-CoA dehydratase in cell extracts suggested that the key enzyme of the HP/HB cycle might be adapted to the aerobic operation of the cycle in N. maritimus. This possibility raised our interest, because closely related clostridial 4-hydroxybutyryl-CoA dehydratases had been reported to be extremely oxygen-sensitive (28, 29). The presence of the oxygen-tolerant 4-hydroxybutyryl-CoA dehydratase in Thaumarchaeota is a biochemical requirement for the operation of the HP/HB cycle under aerobic conditions. The gene encoding 4-hydroxybutyryl-CoA dehydratase (Nmar_0207) was cloned and heterologously expressed in E. coli. The corresponding protein was purified anaerobically, and its properties were compared with the properties of heterologously produced 4-hydroxybutyryl-CoA dehydratase from Clostridium aminobutyricum that participates in 4-aminobutyrate fermentation (28). The two enzymes are highly similar, sharing 58% amino acid sequence identity and 72% sequence similarity. Correspondingly, their Km and Vmax values for 4-hydroxybutyryl-CoA were almost identical (N. maritimus: Vmax 25 ± 2 µmol⋅min−1⋅mg−1 protein, Km 0.06 ± 0.02 mM; C. aminobutyricum: Vmax 19 ± 1 µmol⋅min−1⋅mg−1 protein, Km 0.056 ± 0.03 mM).
However, the two enzymes were strikingly different in their oxygen tolerance. The clostridial enzyme was oxygen sensitive and had a half-life of 14 min under aerobic conditions, whereas the 4-hydroxybutyryl-CoA dehydratase of N. maritimus was almost two orders of magnitude more stable in the presence of oxygen, retaining 50% of activity even after 46 h of incubation in the presence of oxygen (SI Appendix, Fig. S4). These data suggest that the thaumarchaeal 4-hydroxybutyryl-CoA dehydratase is well suited to function in aerobic organisms.
Characterization of Previously Unidentified Enzymes Involved in the HP/HB Cycle of N. maritimus.
Cell extract measurements revealed that the two CoA ligases acting on 3-hydroxypropionate and 4-hydroxybutyrate were ADP-forming (Fig. 1 and Table 1), whereas aerobic Crenarchaeota possess AMP-forming ligases (19, 30, 31). Because this issue is important for evaluating the energy efficiency of the cycle, we studied these enzymes in greater detail. The genome of N. maritimus contains two genes that are homologous to the ADP-forming acetyl-CoA synthetase from Archaeoglobus fulgidus (32), Nmar_1309 (38% identity to AF1938) and Nmar_0206 (41% identity to AF1211) and that may encode the required enzymatic activities. These genes were synthesized, cloned, and heterologously expressed in E. coli. The purified proteins were active and catalyzed the ATP-dependent conversion of 3-hydroxypropionate (Nmar_1309) and 4-hydroxybutyrate (Nmar_0206) with ATP and CoA into 3-hydroxypropionyl-CoA and 4-hydroxybutyryl-CoA, respectively, with ADP and Pi being formed. Although these proteins activated some other organic acids, the catalytic efficiency for these substrates was poor, thus identifying them as 3-hydroxypropionyl-CoA synthetase (Nmar_1309) and 4-hydroxybutyryl-CoA synthetase (Nmar_0206) (Tables 2 and 3). As discussed below, the presence of these two ADP-forming acyl-CoA synthetases is an important feature in making this cycle the most cost-effective autotrophic pathway in aerobes.
Table 2.
General properties of recombinant 3-hydroxypropionyl-CoA synthetase and recombinant 4-hydroxybutyryl-CoA synthetase from N. maritimus
| Property | 3-Hydroxypropionyl-CoA synthetase | 4-Hydroxybutyryl-CoA synthetase |
| Reaction catalyzed | 3-hydroxypropionate + ATP + CoA → 3-hydroxypropionyl-CoA + ADP + Pi | 4-hydroxybutyrate + ATP + CoA → 4-hydroxybutyryl-CoA + ADP + Pi |
| Gene | Nmar_1309 | Nmar_0206 |
| Molecular mass, kDa | 76 | 76 |
| Predicted molecular mass, kDa* | 76.1 | 75.6 |
| Divalent cations required for the activity | Mg2+ or Mn2+ (no activity with Ni2+, Co2+, and Ca2+) | Mg2+ or Mn2+ (no activity with Ni2+, Co2+, and Ca2+) |
Predicted for the native protein without polyhistidine tags.
