Significance
The origin of the 1918 pandemic influenza A virus (IAV) and the reasons for its unusual severity are two of the foremost biomedical mysteries of the past century. We infer that the virus arose via reassortment between a preexisting human H1 IAV lineage and an avian virus. Phylogenetic, seroarcheological, and epidemiological evidence indicates those born earlier or later than ∼1880–1900 would have had some protection against the 1918 H1N1 virus, whereas many young adults born from ∼1880–1900 may have lacked such protection because of childhood exposure to an antigenically distinct H3N8 virus. Our findings suggest that better understanding of how initial exposure shapes lifetime immunity may enhance the prediction and control of future IAV pandemics and seasonal epidemics.
Keywords: phylogeny, cohort immunity, pathogenicity, virulence, reassortment
Abstract
The source, timing, and geographical origin of the 1918–1920 pandemic influenza A virus have remained tenaciously obscure for nearly a century, as have the reasons for its unusual severity among young adults. Here, we reconstruct the origins of the pandemic virus and the classic swine influenza and (postpandemic) seasonal H1N1 lineages using a host-specific molecular clock approach that is demonstrably more accurate than previous methods. Our results suggest that the 1918 pandemic virus originated shortly before 1918 when a human H1 virus, which we infer emerged before ∼1907, acquired avian N1 neuraminidase and internal protein genes. We find that the resulting pandemic virus jumped directly to swine but was likely displaced in humans by ∼1922 by a reassortant with an antigenically distinct H1 HA. Hence, although the swine lineage was a direct descendent of the pandemic virus, the post-1918 seasonal H1N1 lineage evidently was not, at least for HA. These findings help resolve several seemingly disparate observations from 20th century influenza epidemiology, seroarcheology, and immunology. The phylogenetic results, combined with these other lines of evidence, suggest that the high mortality in 1918 among adults aged ∼20 to ∼40 y may have been due primarily to their childhood exposure to a doubly heterosubtypic putative H3N8 virus, which we estimate circulated from ∼1889–1900. All other age groups (except immunologically naive infants) were likely partially protected by childhood exposure to N1 and/or H1-related antigens. Similar processes may underlie age-specific mortality differences between seasonal H1N1 vs. H3N2 and human H5N1 vs. H7N9 infections.
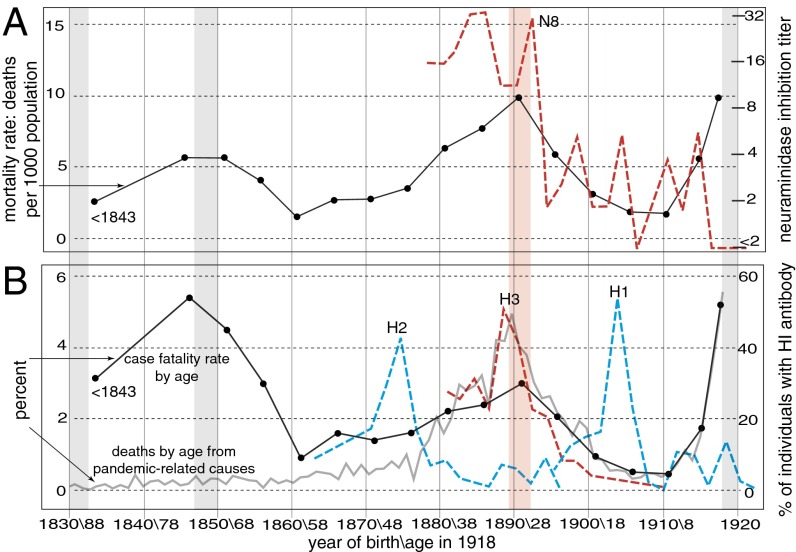
The influenza pandemic of 1918–1920 killed an estimated 50 million people, most during a single wave late in 1918 (1, 2). Its origin, epidemiology, and pathogenesis are still puzzling (3, 4). Unusually for influenza A virus (IAV), which typically kills primarily infants and the elderly, young adults aged about 20–40 y suffered extensive mortality, which peaked in 25- to 29-y-olds (1, 2). The same virus was comparatively mild in those only slightly older or younger (Fig. 1). The very elderly, moreover, suffered less influenza-related mortality during the pandemic than in 1911–1917 (5). Notably, the virus was clinically unremarkable in >95% of patients (2), and almost all fatalities were caused by secondary bacterial pneumonia (6, 7). Any explanation must therefore account not just for the mortality peak in 20- to 40-y-olds but also for the mortality “troughs” in the very elderly and in children ∼5–15 y of age (Fig. 1), as well as for the typical postinfluenza complications (rather than acute viral pathogenesis) that killed most victims. The rapid reversion to more usual IAV mortality patterns by the early 1920s must also be explained.
