Abstract
Life’s origin entails enclosing a compartment to hoard material, energy, and information. The envelope necessarily comprises amphipaths, such as prebiotic fatty acids, to partition the two aqueous domains. The self-assembled lipid bilayer comes with a set of properties including its strong anisotropic internal forces that are chemically or physically malleable. Added bilayer stretch can alter force vectors on embedded proteins to effect conformational change. The force-from-lipid principle was demonstrated 25 y ago when stretches opened purified Escherichia coli MscL channels reconstituted into artificial bilayers. This reductionistic exercise has rigorously been recapitulated recently with two vertebrate mechanosensitive K+ channels (TREK1 and TRAAK). Membrane stretches have also been known to activate various voltage-, ligand-, or Ca2+-gated channels. Careful analyses showed that Kv, the canonical voltage-gated channel, is in fact exquisitely sensitive even to very small tension. In an unexpected context, the canonical transient-receptor-potential channels in the Drosophila eye, long presumed to open by ligand binding, is apparently opened by membrane force due to PIP2 hydrolysis-induced changes in bilayer strain. Being the intimate medium, lipids govern membrane proteins by physics as well as chemistry. This principle should not be a surprise because it parallels water’s paramount role in the structure and function of soluble proteins. Today, overt or covert mechanical forces govern cell biological processes and produce sensations. At the genesis, a bilayer’s response to osmotic force is likely among the first senses to deal with the capricious primordial sea.
Keywords: mechanosensitivity, force sensing, channel gating, bilayer mechanics, touch
To compete successfully, organisms must evolve effective reactions to earth’s physical stimuli: heat, force, voltage, and chemical ligands. Emil Fischer’s lock-and-key binding between molecules is well known and explains such overt senses as vision, smell, and most tastes. We also understand voltage sensing. For example, a charge-bearing helix traversing the electric field across the lipid bilayer can mechanically drive conformational changes of embedded ion channels or enzymes (1). Heat and force are universal, governing all matters and reactions. A challenge to current biology is to understand how Nature chooses and uses her molecular thermometers and force gauges. We review the basis of force sensing, emphasizing the roles of the lipid bilayer mechanics.
Biological Mechanisms Are Ultimately Mechanics
Textbooks describe the energy minimum when atoms are brought to an equilibrium distance (bond length), at which, there is zero force between them because their mutual attraction balances repulsion. Thermal oscillations or external forces change the bond length and generate compressive or expansive forces in the bond, much like with a spring. External force acting on an atom can simply move the whole bonded assembly. For example, pulling one end of an α-helix can tilt it. A force can also strain the bond and displace the partners, like stretching or bending the helix. The bond breaks when work (force times displacement) exceeds the bond energy. For example, it takes ∼4 pN (4 × 10−12 N) to break a hydrogen bond, ∼1,600 pN to break a C–C covalent bond (Fig. S1). Ligand–protein or protein–protein binding also mechanically strains the bonds between protein domains to effect long-range conformational changes. The last 100 y of mechanochemistry have shown how mechanical forces govern even inorganic chemical reactions (2). Many steps in biochemical reactions or other molecular processes can therefore be simulated by explicitly stating the Newtonian forces involved and following the motions of the components—an approach bringing invaluable insights into molecular underpinnings of life. The 2013 Chemistry Nobel was awarded for the development of molecular dynamics and quantum mechanical approaches to simulate complex chemical systems. How the paired atoms move upon a perturbation can be described by a set of laws and numerical parameters called the “force field” (3). In macromolecules, long-distance Lennard-Jones and electrostatic forces come into play during conformational change. Repositioning atoms by iteratively calculating the multiple short- and long-distance forces among atoms and feeding them into Newtonian laws of motion is the basis of molecular dynamics simulation (MDS), which in recent decades has successfully analyzed and explained the many workings of macromolecules and their assembly (4, 5). Some forces relevant to biology and used in MDS are listed in Fig. S1. Some findings might surprise us. For example, most of the time in catalysis is spent on the diffusion to and from the catalytic site, mechanically positioning the substrates and products; whereas the very stage of chemical transformation, of which we tend to fixate, happens almost instantaneously.
The Mechanics of the Lipid Bilayer
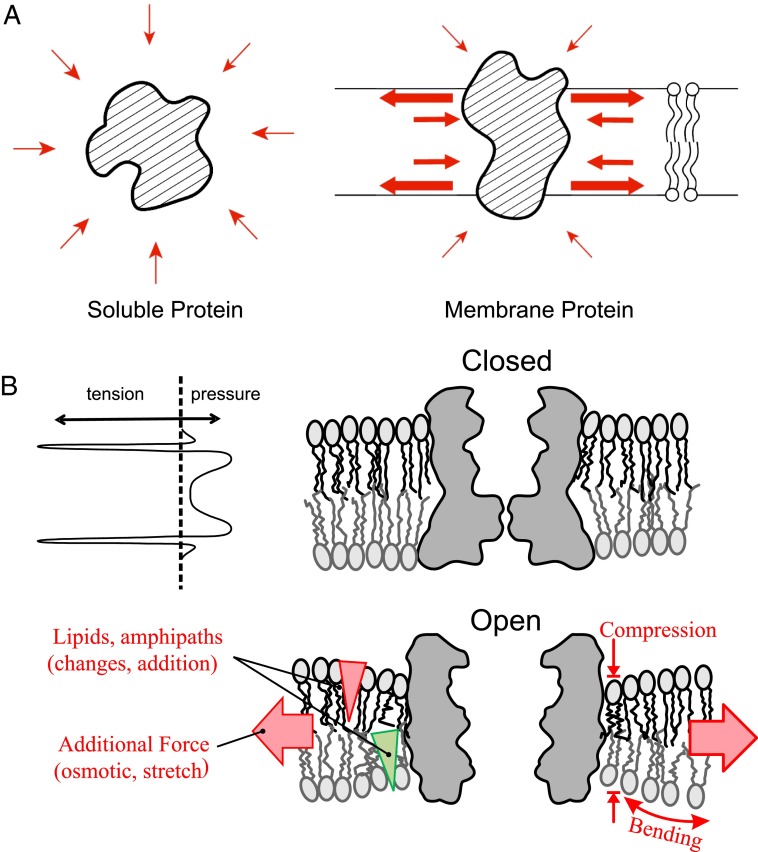
A surface tension appears at any liquid–liquid or liquid–gas interface. Recall that at a hexane–water interface, the surface tension appears because water molecules there are deprived of half of their hydrogen-bonding partners they enjoy in the bulk. Thermal dispersion of the surface also makes molecules less densely packed in the few interfacial layers, thus decreasing the repulsion between the neighbors (6). Qualitatively similar interfacial forces develop in the lipid bilayer. However, there are critical distinctions. Molecules at a liquid interface are not restrained from freely joining the bulk. Water in a sea of hexane will shrink into a sphere. Adding more water increases the sphere’s volume but reduces the relative number of water at the surface, with no change in surface tension. In contrast, the polar lipid head is stuck with its nonpolar tail making the bilayer a “surface without bulk.” Typical membrane lipids tend to compact into a bilayer sheet instead of a droplet. Adding lipids enlarges the sheet but does not change the exposed-to-hidden surface ratio per molecule. Water molecules strongly bond with the polar atoms of the head