Table 3.
Catalytic properties of recombinant 3-hydroxypropionyl-CoA synthetase and recombinant 4-hydroxybutyryl-CoA synthetase from N. maritimus
| Substrate | 3-Hydroxypropionyl-CoA synthetase |
4-Hydroxybutyryl-CoA synthetase |
||||
| Vmax, µmol⋅min −1⋅mg −1 protein | Km, mM | kcat/Km | Vmax, µmol⋅min −1⋅mg −1 protein | Km, mM | kcat/Km | |
| 3-Hydroxypropionate | 0.59 ± 0.03 | 1.2 ± 0.2 | 0.64 | – | – | – |
| 4-Hydroxybutyrate | 0.48 ± 0.04 | 5.6 ± 1.0 | 0.11 | 1.4 ± 0.01 | 0.37 ± 0.06 | 4.88 |
| Acetate | – | – | – | 0.22 ± 0.01 | 200 ± 5 | 0.001 |
| Propionate | 0.50 ± 0.10 | 17.0 ± 6.0 | 0.04 | 0.10 ± 0.01 | 88 ± 7 | 0.002 |
| Butyrate | 0.54 ± 0.01 | 12.4 ± 0.3 | 0.06 | 0.61 ± 0.05 | 5 ± 1 | 0.16 |
| ATP | 0.59 ± 0.03 | 0.6 ± 0.1 | 1.28 | 1.7 ± 0.2 | 0.22 ± 0.07 | 9.96 |
| CoA | 0.60 ± 0.10 | 0.16 ± 0.09 | 4.85 | 1.4 ± 0.1 | 0.16 ± 0.05 | 11.28 |
Neither synthetase was active with the following substrates: crotonate, acetoacetate, (S)-3-hydroxybutyrate, (R)-3-hydroxybutyrate, succinate, (RS)-malate, itaconate, (S)-citramalate. –, no activity detected.
Inspection of the N. maritimus genome sequence revealed that certain genes coding for proteins of the HP/HB cycle occur in distinct clusters. For instance, the gene for the 4-hydroxybutyryl-CoA synthetase is colocalized with the gene for the 4-hydroxybutyryl-CoA dehydratase (Nmar_0207), the latter catalyzing the following reaction of the cycle. The 3-hydroxypropionyl-CoA synthetase gene is colocalized with the gene for an enoyl-CoA hydratase of the crotonase family (Nmar_1308) that may represent a 3-hydroxypropionyl-CoA dehydratase, the next enzyme in the reaction sequence. Indeed, heterologously produced Nmar_1308 catalyzed 3-hydroxypropionyl-CoA dehydration to acryloyl-CoA (Vmax 74 ± 9 µmol⋅min−1⋅mg−1 protein, Km 0.18 ± 0.06 mM) as well as crotonyl-CoA hydration to 3-hydroxybutyryl-CoA (Vmax 3,110 ± 230 µmol⋅min−1⋅mg−1 protein, Km 0.16 ± 0.03 mM). Both activities are essential for the proposed cycle. The ratio of 3-hydroxypropionyl-CoA dehydratase and crotonyl-CoA hydratase activities for the heterologously produced enzyme was similar to the ratio of these activities in cell extracts (Table 1). This similarity suggests that Nmar_1308 is responsible for both conversions in vivo and functions as promiscuous 3-hydroxypropionyl-CoA dehydratase/crotonyl-CoA hydratase (Fig. 1).
Phylogenetic Analysis Indicates Convergent Evolution of the HP/HB Cycle.