Fig. 1.
Mortality in 1918 and seroarcheological patterns. (A) Influenza and pneumonia mortality (solid line) in different age groups in the United States in 1918 (data from ref. 1), and N8 neuraminidase inhibition titers (legend at right) in sera from different age groups (data from ref. 11). Vertical bars indicate the pandemics of 1830–1833, 1847–1850, 1889–1893, and 1918–1920. (Ages/birth years are indicated below B). (B) Case-fatality ratios in 1918 (in black) (data from ref. 1) and percentages of deaths by age from pandemic-related causes in Ontario, Canada (in gray) (data from ref. 33). Also shown are percentages of sera from different age groups with HA inhibition antibody titers (SI Appendix).
Current hypotheses of the origin of the 1918 H1N1 virus range from an introduction of all eight genome segments from an avian source shortly before 1918 (8) to reassortment involving progenitor viruses supposedly circulating in humans and swine for decades before 1918 (9). Here, by using a molecular clock model that explicitly allows different evolutionary rates in different hosts, which we recently demonstrated to be essential for accurate inference of the timing and directionality of IAV host jumps (10), we reconstruct the evolutionary origins of the 1918 pandemic H1N1 virus, the classic swine H1N1 influenza virus, and the postpandemic seasonal H1N1 lineage. We then evaluate numerous observations from epidemiology, seroarcheology, and molecular evolution in light of these findings. Our results suggest that the childhood exposure of various age cohorts to different HA and neuraminidase (NA) subtypes was a key factor underlying the age-specific patterns of fatality not only in 1918 but also during other pandemics and seasonal influenza epidemics.
Results
Evidence That H1 Emerged in Humans Before ∼1907, Not in 1918.
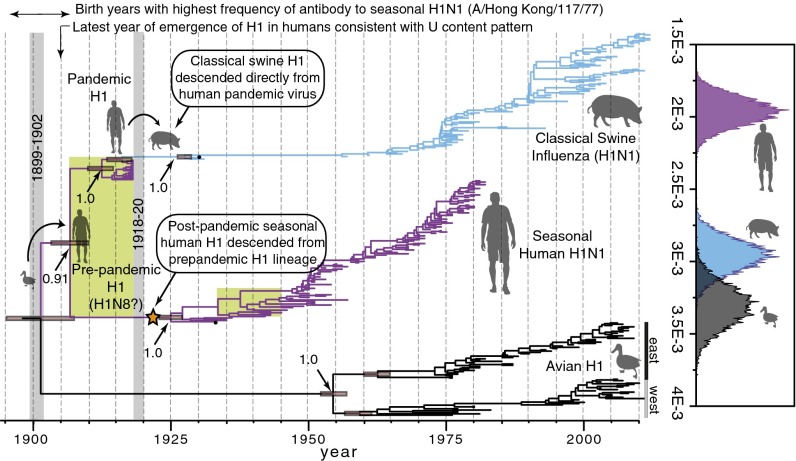
Host-specific local clock (HSLC) analyses of H1 HA data reveal two crucial points (Fig. 2 and SI Appendix, Fig. S1A). First, human H1 emerged from an avian source considerably earlier than 1918, sometime after the human + avian H1 most recent common ancestor (MRCA) at 1901 [95% credible interval (CI): 1895–1907] but before the MRCA of the pandemic and seasonal H1 lineages at 1907 (1903–1910). Second, the classic swine influenza lineage is nested within the 1918 genetic diversity of human H1, whereas the seasonal human H1 HA is distantly related to the pandemic HA. This pattern indicates that the swine influenza lineage emerged directly from the human pandemic virus but that postpandemic seasonal H1N1 did not. Rather, there is strong phylogenetic evidence that it descended from a distinct H1 lineage that shared a common ancestor with the 1918 pandemic HA in ∼1907 (i.e., both the seasonal and pandemic HA genes were evidently drawn from >10 y of accumulated H1 HA genetic diversity circulating in the human population in 1918). This extensive diversity is consistent with the 6–12 y of circulating seasonal H1 diversity seen in the 1930s, 1940s, and 1950s, but it is distinctly deeper than the just 1–2 y of circulating HA diversity sampled in all later pandemic years [Fig. 2 (green rectangles) and SI Appendix, Fig. S1B].
Fig. 2.
Maximum clade credibility (MCC) tree of the H1 subtype of HA. (Right) Clade-specific rate distributions (in substitutions per site per year). (Left) Time window of the pandemic of 1918–1920 is shown with a gray bar, as is that of the putative pandemic around 1900. The posterior probability of each node and the 95% CIs on node dates are shown. The orange star indicates the H1 variant that evidently gave rise to postpandemic seasonal H1N1 lineage in humans. The widths of the green rectangles indicate the comparable extent of H1 genetic diversity in 1918 and 1945 (more than 10 y in each case).