groups or the glycerol, although barred from the nonpolar hydrocarbon tails. Thus, a sharp lateral tension develops at the level of the lipid neck. As a self-assembled entity at equilibrium, this tension is balanced by the large repulsion from interior tail movement and the smaller head–head repulsion in a relaxed membrane. The relative contribution of the two repulsions depends on the head-to-tail width ratio, which corresponds to the spontaneous curvature of the lipid leaflet, with a larger head favoring positive curvature. This layered internal force distribution has been calculated from thermodynamics (7, 8) and examined by MDS (9, 10) (Fig. 1B, Upper Left). Thus, unlike soluble proteins, which are bombarded by particles from all directions (Fig. 1A, Left), embedded proteins as a part of the membrane ensemble are subjected to anisotropic push and pull (Fig. 1A, Right), especially sharply focused suction. A stretch will deform the water sphere in hexane imagined above, changing the surface-to-volume ratio but not the surface tension. However, a stretch force can only thin the bilayer (until it breaks) and thereby increase the lateral tension. This general description should not obscure local variations. The contour and hydrophobicity of the protein surface need to match that of the lipids. The bilayer structure can therefore be distorted, e.g., being curved, compressed, or otherwise deformed at the protein-lipid interface. The distortion spreads over a few layers of annular lipids and even enables a mechanical cross-talk between the proteins or protein domains through the lipid medium (11). Added external stretch can thin the bilayer generating a hydrophobic mismatch at the interface, driving the protein to take a better-matched conformation. Reducing the acyl chain length from PC20 to PC18 converts the reconstituted gramicidin A from a stretch-activated to a stretch-inactivated channel (12). Besides thinning, a stretch can also change the magnitude and direction of the force vectors on the protein. Chemically changing bilayer composition by inserting impurities or through lipid metabolism can have the same effect (Fig. 1B). Although these principles are general, we chose to discuss ion channels among membrane proteins because their activities can be examined easily and quantitatively, even at the level of single proteins and down to a microsecond time resolution (13) by electric means.
Fig. 1.
Anisotropic forces of the lipid bilayer and their changes that can reshape embedded proteins. (A) A diagram comparing a protein in the cytoplasm, near-isotropically compressed by the bombardment of neighboring particles (Left) and one embedded in a bilayer, subjected to large lateral tension at the two polar–nonpolar interfaces opposed by compression forces at the other levels of the bilayer (Right). (B) The lateral tension at the interface is balanced by the pressure in the head groups and hydrophobic tails (Upper Left) and this equilibrium can match a certain protein conformation, say, the closed state of a channel protein (Upper Right). External stretch force (Lower, broad red arrows) and/or amphipaths with positive (red triangle) or negative (green triangles) spontaneous curvature asymmetrically added to one leaflet can thin and bend the bilayer at the protein–lipid interface, changing the force vectors (small arrows) and therefore prefer a better matched protein conformation, say, the open state.
The Force-from-Lipid Principle
The force-from-lipid principle was established some 25 y ago from the study of the mechanosensitive channels from Escherichia coli (14, 15). Their discovery, atomic structures, and biological roles have been periodically reviewed (see refs. 16 and 17 and citations therein). Briefly, the key observation is that purified MscL or MscS can be reconstituted into artificial bilayers and retain their mechanosensitivity, excluding the need of cytoskeleton, external tether, or accessory channel subunits as force transmitters (18, 19). Because bilayers of phospholipids of different head groups (20) or fatty-acid lengths (21) support the mechanosensitivity of MscL, a lock-and-key ligand-gated mechanism need not apply. Judging by the slope of the Boltzmann distribution of the open probability against bilayer tension, MscL protein expands by ∼20 nm2. This expansion in area (ΔA) requires 220 pN to stretch its 22-nm perimeter, doing 2 × 10−23 J (50 kT) work. Half of the channels are open at 10–12 pN/nm, not far from the lytic tension of lipid bilayer (22, 23). The ΔA estimated from activities approximates the structural estimate from crystallography (24) and electron paramagnetic resonance spectroscopy (25). MscL is a homopentamer of subunits with two transmembrane helices, M1 and M2. To open, these helices apparently lean down and move outward making a much thinner structure, as if to meet a thinned bilayer (25). Bilayers with shorter chain length favor opening, but tension is still required. However, an asymmetric addition of lysolipids to either one of the monolayers opens the channel in a relaxed membrane (21), suggesting involvement of additional physical parameters, such as a mismatch of tension in the two leaflets and/or bending of annular lipids favored by positive curvature. Asymmetric insertions of exogenous amphipaths activate these channels (14), consistent with the bilayer-coupling hypothesis (26). The lipid force profile and how stretch relates to MscL gating has been examined by MDS (10). In line with the role of the bilayer’s focused lateral tension (Fig. 1B), random and scanning mutagenesis showed that replacing hydrophobic with hydrophilic residues in M1 or M2 at the outer rim of MscL removes mechanosensitivity in vitro and in vivo (27).
The miniprotein antibiotic gramicidin A from Bacillus brevis readily forms cation channels in bilayers by pair-wise stacking. It has been used to examine the role of various chemical and physical properties of the bilayer in quantitative details (28). Over the years, the force-from-lipid principle has gradually been used to understand eukaryotic processes as well. More recently, this principle has been rigorously extended to animal ion channels as summarized below.