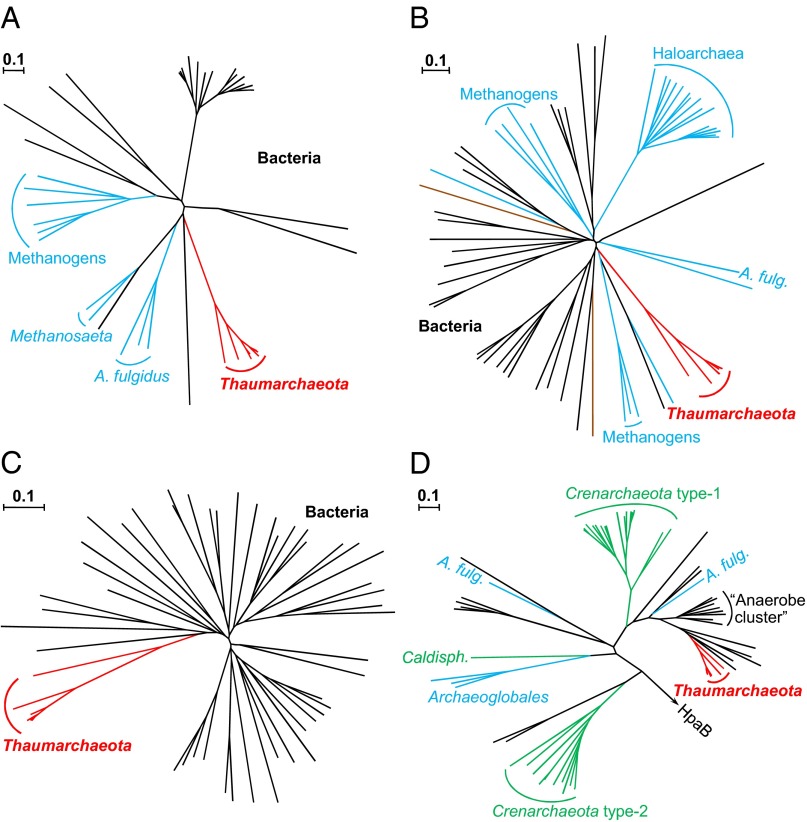
In addition to verifying experimentally the presence of three previously unaccounted genes of the HP/HB cycle in N. maritimus, we were able to identify most other genes for the enzymes of this pathway bioinformatically (Table 1). Using this information, we performed a comparative phylogenetic analysis to trace the evolution of the HP/HB cycle (Fig. 2 and see SI Appendix, Figs. S6–S13 and SI Appendix, SI Text for details). The identified genes of the HP/HB cycle were found in the genomes of all sequenced representatives of the phylum Thaumarchaeota, suggesting a common ability to grow autotrophically via this variant of the HP/HB cycle. Additionally, the corresponding proteins formed separate “thaumarchaeal clusters,” which were not specifically associated with crenarchaeal sequences. Even for the key enzyme of the cycle, 4-hydroxybutyryl-CoA dehydratase, thaumarchaeal proteins cluster together with bacterial representatives rather than with crenarchaeal ones (Fig. 2D). This clustering suggests a convergent evolution of autotrophic CO2 fixation cycles in Cren- and Thaumarchaeota.
Fig. 2.
Phylogenetic trees of N. maritimus HP/HB cycle enzymes identified in this study and of the key enzyme of the cycle, 4-hydroxybutyryl-CoA dehydratase. (A) Homologs of 3-hydroxypropionyl-CoA synthetase from Nmar_1309. (B) Homologs of the 4-hydroxybutyryl-CoA synthetase (Nmar_0206). (C) Homologs of 3-hydroxypropionyl-CoA dehydratase/crotonyl-CoA hydratase (Nmar_1308). (D) Homologs of 4-hydroxybutyryl-CoA dehydratase (Nmar_0207). Thaumarchaeal sequences are shown in red, crenarchaeal in green, euryarchaeal in blue, eukaryotic in brown, and bacterial in black. The tree is based on amino acid sequence analysis. Tree topology and evolutionary distances are given by the neighbor-joining method with Poisson correction. (Scale bars: a difference of 0.1 substitutions per site.) For details of the tree construction, see SI Appendix, Figs. S6 and S10–S12. The accession numbers of the sequences used for the construction of the tree are listed in SI Appendix, Table S4. A. fulg., A. fulgidus; Caldisph., Caldisphaera.