Additional analyses indicate that these inferences are robust to the exclusion of potentially laboratory-adapted human and swine strains from the 1930s and several short 1918 HA sequence fragments (7) (SI Appendix, Fig. S2) and to the inclusion of human H1N1 sequences only from 1918 to 1957 (before the reemergence of H1N1 in 1977) (SI Appendix, Fig. S3). The results cannot be explained as an artifact arising from an adaptive burst of amino acid-changing substitutions in the rapidly evolving HA globular head domain because an independent analysis of the conserved stalk domain yields similar results (SI Appendix, Fig. S4) and there is no evidence of episodic diversifying selection (SI Appendix, Fig. S5). Accordingly, both third codon position sites (at which 97% of substitutions are synonymous) and silent third position sites give virtually identical timing and topology estimates as the full dataset (SI Appendix, Fig. S6).
These results are consistent with an avian-to-human movement of H1 in the first decade of the 20th century. This decade is an interval during which a putative influenza pandemic occurred, in ∼1900 (11–13). It was retrospectively identified on the basis of increased pneumonia and influenza mortality in North America, England, Ireland, and elsewhere but is not universally considered a bona fide pandemic. It was thought to have occasioned the emergence of an H3 pandemic virus until subsequent reinterpretation of H3 seroarcheological observations compellingly indicated H3’s emergence during the 1889–1893 “Russian” influenza pandemic (14). Analysis of uracil (U) content, which tends to increase after avian IAV segments are transmitted to mammals (15), corroborates these phylogenetic results. Human N1 U content in the 1918 sequence is within the avian range (SI Appendix, Fig. S7), and there are avian sequences with a virtually identical base composition to the A/Brevig Mission/1/1918 NA at all four nucleotides (e.g., A/duck/NZL/76/1984). Thus, seven of the eight segments of the 1918 human virus, encoding the polymerase proteins (PB2, PB1, PA); the nucleocapsid protein (NP); the matrix proteins (M1/2); and the nonstructural proteins (NS1/2) (10) as well as NA, exhibit avian-like U content. The HA-encoding segment is the only one in the genome of the 1918 virus with a U content significantly above the avian distribution (P = 0.0003), consistent with it emerging in humans earlier than the other segments (SI Appendix, Fig. S7). We estimate this host jump would have had to occur by 1905 or earlier to allow sufficient time to reach such a high U content by 1918.
HA Seroarcheology Corresponds to Phylogeny.
Many findings from seroarcheology and epidemiology, although overlooked in recent years, also point to this earlier period, and not to 1918, for the introduction of H1 into the human population (11, 13, 16–24) (Fig. 1 and SI Appendix, SI Text): (i) Seroarcheological results point to an introduction of H1 between 1896 and 1907, not in 1918 (16–18, 21, 25) (note the peak in antibodies to seasonal H1 in those born around 1904 in Fig. 1B, a pattern that seems inexplicable if H1 were new to humans in 1918); (ii) seroarcheology suggests the disappearance of H3 not in 1918 but shortly after ∼1900 (11, 13, 18, 19) (Fig. 1B); and (iii) mortality patterns in 1968–1970 indicate that the childhood exposure of those born after ∼1900 was to a non-H3 virus, which offered no protection from the 1968 H3N2 virus (23, 24), whereas those born before 1900 enjoyed considerable protection from prior exposure to H3 antigens.
The prominent peaks in antibody titers against H3 and N8 in those born in and around 1889 (Fig. 1) and the low mortality of those aged >70 y in 1968–1970 provide compelling evidence that the 1889–1893 pandemic was caused by an H3N8 virus (11, 13, 18, 26). It is widely assumed that the 1889 virus circulated until the 1918 pandemic. However, only about half of those born in 1893 were primed with H3 (14). Given the high attack rates of IAV (22), this observation seems incompatible with an H3 virus circulating until 1918 because it would require that half of 25-y-olds remained immunologically naive to IAV in 1918. We therefore hypothesize that the period around 1900, not 1918, occasioned the disappearance of H3 and the reemergence of H1 in humans. However, such seroepidemiological results can be difficult to interpret with high precision, not least because particular viruses can greatly influence the conclusions of such studies. For example, it is conceivable (although we think unlikely) that an H3N8 virus circulated from 1889 right up until ∼1918 but that its HA underwent such extensive antigenic drift after ∼1900 that antibodies against it fail to bind to either 1968 human H3 or 1963 equine H3 HA antigens, which are phylogenetically very divergent (10).