Mechanosensitive Two-Pore-Domain K+ Channels
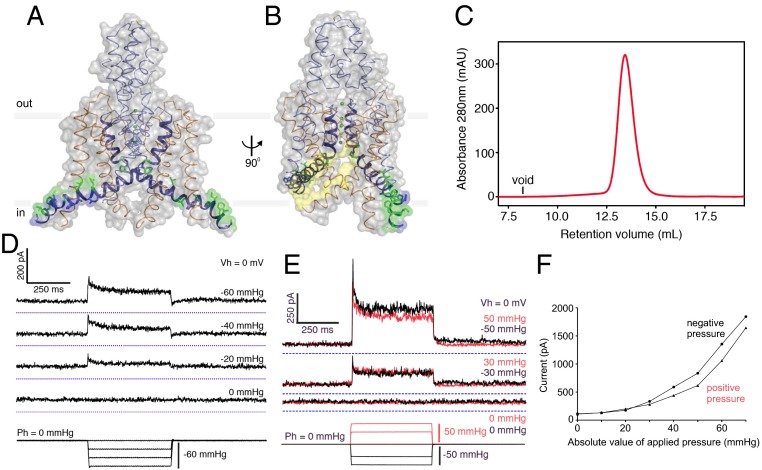
Microbes have more varieties of K+ channels than animals (29). The simplest are homotetramers of S1-P-S2 subunits (“S” for “segment” in “α-helical segment”; “P” for “pore” in “pore domain”, the filter structure), such as KcsA from the Gram-positive Streptomyces, the first ion channel of known crystal structure (30). A variant of this motif are two-pore-domain K+ channel (K2p) dimers of S1-P1-S2-S3-P2-S4 subunits. Human K2p family comprises 15 fairly disparate members, several of which can be stretched open in clamped patches. The structures of two K2p (31–33) are now known. The mechanosensitive TRAAK (TWIK-related arachidonic acid-stimulated K+) (34) has been solved in open states at 3.8 Å (32) (Fig. 2 A and B) and then at 2.75 Å resolution revealing a domain swap (33). TRAAK has features not found in KcsA, two of which may be related to mechanosensitivity: Of the four inner helices, the two S2 are long, each having a kink that makes the lower portion lying almost flat. This portion is amphipathic with hydrophilic basic residues facing the membrane–cytoplasm interface (Fig. 2A, purple) and hydrophobic residues facing the membrane interior (green), placing the two peptides at the membrane–cytoplasm interface of the inner leaflet, rich in acidic lipids in vivo. Interestingly, the inner transmembrane half of K2p channels (31) is even fenestrated. In TRAAK (32, 33), a sizable portion of channel’s lower half is not enclosed by protein, leaving a 5-Å-wide gap presumably filled with lipids in vivo, extending from the bottom of the filter to the lower edge of the channel (Fig. 2B, yellow).
Fig. 2.
The atomic structures and the reconstituted activities of TRAAK. (A and B) TRAAK structures from two perspectives showing the two S2 amphipathic peptides [hydrophobic (green) and charged (purple) residues] and one of the two prominent fenestrations (yellow) (from ref. 32). (C) Monodispersion of column eluate indicates the purity of the TRAAK protein produced. (D) Such protein is reconstituted into liposomes. TRAAK channels in a patch excised from such a proteoliposome are activated (upper traces) in proportion to the suction applied to patch (bottom traces). (E) TRAAKs in a patch excised from an expressing CHO cell, in which they should have the same orientation, respond (upper traces) to either suction (black) or pressure (red). (F) Activation by pressures in either direction are quantitatively similar, indicating that it is the membrane stretch that activates (modified from ref. 46).
K2p control the resting membrane potential by passing leak current (35). They are regulated by disparate stimuli: pH, temperature, lipidic ligands, and membrane stretch. Among the diverse K2p, the mechanosensitive TREK-1 (TWIK-related K+-1) and TRAAK have been extensively studied by Patel, Honore, and coworkers (36, 37). Whole-cell TREK-1 current is strongly suppressed by hyperosmolarity. In patches from expressing cells, pressure in either direction activates TREK-1, although suction is more effective. It is also activated by negatively charged phospholipids such as PIP2, by lysophospholipids, polyunsaturated fatty acids (PUFAs), volatile general anesthetics (36, 37) and morphine (38).
Reconstitution of Vertebrate Mechanosensitive K+ Channels, K2p
Although there are antecedents (39, 40), recent works generate new excitement because of the deep knowledge on the structure and function of K2p summarized above. Berrier et al. (41) have recently reconstituted an enriched fraction of mouse TREK-1 into liposomes and examined patches excised from them. They observed the ∼80-pS K+-specific outward-rectifying chlorpromazine-sensitive conductance. Surprisingly, these channels displayed spontaneous activities that could not be further increased by pipet suctions. Positive pipet pressure pulses, however, could proportionally reduce their activity to total closure. These authors reasoned that open probability has been maximized by inherent tension in the patches (41). Such a tension is likely generated during the gigaOhm-seal formation, bonding the bilayer lipids to the pipet glass surface (42–45).
In a recent rigorous study, Brohawn et al. (46) purified zebrafish TREK-1 and human TRAAK to homogeneity, as evidenced by monodispersion of column-elution profiles (Fig. 2C), and also reconstituted them into liposomes made of mixed-length phophatidylcholine extracted from soybean azolectin. In excised patches with multiple channels, they found the channels to respond instantaneously to applied force (Fig. 2D). These channels are also activated by arachidonic acid (the PUFA embedded in the name TRAAK). These K2p respond proportionally to either negative or positive pressures much like the bacterial MscL in a similar setting (21). This is also true when TRAAKs are expressed in cultured cells, where they should be in the same orientation (Fig. 2 E and F). This indifference to pressure direction leaves no doubt that it is the added bilayer tension that gates the channels. Beyond demonstrating the force-from-lipid biophysical principle for animal channels, these authors also expressed TRAAK in cultured mouse Neuro2A cells. Prodding them with a probe evokes a K2p-dependent outward current that counteracts the native inward current through Piezo1. Details aside, both sets of reconstitution experiments clearly showed that vertebrate K2p, much like MscL and MscS, are themselves sensitive to stretch force from the lipid bilayer, requiring no additional subunits, cytoskeleton, or extracellular matrix tethers.
Precisely how lipid forces operate TREK-1 or TRAAK is not yet clear. Like many K+ channels, including the budding-yeast K2p (47), TREK-1 apparently uses a dynamic filter, where several gain-of-function mutations are found (48). Its quaternary ammonium (QA)-binding site seems accessible from the inside before channel activation, suggesting a static open inner gate (49), although QAs could reach the site through the fenestration now shown in the atomic structure (32, 33) (Fig. 2B). TREK-1 is activated by negatively charged phospholipids such as PIP2, and inhibited by polycations or agents that cause PIP2 hydrolysis (36, 37). These observations can be explained by the interaction of the negatively charged inner leaflet with a proximal peptide segment, rich in basic amino acids, which immediately follows the end of the inner helix S4. The bilayer crenators (trinitrophenol, lysolecithin) activate, whereas cup formers (chlorpromazine, tetracaine) inhibit TREK-1 (36, 37), consistent with the bilayer-coupling hypothesis (14, 26). TREK-1 is estimated to expand by ∼2 to 4 nm2 in the membrane plane when opens (45). It remains reasonable that the inner helices (S2 and/or S4) converge to close and diverge to open like other K+ channels as was supposed by earlier homology models (50), even if the inner gate cooperates with the filter gate. The two S2 inner helices, having amphipathicity (Fig. 2 A and B) that matches the inner leaflet naturally rich in acidic phospholipids and being at the level where peak lateral tension is expected, would predict that they are subjected to that tension and its changes during membrane stretch.