Comparison of the Growth Yield of AOA and AOB.
AOB use the costly Calvin–Benson cycle, whereas AOA use the identified cost-effective pathway, features that should severely affect the growth yields of these two groups. To compare the growth yield of AOA and their bacterial counterparts, we grew N. maritimus strain SCM1 and the AOB Nitrosococcus oceani in unbuffered synthetic medium containing 0.5 mM NH4Cl (SI Appendix, Fig. S5). The doubling time of N. oceani was significantly shorter than the doubling time of N. maritimus (15 h versus 28 h). However, the specific growth yield of N. maritimus was significantly greater, 1.3 g dry mass formed per mole of ammonia oxidized in contrast to only 0.8 g/mol for N. oceani. Therefore, N. maritimus couples ammonia oxidation with biomass formation in a more efficient way than N. oceani.
Discussion
Method-related challenges of this study were associated with the extremely small cell dimensions of N. maritimus and its oligotrophic character, resulting in growth with a generation time of more than a day and maximum cell densities of only 5 × 107 cells/mL. In addition to direct biochemical analysis using cell extracts generated from 12–15 L batch cultures, we further validated enzyme function and gene annotation by expressing four thaumarchaeal enzymes in E. coli. Together, the detailed biochemical characterization of carbon fixation in N. maritimus provided compelling evidence for the operation of an energy-efficient variant of the HP/HB cycle in Thaumarchaeota, both confirming and extending a mechanism of CO2 fixation only suggested by genomic analysis.
What makes the thaumarchaeal HP/HB cycle so special? This autotrophic cycle is more energy efficient than any other aerobic carbon-fixation pathway. The efficiency can be explained by three specific adaptions. First, the activation of 3-hydroxypropionate and 4-hydroxybutyrate proceeds via ADP- rather than AMP-producing enzymes; hence, one turn of the cycle requires two high-energy bonds less than one turn of the crenarchaeal HP/HB pathway (Fig. 1). Second, the participation of promiscuous enzymes catalyzing multiple reactions in the thaumarchaeal HP/HB cycle, such as acetyl-CoA/propionyl-CoA carboxylase and 3-hydroxypropionyl-CoA dehydratase/crotonyl-CoA hydratase, reduces the cost of protein biosynthesis. Third, the oxygen stability of 4-hydroxybutyryl-CoA dehydratase that until now has been assumed to require anoxic conditions because of its radical mechanism (33) is a prerequisite for the aerobic operation of the thaumarchaeal HP/HB cycle, lowering enzyme maintenance and turnover costs further. Interestingly, 4-hydroxybutyryl-CoA dehydratase from M. sedula also is robust in the presence of oxygen, as reported during revision of this work (34).
Comparative phylogenetic analyses pointed to convergent evolution of the HP/HB cycle, almost certainly having been invented independently in the crenarchaeal and thaumarchaeal lineages. The thaumarchaeal and crenarchaeal variants of the HP/HB cycle involve analogous reactions to fix CO2, but they use a divergent set of mostly unrelated enzymes. Thus, the advantage in energy conferred by this pathway explains its thrice-repeated independent emergence during evolution, namely in the thaumarchaeal and crenarchaeal versions of the HP/HB cycle and in the 3-hydroxypropionate bicycle in Chloroflexi (14, 19, 35). Independent emergence of the HP/HB cycle provides additional support for the hypothesis of an early evolutionary divergence of the Thaumarchaeota and its status as a separate phylum (1). Still, only few full-genome sequences of the representatives of Thaumarchaeota are available, and further phylogenetic analyses that include new members of the Thaumarchaeota are required to confirm these conclusions.
The development of this economical cycle no doubt has contributed to the ecological success of AOA. Accordingly, CO2 fixation through the thaumarchaeal HP/HB cycle requires one-third less energy than via the Calvin–Benson cycle functioning in AOB (36) (Table 4). Furthermore, CO2 fixation via the HP/HB cycle is not accompanied by losses caused by the oxygenase side-reaction of ribulose-1,5-bisphosphate carboxylase/oxygenase, which leads to an additional loss of about 20% of fixed carbon in organisms using the Calvin–Benson cycle (15, 37). In fact, comparison between the specific growth yields obtained from cultures of N. maritimus and the marine AOB N. oceani grown under exactly the same conditions indicates that N. maritimus uses the energy gained by ammonia oxidation for biomass formation in a much more efficient way than its bacterial counterpart (SI Appendix, Fig. S5). The specific growth yield of N. maritimus was ∼1.5 higher than the value determined for N. oceani. (Note, however, that the growth rate of N. oceani was significantly higher than the growth rate of N. maritimus.) Therefore, the growth yields of AOA and AOB distinctly mirror the different energy requirements of the corresponding autotrophic pathways. Consequently, these data offer a biochemical explanation for the dominance of AOA, relative to AOB, in environments with low ammonia availability such as open ocean water (7, 10), where they often account for more than 20% of all planktonic microorganisms (6).
Table 4.
Comparison of the energy efficiency of aerobic autotrophic CO2 fixation cycles
| 1 mole precursor or 1 g biomass | Calvin–Benson cycle* | 3-Hydroxypropionate bicycle | Crenarchaeal HP/HB cycle | Thaumarchaeal HP/HB cycle |
| Acetyl-CoA | 7 | 7 | 6 | 4 |
| Pyruvate | 7 | 7 | 9–10† | 5 |
| Phosphoenolpyruvate | 8 | 9 | 10–11† | 7 |
| Oxaloacetate | 8 | 9 | 9–10† | 6 |
| 2-Oxoglutarate | 15 | 16 | 15–16† | 10 |
| Biomass (1 g) | 0.12* | 0.13 | 0.13–0.15† | 0.09 |
The numbers represent moles of high-energy anhydride bonds of ATP required to form 1 mole of central precursor metabolite or of the main precursors for the synthesis of 1 g of dry biomass. For the details of calculations, see SI Appendix, SI Text. The estimated amount of the central metabolic precursors acetyl-CoA, pyruvate, phosphoenolpyruvate, oxaloacetate, and 2-oxoglutarate necessary for the synthesis of 1 g of dry cells was taken from ref. 42.
Note that the costs of the oxygenase side reaction of the key enzyme of the Calvin–Benson cycle (ribulose 1,5-bisphosphate carboxylase) are not taken into account in this table. C3 plants lose about 20% of fixed carbon in photorespiration (15, 37).
The costs of the synthesis of pyruvate, phosphoenolpyruvate, oxaloacetate, and 2-oxoglutarate in M. sedula vary depending on the pathway of succinyl-CoA conversion to succinate (27).
The autotrophic lifestyle of marine AOA was confirmed earlier by radiocarbon studies on diagnostic archaeal lipids revealing that more than 80% of the thaumarchaeal biomass in natural seawater originates from inorganic carbon and by incorporation of radiolabeled bicarbonate (11, 38). Estimations suggest that Thaumarchaeota contribute to around 1% to the total marine primary production and contribute significantly to the global carbon cycle (11, 38). Because the relative abundance of Thaumarchaeota increases with depth (up to 40% of all pelagic microorganisms; ref. 6), the thaumarchaeal variant of the HP/HB cycle probably represents the most important CO2-fixation pathways below the photic zone in the meso- and bathypelagic realm of the ocean.