The Voltage-Dependent K+ Channel Is as Sensitive to Bilayer Force as It Is to Voltage
The celebrated voltage-dependent K+ channel, Kv, is a tetramer of S1-S2-S3-S4-S5-P-S6 subunits in essence (1). Like the S1-P-S2 of KcsA, the four S5-P-S6 helical pairs come together to form the pore domain housing the filter and the gate. The four S1-S4 form the four peripheral domains, each bearing a voltage sensor. Driven by the resting membrane potential, the voltage sensors, acting through the S4-S5 linkers, mechanically constrict the lower gate comprising the four S6. Depolarization moves the sensors and linkers, releasing the constriction (51). These actions likely also make use of the intimately attached lipids (52). Here the channel opens not by removing a plug, but by a lateral expansion of the cytoplasmic side of the channel with a ΔA. If Kv is built only for interactions between its protein domains, one might expect the domains to be densely packed to minimize the contact with the membrane—a potential source of noise. However, the association between the pore and the peripheral domains is limited to the inner S4-S5 link and a contact between S1 and S6 toward the outer side (52, 53). As a result, all domains, including the gate-bearing core are in extensive contact with lipids. This unusual design implies roles of lipids in Kv function. Indeed, the action of the voltage sensor with its positive arginines (the gating charges) engages the negative phosphate oxygens at the lipid neck (54, 55). However, why should the pore domain also be surrounded by lipids?
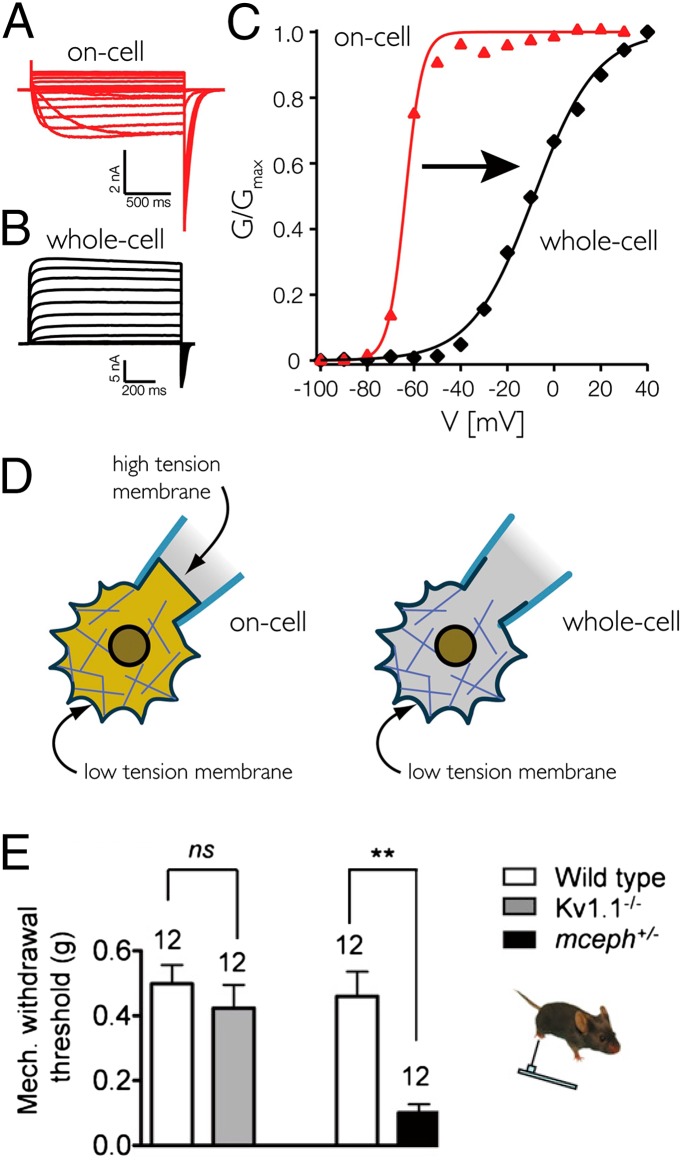
The free-energy change associated with the bilayer deformation is estimated to be comparable to that of the voltage-dependent part of the total gating energy (56). Although not commonly known, Kv (e.g., the Kv1.2 paddle chimera of known crystal structure) (52) shows drastically different behavior in whole cells, in patches, or upon reconstitution into planar bilayers. MacKinnon and coworkers found the differences best explained by changes in tension on the membrane, held low when it is locally constrained by subcortical cytoskeleton, but increases when the membrane is detached and adheres to the surface of the glass electrode (43, 44). For example, the tendency to open, as indicated by the Boltzmann slope and midpoint voltage, is much higher for channels in an on-cell patch than those in the rest of the same cell after the patch is broken (Fig. 3 A–D). Quantitative analyses of kinetic schemes indicate that the tension-sensitive step is the final opening of the gate after the four voltage sensors have moved, i.e., at the point when ΔA takes place. Thus, tension mainly acts on coupling the sensors to the gate, presumably through lipids, and not on the sensors themselves. The tension in the patch generated by lipid adhesion to glass is only about 0.5–4 mN/m (42). Free-energy changes, as estimated from the changes of the rate of opening step with or without added tension, indicate a ΔA of some 3–4 nm2, consistent with structural information (44). Because this tension-dependent behavior is observed in excised patches and varies with lipid compositions upon Kv reconstitution (43), the tension must come from the lipids. The effect of this small tension is not trivial. “Kv channel is as much a mechanosensitive channel as it is a voltage-dependent channel” (44, p. 10356); open probability rises from 0 to 0.65 between −60 and +50 mV, but rises from 0 to 1.0 upon suction at midrange voltages. Kv, being a model for Nav, Cav, and TRPs, indicates that the lipid-tension effect is much more general than we commonly thought. The generality of channel mechanosensitivity has been advocated by Morris and coworkers (ref. 57 and references therein).
Fig. 3.
The mechanosensitivity of Kv. (A–C) The Kv1.2 paddle chimera expressed in an Sf-9 cell responds to voltage steps ranging from −100 to 40 mV. The responses in on-cell mode (A) are drastically different from those in the whole-cell mode (B). The normalized conductances are graphically compared in C. As interpreted in D, the large differences originate from the high pipet tension in the on-cell patch due to lipid–glass adhesion, but the lack of it in the whole-cell membrane, where the cortical cytoskeleton prevents the local membranes from being stretched (from ref. 44). (E) The role of Kv1.1’s mechanosensitivity in countering impact-induced excitation in vivo is made evident when a copy of a dominant-negative allele of Kv1.1 is expressed in the mouse (mceph+/−) causing it to be overly sensitive to gentle poking, as indicated by the low limb-withdrawal threshold of prod strength (from ref. 58). ns, not significant; **P < 0.01.
Physics or Physiology?