Apart from providing an explanation for the capacity of the Thaumarchaeota to thrive in extreme oligotrophic environments, there are clear biotechnological applications that could derive from the present study. In recent years, carboxylating enzymes and autotrophic CO2-fixation pathways have attracted much attention because of their potential future applications for the production of industrial chemicals using synthetic biology approaches (39–41). Autotrophy based on acetyl-CoA/propionyl-CoA carboxylase rather than on ribulose-1,5-bisphosphate carboxylase/oxygenase would seem to be an attractive model for the development of a synthetic carbon-fixation pathway. The HP/HB cycle already has been suggested as a potential tool for biotechnological applications (40, 41), and we suspect that, apart from the biological importance, our discovery could have significant applied value.
Materials and Methods
N. maritimus strain SCM1 was cultivated autotrophically under aerobic conditions with ammonia as a sole energy source at 28 °C as described previously (3, 5). Cell extracts were prepared using a French pressure cell. 3-Hydroxypropionate conversion to propionyl-CoA and 4-hydroxybutyrate conversion to acetyl-CoA were measured by following the formation of propionyl-CoA and acetyl-CoA, respectively, with HPLC. Ribulose-1,5-bisphosphate carboxylase/oxygenase and acetyl-CoA and propionyl-CoA carboxylases were measured radiochemically by determining substrate-dependent fixation of 14CO2. Other enzyme assays were performed using standard biochemical techniques either spectrophotometrically or by following the product formation in an ultra-performance liquid chromatography (UPLC)-based assay. HPLC and UPLC were performed using a reversed-phase C18 column, the reaction products were detected by absorbance at 260 nm, and the amount of product(s) was calculated from the relative peak area. The genes for Nmar_1308 and Nmar_0207 were amplified from genomic DNA of N. maritimus, and the genes for Nmar_1309 and Nmar_0206 were synthesized by Eurofins MWG Operon. The codon use in the genes was optimized for the expression in E. coli. The gene for clostridial 4-hydroxybutyryl-CoA dehydratase was amplified from genomic DNA of C. aminobutyricum. The genes from N. maritimus were cloned in the expression vector pET16b containing an N-terminal His-tag, and the clostridial gene was cloned in the expression vector pET28b (N-terminal His-tag). All proteins were heterologously overproduced in E. coli and purified using Ni-NTA column. Detailed materials and methods can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank G. Fuchs of Freiburg University and K.-U. Hinrichs of Bremen University for discussions, suggestions, and critical reading of the manuscript; W. Schmieder, M. Glinka, C.-E. Wegner, and K. Egloff for help with some experiments; W. Buckel and J. Zhang of Marburg University for providing genomic DNA of C. aminobutyricum; T. Selmer of Aachen University for providing plasmid pASK-7plus-pct; Eva Spieck, Hamburg University, for providing Nitrosococcus oceani; and S. M. Sievert of the Woods Hole Oceanographic Institution for financial support. This work was funded by Deutsche Forschungsgemeinschaft (Grants BE 4822/3-1 and BE 4822/1-1 to I.A.B., KO 3651/1-1 to M.K., and a Gottfried Wilhelm Leibniz Prize to K.-U. Hinrichs); and in part by the United States National Science Foundation Grants MCB-0920741 (to D.A.S.) and OCE-0623908. L.S.v.B. and T.J.E. were funded by the Schweizerische Nationalfonds Ambizione Grant PZ00P3_136828/1 and Eidgenössische Technische Hochschule (ETH) Research Grant ETH-41 12-2 (both to T.J.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
1On leave from M. V. Lomonosov Moscow State University, Moscow 119234, Russia.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402028111/-/DCSupplemental.
References
- 1.Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6(3):245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- 2.Vajrala N, et al. Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA. 2013;110(3):1006–1011. doi: 10.1073/pnas.1214272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 4.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461(7266):976–979. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 6.Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature. 2001;409(6819):507–510. doi: 10.1038/35054051. [DOI] [PubMed] [Google Scholar]
- 7.Stahl DA, de la Torre JR. Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol. 2012;66:83–101. doi: 10.1146/annurev-micro-092611-150128. [DOI] [PubMed] [Google Scholar]
- 8.Schleper C, Nicol GW. Ammonia-oxidising archaea—physiology, ecology and evolution. Adv Microb Physiol. 2010;57:1–41. doi: 10.1016/B978-0-12-381045-8.00001-1. [DOI] [PubMed] [Google Scholar]
- 9.Leininger S, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442(7104):806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 10.Wuchter C, et al. Archaeal nitrification in the ocean. Proc Natl Acad Sci USA. 2006;103(33):12317–12322. doi: 10.1073/pnas.0600756103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingalls AE, et al. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc Natl Acad Sci USA. 2006;103(17):6442–6447. doi: 10.1073/pnas.0510157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakimov MM, et al. Contribution of crenarchaeal autotrophic ammonia oxidizers to the dark primary production in Tyrrhenian deep waters (Central Mediterranean Sea) ISME J. 2011;5(6):945–961. doi: 10.1038/ismej.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratscher J, Dumont MG, Conrad R. Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc Natl Acad Sci USA. 2011;108(10):4170–4175. doi: 10.1073/pnas.1010981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg IA, et al. Autotrophic carbon fixation in archaea. Nat Rev Microbiol. 2010;8(6):447–460. doi: 10.1038/nrmicro2365. [DOI] [PubMed] [Google Scholar]
- 15.Berg IA. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol. 2011;77(6):1925–1936. doi: 10.1128/AEM.02473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hügler M, Sievert SM. Beyond the Calvin cycle: Autotrophic carbon fixation in the ocean. Annu Rev Mar Sci. 2011;3:261–289. doi: 10.1146/annurev-marine-120709-142712. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Even A, Flamholz A, Noor E, Milo R. Thermodynamic constraints shape the structure of carbon fixation pathways. Biochim Biophys Acta. 2012;1817(9):1646–1659. doi: 10.1016/j.bbabio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs G. Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life? Annu Rev Microbiol. 2011;65:631–658. doi: 10.1146/annurev-micro-090110-102801. [DOI] [PubMed] [Google Scholar]
- 19.Berg IA, Kockelkorn D, Buckel W, Fuchs G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science. 2007;318(5857):1782–1786. doi: 10.1126/science.1149976. [DOI] [PubMed] [Google Scholar]
- 20.Hallam SJ, et al. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biol. 2006;4(4):e95. doi: 10.1371/journal.pbio.0040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blainey PC, Mosier AC, Potanina A, Francis CA, Quake SR. Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS ONE. 2011;6(2):e16626. doi: 10.1371/journal.pone.0016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spang A, et al. The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: Insights into metabolic versatility and environmental adaptations. Environ Microbiol. 2012;14(12):3122–3145. doi: 10.1111/j.1462-2920.2012.02893.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg IA, Ramos-Vera WH, Petri A, Huber H, Fuchs G. Study of the distribution of autotrophic CO2 fixation cycles in Crenarchaeota. Microbiology. 2010;156(Pt 1):256–269. doi: 10.1099/mic.0.034298-0. [DOI] [PubMed] [Google Scholar]
- 24.Könneke M, Lipp JS, Hinrichs KU. Carbon isotope fractionation by the marine ammonia-oxidizing archaeon Nitrosopumilus maritimus. Org Geochem. 2012;48(1):21–24. [Google Scholar]