Any entity that deforms in the direction of force is, by physical definition, mechanosensistive. So does the mechanosensitivity of Kv simply reflect its necessary expansion in the membrane plane under stress, or does it have any biological significance? As described above, heterologous TRAAK can pass outward current that counteracts in piezo1 inward current in cells responding to external force (46). Given evolution’s opportunism, it seems unlikely that this exquisite sensitivity is not exploited. Hypoosmotic swelling opens Kv in cultured cells (44), presumably by stretching small membrane domains confined by cytoskeletons (Fig. 3D). Thus, osmotic changes, muscle contractions, or lipid-composition changes (below) may activate or modulate Kv, Cav, etc., at least in principle. Mechanosensitivity is most “palpable” in the sense of touch. Interestingly, a recent report shows that, when certain dorsal-root-ganglion neurons are prodded, channels with Kv1.1 subunits pass K+ outward, acting as a brake (58) to oppose the depolarization by the inward current through other touch-sensitive channels yet to be defined. It is reasoned that these Kv1.1 subunit-containing channels therefore set the threshold of the touch-induced action potential or tune the firing adaptation. Mice expressing a dominant negative allele of Kv1.1 suffer severe mechanical allodynia, interpreting touch as hurt (58) (Fig. 3E).
Evolution also exploits Kv’s mechanosensitivity negatively. The Chilean rose tarantula paralyzes its preys with venom that includes the toxin VSTx1, which enters the membrane to bind the Kv2.1 sensor at 10−8 M specificity (59). Strangely, VSTx1 has no effect on Kv in slack membranes, and causes the channel in the tense bilayer to behave as in a slack membrane (43), as if the toxin perturbs the local lipid arrangement to block voltage sensor-gate coupling and/or the final gate opening.
The Photomechanics of Fly Vision
In the 1970s, the Pak laboratory and others (60) began a systematic genetic dissection of the Drosophila visual transduction. Blind flies were sorted by complementation grouping and by the wave of discharges from the compound eye during a light flash (electroretinography). Mutants display different corruptions of the wild-type waveforms. For examples: “norpA” for “no receptor Potential, complementation group A” later proves to encode phospholipase C; “innaE” for “inactivation, no afterpotential, group E” encodes a diacylglycerol lipase, etc., implicating the involvement of lipid metabolism.
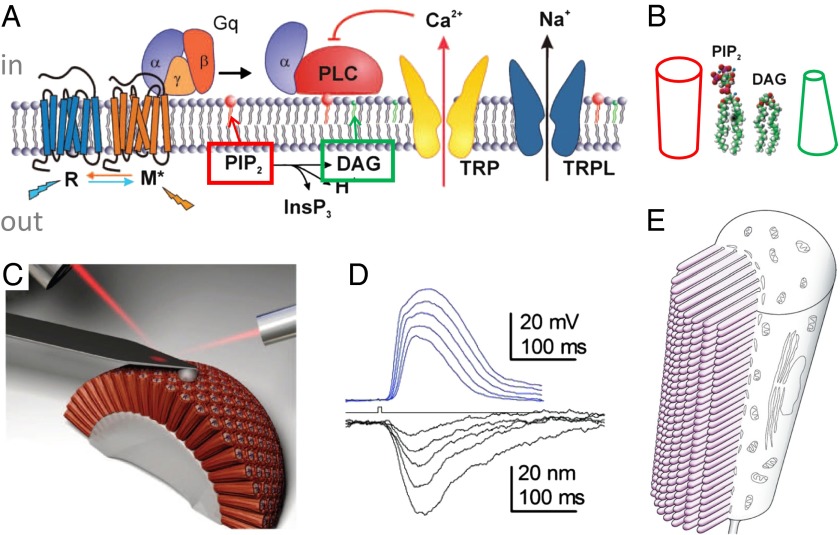
“trp” stands for “transient receptor potential” (61), which was the first blind fly gene cloned (62). trp and its homolog trpl (trp-like) were later shown to encode pore-forming channel subunits (63). Much research implicates the cascade of rhodopsin-Gq-phospholipase C (PLC, from norpA) hydrolyses PIP2 (phosphatidylinositol 4,5-bisphosphate) into DAG (diacylglycerol), although how these lipids activate the channels was unclear. Thinking being chemical in our era, one tends to assume that these lipids bind a specific site to control the channels. It is therefore a great surprise when Hardie and Franze (64) showed that there is a global physical change in the membrane. Astonishingly, light induces a near-micrometer shrinkage of the ommatidia (units in the compound eye) that is directly visible under a light microscope and measured with an atomic force microscope (Fig. 4 C and D). This contraction begins before the channel current. Deleting the PLC enzyme (norpA−/−) takes away the contraction; deleting the channels does not. The authors reason that beheading PIP2 to make the smaller DAG increases tension in the inner membrane leaflet to pull open channels (Fig. 4A). To test, the well-established mechanosensitive channel, gramicidin A (28) was added to the photoreceptor cell and found to pass current proportional to light intensity. Note that going from PIP2 to DAG is not an isometric shrinkage. That PIP2 rod becoming the DAG cone (Fig. 4B) should increase bilayer asymmetry and curvature, and would change the bilayer force profile (Fig. 1). Although we do not know the exact changes in membrane thickness, curvature, or forces in the channel’s vicinity, diverse cationic amphipaths that are expected to enter and crowd the inner leaflet (thus reducing lateral tension there) did inhibit light-induced current. The case of membrane forces controlling TRP/TRPL therefore seems convincing. Still, H+ (65) and PUFAs (products of innaE) (66) are also involved, so photomechanics may not be the whole story.
Fig. 4.
The photomechanical transduction pathway in the Drosophila compound eye. (A) A diagram showing the pathway, where the G protein-activated PLC converts PIP2 to DAG, concentrated in the inner leaflet (upper half of the bilayer in this diagram). (B) Lipid structures emphasize that the beheading of the rod-shaped PIP2 converts it into the cone-shaped DAG, yielding change in both the volume of lipid and spontaneous curvature in the inner leaflet (modified from ref. 64). (C) The arrangement of the cantilever of an atomic force microscope on a retina. (D) Brief flashes (bump on the center line) leads to shrinkages as measured by the atomic force microscope, proportional to light intensity (lower curves). The shrinkage precedes the phototransduction current (upper curves) (from ref. 64). E shows the structure of an ommatidium, the unit of the compound eye, which comprises thousands of microvilli where the phototransduction takes place. It is the sum of the small shrinkage in each microvillus that is measured by the atomic force microscope (modified from ref. 100).
The Drosophila work coined the term “TRP,” which is honored as the founder of the TRPC subfamily (below), with “C” being “canonical”. Canonical or not, is this a special case? Rods and cones do not work this way: The rhodopsin-Gt-phosphodiesterase cascade leads to a cGMP drop that closes the cGMP-gated channel, shutting off dark current. However, the vertebrate retina also contains non–image-forming but intrinsically photosensitive ganglion cells for circadian entrainment. Here the molecular pathway directly parallels that of the insect, beginning with melanopsin and ending in the activation of TRPC6/7 (67). The conservation is striking, with melanopsin being far more similar to the insect rhodopsin than vertebrate rhodopsin (68). Similar pathways may be used for other TRP channels in melanocyte (69) and keratinocyte (70) of the skin.