- 25.van der Meer MT, Schouten S, Rijpstra WI, Fuchs G, Sinninghe Damsté JS. Stable carbon isotope fractionations of the hyperthermophilic crenarchaeon Metallosphaera sedula. FEMS Microbiol Lett. 2001;196(1):67–70. doi: 10.1111/j.1574-6968.2001.tb10542.x. [DOI] [PubMed] [Google Scholar]
- 26.Cronan JE, Jr, Waldrop GL. Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res. 2002;41(5):407–435. doi: 10.1016/s0163-7827(02)00007-3. [DOI] [PubMed] [Google Scholar]
- 27.Estelmann S, et al. Labeling and enzyme studies of the central carbon metabolism in Metallosphaera sedula. J Bacteriol. 2011;193(5):1191–1200. doi: 10.1128/JB.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerhardt A, Cinkaya I, Linder D, Huisman G, Buckel W. Fermentation of 4-aminobutyrate by Clostridium aminobutyricum: Cloning of two genes involved in the formation and dehydration of 4-hydroxybutyryl-CoA. Arch Microbiol. 2000;174(3):189–199. doi: 10.1007/s002030000195. [DOI] [PubMed] [Google Scholar]
- 29.Scherf U, Söhling B, Gottschalk G, Linder D, Buckel W. Succinate-ethanol fermentation in Clostridium kluyveri: Purification and characterisation of 4-hydroxybutyryl-CoA dehydratase/vinylacetyl-CoA delta 3-delta 2-isomerase. Arch Microbiol. 1994;161(3):239–245. doi: 10.1007/BF00248699. [DOI] [PubMed] [Google Scholar]
- 30.Alber BE, Kung JW, Fuchs G. 3-Hydroxypropionyl-coenzyme A synthetase from Metallosphaera sedula, an enzyme involved in autotrophic CO2 fixation. J Bacteriol. 2008;190(4):1383–1389. doi: 10.1128/JB.01593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins AS, Han Y, Bennett RK, Adams MW, Kelly RM. Role of 4-hydroxybutyrate-CoA synthetase in the CO2 fixation cycle in thermoacidophilic archaea. J Biol Chem. 2013;288(6):4012–4022. doi: 10.1074/jbc.M112.413195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musfeldt M, Schönheit P. Novel type of ADP-forming acetyl coenzyme A synthetase in hyperthermophilic archaea: Heterologous expression and characterization of isoenzymes from the sulfate reducer Archaeoglobus fulgidus and the methanogen Methanococcus jannaschii. J Bacteriol. 2002;184(3):636–644. doi: 10.1128/JB.184.3.636-644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckel W, Golding BT. Radical enzymes in anaerobes. Annu Rev Microbiol. 2006;60:27–49. doi: 10.1146/annurev.micro.60.080805.142216. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins AB, Adams MW, Kelly RM. Conversion of 4-hydroxybutyrate to acetyl coenzyme A and its anapleurosis in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate carbon fixation pathway. Appl Environ Microbiol. 2014;80(8):2536–2545. doi: 10.1128/AEM.04146-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarzycki J, Brecht V, Müller M, Fuchs G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci USA. 2009;106(50):21317–21322. doi: 10.1073/pnas.0908356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bock E, Wagner M. 2006. Oxidation of inorganic nitrogen compounds as an energy source. The Prokaryotes: Vol. 2: Ecophysiology and Biochemistry, eds Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (Springer, New York), pp. 457–495.
- 37.Cegelski L, Schaefer J. NMR determination of photorespiration in intact leaves using in vivo 13CO2 labeling. J Magn Reson. 2006;178(1):1–10. doi: 10.1016/j.jmr.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Herndl GJ, et al. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl Environ Microbiol. 2005;71(5):2303–2309. doi: 10.1128/AEM.71.5.2303-2309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erb TJ. Carboxylases in natural and synthetic microbial pathways. Appl Environ Microbiol. 2011;77(24):8466–8477. doi: 10.1128/AEM.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuzawa S, Chiba N, Katz L, Keasling JD. Construction of a part of a 3-hydroxypropionate cycle for heterologous polyketide biosynthesis in Escherichia coli. Biochemistry. 2012;51(49):9779–9781. doi: 10.1021/bi301414q. [DOI] [PubMed] [Google Scholar]
- 41.Keller MW, et al. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc Natl Acad Sci USA. 2013;110(15):5840–5845. doi: 10.1073/pnas.1222607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the Bacterial Cell: A Molecular Approach. Sunderland, MA: Sinauer; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.