That the ∼5-Å PIP2-to-DAG head shrink can become a near-micrometer contraction has to do with the unusual anatomy of the insect photoreceptor cell, which sums the minute shrinkage of the thousands of stacked microvilli it bears (Fig. 4E). Potentially, there is a direct mechanical link between the accumulation of DAG and the size of a stack of microvilli. For example, increased content of this lipid with negative spontaneous curvature in the inner monolayer would favor higher curvature, thinner microvilli, and lateral-stack shrinkage. Elsewhere, isolated microvilli, cilia, filopodia, or dendritic spines are common, where such physical movement cannot easily be detected. Nonetheless they house many signal-transduction pathways that deploy receptors, G proteins, PLC, and TRPs. Such pathways are commonly explored according to the current dominant paradigm, with the ultimate explanation being protein phosphorylations due to PLC’s activation of protein kinase C (PKC) (71). One wonders how much the PLC-induced changes in bilayer tension discussed here in fact participate in these pathways (72).
TRP Superfamily
TRPs form a loosely connected family: The mammalian subtype TRP-A, -C, -M, -ML, -N, -P, and -V are only similar in the S5-P-S6 core sequence. Although known since the 1970s, it is the 1997 discovery of the “pepper channel” (TRPV1) being a molecular thermometer (73) that caught imagination and popularized TRP research, resulting in copious and complex findings that defy any comprehensive review today. Besides the subfamily diversities, TRP channels are polymodal, meaning that each can receive and integrate multiple inputs, including cytoplasmic ligands, pH, Ca2+, membrane lipids and other amphipaths, temperature, osmolarity, and mechanical force. TRPC1, C6, M3, M4, M7, P2, V1, V2, and V4 are judged as mechanosensitive, by widely different criteria. The risk seems not that their mechanosensitivity is in doubt but that other TRPs are not so assigned, given that the mechanosensitivity can so easily be masked, as in Kv and the Drosophila TRP. For example, TRPV4 has long been thought to be activated by a lipid ligand (74) but can in fact be stretched open (75, 76).
Besides mammals and insects, TRPs are found in protists, algae, diatoms, fungi, etc., although not in prokaryotes (16). The extensively studied TRPY1 (77) in the vacuolar membrane of the budding yeast presents a 300-pS inward rectifier, favoring the flow of cations including vacuolar Ca2+ into the cytoplasm, observed upon hyperosmotic shock. It opens to membrane stretch in excised patches. Forward and reverse genetics point to the importance of aromatic residues, which tend to locate at the polar–nonpolar interfaces of the bilayer. The S5-P-S6 core appears to be the force sensor because insertion of amorphous peptides covalently disrupting the connection between the core from the S1-S4 peripheral domains has little effect on channel mechansoensitivity (78).
The atomic structure of TRPV1 has recently been solved by cryoelectron microscopy showing a Kv-like basic structure with several differences (79, 80). It has also been purified and reconstituted into lipid bilayers, showing that this minimal assembly is sufficient for temperature sensing (81). There has been no report on TRP channel reconstitution experiments to directly test the principle of lipid-force sensing. However, indirect evidence is accumulating, arguing against force transmission from partner proteins. For example, rat TRPV4 has been expressed in budding yeast and remains responsive to osmotic stimulation (82) and in Xenopus oocytes displaying mechanosensitive unitary conductances (75, 76). Given the divergence among yeast, toad, and rat, it is difficult to imagine how heterologous cytoskeleton or other tether can reconnect to transmit force.
Accessories
Besides the definitive reconstitution experiments, there are other evidences that general physical properties instead of specific chemical binding govern mechanosensitivity. By various criteria, reported mechanosensitive channels include MscL, MscS, Mec-4/10, TRP, Piezo, K2p, Kv, KCa, Nav, NMDA receptor, CFTR, AChR, etc. (see ref. 83 for references). These proteins have no similarities and no recognizable “force-sensing domain” comparable to voltage sensor or ligand-binding sites. We emphasize the nonspecific physical interaction between embedded protein and the bilayer here partly because it is often ignored. There are, of course, examples of specific binding of certain lipids (e.g., PIP2), anesthetics, or drugs, that act on membrane proteins.
The cortical cytoskeleton protects the membrane from being stretch (Fig. 3 A–D) in animal cells in general. Treatment with cytochalasins, actin-filament dissociating agents, ease the stretch activation of myocyte’s native unitary conductances, the first such activation ever described (84). Similar observations have also been made on mechanosensitive channels of known molecular identity. For example, treatment with latrunculin, another such agent, greatly enhances the suction-induced current through TREK-1–expressed in COS cells (85). Mutating β-spectrin, which links the membrane to the cytoskeleton, reduces the force needed to pull membrane tether from cultured touch-receptor neurons of the worm with an atomic force microscope (86).
The elaborate anatomy of the vertebrate cochlear or the insect Johnston’s organ illustrates how additional structures are built to enhance mechanosensations. At the level of molecules, channels are accessorized to harness or amplify the signals. Some channels clearly choose their own lipids, e.g., the negatively charged PIP2 as deduced by its effects. The binding can even be tight enough for resolution in crystal structure, such as Kir (87). Tightly bound lipids can be considered subunits of such proteins. Many channels seem to preferentially reside in raft-like microdomains. Mechanosensitive channels are found to be in ordered and thicker domains with cholesterol and sphingolipids (83). Accessory proteins such as cadherins or integrins also form tethers to transmit force inward. There is little doubt that the transmitted force gate mechanosensitive channels, as in the case of the hair-cell transduction channel. However, it is unclear whether the channels bind the tether directly or indirectly through other proteins to receive the force. The channel can even receive the force from a lipid microdomain, which is pulled by the tether (83). The hair-cell tip link produces tenting that appears to tense up the membrane (88). For cells less specialized than the hair cells, a common conception is that the channel is pulled by the cytoskeleton. As described above, the cortical cytoskeleton prevents rather than activates mechanosensitive channels (44) (Fig. 3 A–D). Although tugging on the stress fibers can indeed open certain channels (89), it is again unclear whether we are pulling the channel or the surrounding lipids.
If a channel with a small free-energy difference between its closed and open state has to expand a sizable gate to allow ion passage, it must be very sensitive to membrane tension, as in the case of Kv. Additional structures may well be needed to prevent accidental opening. The peripheral domains in Kv and its related tetrameric channels may well be such structures. A major role of the cholesterol-rich stiffen platform is likely to enforce order and suppress mechanical noise (83).
The Origin
Homo sapiens is but ∼4 × 105-y-old, whereas Australian slime-mat fossils date back to ∼4 × 109 y, near the earth’s formation at ∼4.5 × 109 y. Life’s origin requires an early step to segregate and concentrate the self-replicators, presumably RNAs, and their metabolites. Segregation of an aqueous compartment requires enclosure with an amphipathic material. Thus, life’s abiotic origin is not just a chicken-and-egg problem, but also a yolk-and-shell problem. Besides the RNA-world, there is in fact a lipid-world theory that argues for the early appearance of vesicles and protocells (90, 91). Venter and coworkers’ Mycoplasma laboratorium experiment (92) or Michael Crichton’s Jurassic Park fiction both require placing remodeled or renewed DNAs into a preexisting cell. To completely create life in vitro, synthetic RNAs need to be wrapped inside a man-made bilayer vesicle, as being attempted in the Szostak laboratory (93, 94) and elsewhere (95). Mother Nature took her time with available material. Abiotic Miller–Urey experiments produce amino acids but also long-chain hydrocarbons, their derivatives, and even lipid-like amphipaths. Some meteorites carry much more fatty acid than amino acids. Such lipid-like molecules undergo spontaneous noncovalently linked aggregations to form micelles, bilayers, or vesicles through entropy-driven hydrophobic interactions. This spontaneity is the key, requiring no additional material or information. The self-assembled bilayer comes with its physical properties and abilities. Even in a naked lipid bilayer, lateral forces profile respond to deformation (10), heat (96), pH (97), ions (98), chemical modifications by light (99), etc. It could work as an elementary sensor in response to changes, including osmotic changes, in the violent primordial soup. The responses may appear crude now, but allow for evolutionary improvements by the embedment of impurities, such as peptides. We are now at awe by the sophistication of modern membranes, which house the key energy converters: photosynthesis and oxidative phosphorylation. However, at the most basic level, we are still struggling to understand how physical forces govern various cell biological, genetic, and developmental processes. The 21st century remains surprisingly ignorant of mechanosensitive processes. We know not the molecular bases of touch, hearing, the Bayliss effect, electromechanical feedback of the heart, the monitoring of blood pressure. Nor do we know how organisms sense gravitation, wind, waves, drought, and flood. The growing literature on the importance of lipids and amphipaths leads us to advocate a paradigm that combines the chemistry and the mechanics of the bilayer beyond lock-and-key thinking. We review here the recent extension of the force-from-lipid principle from bacterial to animal ion channels. This extension showed that membrane proteins are universally governed by the mechanical properties of surrounding lipids throughout evolution. This review on the extension of force-from-lipid principle is meant to remind us that certain physical principles are so basic that they underlie all molecules, whether they reside in bacteria, however lowly, or in humans, however lofty.
Supplementary Material
Acknowledgments
Work in our laboratories is supported by the Huck Institute of Life Sciences (A.A.) and National Institutes of Health Grant GM096088 and the Vilas Trust of the University of Wisconsin–Madison (to C.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313364111/-/DCSupplemental.
References
- 1.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309(5736):897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 2.Beyer MK, Clausen-Schaumann H. Mechanochemistry: The mechanical activation of covalent bonds. Chem Rev. 2005;105(8):2921–2948. doi: 10.1021/cr030697h. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Donini O, Reyes CM, Kollman PA. Biomolecular simulations: Recent developments in force fields, simulations of enzyme catalysis, protein-ligand, protein-protein, and protein-nucleic acid noncovalent interactions. Annu Rev Biophys Biomol Struct. 2001;30:211–243. doi: 10.1146/annurev.biophys.30.1.211. [DOI] [PubMed] [Google Scholar]
- 4.Khalid S, Bond PJ. Multiscale molecular dynamics simulations of membrane proteins. Methods Mol Biol. 2013;924:635–657. doi: 10.1007/978-1-62703-017-5_25. [DOI] [PubMed] [Google Scholar]
- 5.Stansfeld PJ, Sansom MS. Molecular simulation approaches to membrane proteins. Structure. 2011;19(11):1562–1572. doi: 10.1016/j.str.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Alejandre J, Tildesley DJ, Chapela GA. Molecular dynamics simulation of the orthobaric densities and surface tension of water. J Chem Phys. 1995;102(11):4574–4583. [Google Scholar]
- 7.Cantor RS. Lateral pressures in cell membranes: A mechanism for modulation of protein function. J Phys Chem B. 1997;101:1723–1725. [Google Scholar]
- 8.Cantor RS. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem Phys Lipids. 1999;101(1):45–56. doi: 10.1016/s0009-3084(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl E, Edholm O. Spatial and energetic-entropic decomposition of surface tension in lipid bilayers from molecular dynamics simulations. J Chem Phys. 2000;113(9):3882–3893. [Google Scholar]
- 10.Gullingsrud J, Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J. 2004;86(6):3496–3509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459(7245):379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinac B, Hamill OP. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc Natl Acad Sci USA. 2002;99(7):4308–4312. doi: 10.1073/pnas.072632899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapovalov G, Lester HA. Gating transitions in bacterial ion channels measured at 3 microns resolution. J Gen Physiol. 2004;124(2):151–161. doi: 10.1085/jgp.200409087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348(6298):261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 15.Markin VS, Martinac B. Mechanosensitive ion channels as reporters of bilayer expansion. A theoretical model. Biophys J. 1991;60(5):1120–1127. doi: 10.1016/S0006-3495(91)82147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 17.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436(7051):647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 18.Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: The MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 19.Nomura T, et al. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci USA. 2012;109(22):8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: Effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44(36):12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 21.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9(9):696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 22.Sukharev SI, Sigurdson WJ, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J Gen Physiol. 1999;113(4):525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang CS, Anishkin A, Sukharev S. Gating of the large mechanosensitive channel in situ: Estimation of the spatial scale of the transition from channel population responses. Biophys J. 2004;86(5):2846–2861. doi: 10.1016/S0006-3495(04)74337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science. 1998;282(5397):2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 25.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418(6901):942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 26.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura K, Nomura T, Sokabe M. Loss-of-function mutations at the rim of the funnel of mechanosensitive channel MscL. Biophys J. 2004;86(4):2113–2120. doi: 10.1016/S0006-3495(04)74270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundbaek JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J R Soc Interface. 2010;7(44):373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinac B, Saimi Y, Kung C. Ion channels in microbes. Physiol Rev. 2008;88(4):1449–1490. doi: 10.1152/physrev.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 31.Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335(6067):432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- 32.Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335(6067):436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brohawn SG, Campbell EB, MacKinnon R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc Natl Acad Sci USA. 2013;110(6):2129–2134. doi: 10.1073/pnas.1218950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274(3):1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 35.Enyedi P, Czirják G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol Rev. 2010;90(2):559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 36.Patel AJ, Lazdunski M, Honoré E. Lipid and mechano-gated 2P domain K(+) channels. Curr Opin Cell Biol. 2001;13(4):422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 37.Honoré E. The neuronal background K2P channels: Focus on TREK1. Nat Rev Neurosci. 2007;8(4):251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 38.Devilliers M, et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat Commun. 2013;4:2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 39.Kloda A, Lua L, Hall R, Adams DJ, Martinac B. Liposome reconstitution and modulation of recombinant N-methyl-D-aspartate receptor channels by membrane stretch. Proc Natl Acad Sci USA. 2007;104(5):1540–1545. doi: 10.1073/pnas.0609649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 41.Berrier C, et al. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J Biol Chem. 2013;288(38):27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opsahl LR, Webb WW. Lipid-glass adhesion in giga-sealed patch-clamped membranes. Biophys J. 1994;66(1):75–79. doi: 10.1016/S0006-3495(94)80752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt D, MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci USA. 2008;105(49):19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt D, del Mármol J, MacKinnon R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc Natl Acad Sci USA. 2012;109(26):10352–10357. doi: 10.1073/pnas.1204700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honoré E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci USA. 2006;103(18):6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK 1 K+ channels. Proc Natl Acad Sci USA. 2014;111(9):3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loukin SH, Saimi Y. Carboxyl tail prevents yeast K(+) channel closure: Proposal of an integrated model of TOK1 gating. Biophys J. 2002;82(2):781–792. doi: 10.1016/S0006-3495(02)75440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30(17):3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piechotta PL, et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30(17):3607–3619. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milac A, et al. Structural models of TREK channels and their gating mechanism. Channels (Austin) 2011;5(1):23–33. doi: 10.4161/chan.5.1.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: Structural basis of electromechanical coupling. Science. 2005;309(5736):903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 52.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 53.Lee SY, Banerjee A, MacKinnon R. Two separate interfaces between the voltage sensor and pore are required for the function of voltage-dependent K(+) channels. PLoS Biol. 2009;7(3):e47. doi: 10.1371/journal.pbio.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt D, Jiang QX, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444(7120):775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Wanderling S, Sompornpisut P, Perozo E. Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain. Nat Struct Mol Biol. 2014;21(2):160–166. doi: 10.1038/nsmb.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reeves D, Ursell T, Sens P, Kondev J, Phillips R. Membrane mechanics as a probe of ion-channel gating mechanisms. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78(4 Pt 1):041901. doi: 10.1103/PhysRevE.78.041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris CE. Voltage-gated channel mechanosensitivity: Fact or friction? Front Physiol. 2011;2:25. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao J, et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77(5):899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 59.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430(6996):232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 60.Pak WL. Study of photoreceptor function using Drosophila mutants. In: Breakfile X, editor. Neurogenetics, Genetic Approaches to the Nervous System. Amsterdam: Elsevier North-Holland Publishing Co; 1979. pp. 67–99. [Google Scholar]
- 61.Minke B, Wu C-F, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258(5530):84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 62.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2(4):1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 63.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8(4):643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 64.Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338(6104):260–263. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- 65.Huang J, et al. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20(3):189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Lev S, Katz B, Tzarfaty V, Minke B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: Implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J Biol Chem. 2012;287(2):1436–1447. doi: 10.1074/jbc.M111.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes S, Hankins MW, Foster RG, Peirson SN. Melanopsin phototransduction: Slowly emerging from the dark. Prog Brain Res. 2012;199:19–40. doi: 10.1016/B978-0-444-59427-3.00002-2. [DOI] [PubMed] [Google Scholar]
- 68.Sexton T, Buhr E, Van Gelder RN. Melanopsin and mechanisms of non-visual ocular photoreception. J Biol Chem. 2012;287(3):1649–1656. doi: 10.1074/jbc.R111.301226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA. 2013;110(6):2383–2388. doi: 10.1073/pnas.1215555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore C, et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci USA. 2013;110(34):E3225–E3234. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton AC. Protein kinase C: Poised to signal. Am J Physiol Endocrinol Metab. 2010;298(3):E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liman ER. Cell signaling. Putting the squeeze on phototransduction. Science. 2012;338(6104):200–201. doi: 10.1126/science.1229909. [DOI] [PubMed] [Google Scholar]
- 73.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 75.Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem. 2010;285(35):27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loukin S, Su Z, Kung C. Increased basal activity is a key determinant in the severity of human skeletal dysplasia caused by TRPV4 mutations. PLoS ONE. 2011;6(5):e19533. doi: 10.1371/journal.pone.0019533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su Z, et al. The use of yeast to understand TRP-channel mechanosensitivity. Pflugers Arch. 2009;458(5):861–867. doi: 10.1007/s00424-009-0680-0. [DOI] [PubMed] [Google Scholar]
- 78.Su Z, Anishkin A, Kung C, Saimi Y. The core domain as the force sensor of the yeast mechanosensitive TRP channel. J Gen Physiol. 2011;138(6):627–640. doi: 10.1085/jgp.201110693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504(7478):113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77(4):667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009;583(4):754–758. doi: 10.1016/j.febslet.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anishkin A, Kung C. Stiffened lipid platforms at molecular force foci. Proc Natl Acad Sci USA. 2013;110(13):4886–4892. doi: 10.1073/pnas.1302018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauritzen I, et al. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6(7):642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krieg M, Dunn AR, Goodman MB. Mechanical control of the sense of touch by β-spectrin. Nat Cell Biol. 2014;16(3):224–233. doi: 10.1038/ncb2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 2011;477(7365):495–498. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7(6):985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- 89.Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121(Pt 4):496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- 90.Segré D, Ben-Eli D, Deamer DW, Lancet D. The lipid world. Orig Life Evol Biosph. 2001;31(1-2):119–145. doi: 10.1023/a:1006746807104. [DOI] [PubMed] [Google Scholar]
- 91.Chen IA, Walde P. From self-assembled vesicles to protocells. Cold Spring Harb Perspect Biol. 2010;2(7):a002170. doi: 10.1101/cshperspect.a002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibson DG, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 93.Adamala K, Szostak JW. Nonenzymatic template-directed RNA synthesis inside model protocells. Science. 2013;342(6162):1098–1100. doi: 10.1126/science.1241888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Service RF. The life force. Science. 2013;342(6162):1032–1034. doi: 10.1126/science.342.6162.1032. [DOI] [PubMed] [Google Scholar]
- 95.Black RA, et al. Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc Natl Acad Sci USA. 2013;110(33):13272–13276. doi: 10.1073/pnas.1300963110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simon SA, Advani S, McIntosh TJ. Temperature dependence of the repulsive pressure between phosphatidylcholine bilayers. Biophys J. 1995;69(4):1473–1483. doi: 10.1016/S0006-3495(95)80017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gong K, Feng S-S, Go ML, Soew PH. Effects of pH on the stability and compressibility of DPPC/cholesterol monolayers at the air–water interface. Colloids Surf A Physicochem Eng Asp. 2002;207(1–3):113–125. [Google Scholar]
- 98.Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys J. 2010;98(6):1018–1027. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Girotti AW. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B. 2001;63(1-3):103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 100.Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35(6):356–363. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.