Interestingly, Kendal et al. (11) found that although H3 antibodies were rare or absent in those born after 1900, N8 “persisted in a virus prevalent from the early 1900s until 1916 or 1917” (also Fig. 1A). Based on the intersection of the phylogenetic and seroarcheological evidence, we therefore propose that this N8 NA may have been carried over from a putative H3N8 virus that circulated from 1889 until ∼1900 to an H1N8 virus that emerged in the first decade of the 20th century. If so, prior IAV exposure of the ∼1900–1918 cohort would have been to this putative H1N8 virus. The seroarcheological data (Fig. 1B) suggest that H1 had largely displaced H3 by about 1905. This idea is consistent with the phylogenetic evidence (Fig. 2); the high U content of the 1918 HA (SI Appendix, Fig. S7); the lack of protection of those born after ∼1900 to the 1968 H3 virus (23, 24); and, as described below, the mortality patterns in 1918.
Origin and Emergence of the 1918 Virus.
The time of the most recent common ancestor (TMRCA) of H1 in humans, at 1907 (1904–1910) (Fig. 2), significantly predates the TMRCA of the human and avian N1 lineages, at 1913 (1911–1916) (P = 0.008; SI Appendix, Fig. S8). Hence, and this is a crucial point, there is strong phylogenetic evidence that N1 was introduced from an avian virus only after H1 was already established in humans. The human H1 lineage also significantly predates the human + avian TMRCA of PB1 at 1914 (1911–1916) (P = 0.006; SI Appendix, Fig. S9), suggesting that PB1, too, was transmitted from an avian host via reassortment with a human prepandemic H1 lineage. The remaining internal genes also evidently arose from a Western hemispheric lineage of avian influenza virus (10). Their nearly identical phylogenetic patterns and their avian-like U contents (10) suggest they may have been transmitted during the same reassortment event as PB1. The most parsimonious scenario is that a single event in ∼1915 (see below) brought together a human H1 with the remaining seven avian segments, possibly via an H7N1 virus.
If the avian segments had a single source, the human-avian virus reassortment event must have occurred after ∼1914 but before the TMRCA of the human and swine lineages of each segment. To estimate these dates, we conducted an analysis of each segment, including only human and swine H1N1 viruses, allowing a separate rate for each host using the HSLC model. SI Appendix, Fig. S10 shows that the root node TMRCAs for the swine + human trees are remarkably consistent across segments: PB2, 1915 (1913–1917); PB1, 1915 (1913–1918); PA, 1914 (1912–1916); HA, 1914 (1912–1916); NP, 1914 (1910–1916); NA, 1914 (1911–1916); M1/2, 1914 (1909–1917); and NS1/2, 1915 (1911–1918) (full trees are shown in SI Appendix, Fig. S11). This result suggests an emergence of the 1918 pandemic virus ancestor in ∼1915, with a window from 1913 to 1916 overlapping among all eight 95% CIs and each segment. Thus, with the important exception of the preexisting human H1 HA, our conclusions support an avian origin of the virus shortly before 1918. This conclusion suggests that its genesis was similar to that of the pandemic viruses of 1957 and 1968, which emerged via reassortment of avian and preexisting human viruses within human hosts (26, 27), but with seven segments of avian origin acquired compared with three in 1957 and two in 1968.
Classic Swine Influenza and Postpandemic H1N1.
For every segment, the human + swine root node date (or, in the case of HA, the TMRCA of the swine and pandemic H1 lineage) significantly postdates the TMRCA of the pandemic and seasonal human H1 lineages (SI Appendix, Fig. S10). A separate analysis of HA excluding numerous 189-nt sequence fragments (7) similarly reveals a strongly supported swine + 1918 (human) clade (posterior probability = 1.0) dated at 1917 (1916–1918) (SI Appendix, Fig. S2). In other words, the human H1 HA genetic diversity is significantly older than the MRCA of the human and swine lineages for all eight segments (SI Appendix, Fig. S10). These observations suggest that swine H1N1 descended from the human virus, not vice versa. There is no evidence supporting the hypothesis (9) that reassortment between decades-old human and swine viruses played a role in the origin of the 1918 pandemic lineage. In addition, unlike the conclusions of Smith et al. (9), these phylogenetic results agree with on-the-ground observations in 1918 that the disease was new to swine and was caused by the same agent as the concurrent human influenza pandemic (16, 28).
Unlike HA, the remaining segments of the seasonal H1N1 virus were evidently direct descendants of the pandemic virus (SI Appendix, Fig. S10). This finding suggests that during the pandemic, reassortment occurred between the pandemic lineage and a cocirculating, antigenically distinct H1 virus, creating the seasonal H1N1 ancestor. This scenario parallels the emergence of the 1946–1947 H1N1 lineage and the associated worldwide vaccine failure (29). In both cases, rather than evolving directly from its predecessor via antigenic drift, a new lineage with distinct antigenic properties evidently arose via the acquisition of an “old” and rare homosubtypic H1 HA variant by intrasubtype reassortment. The replacement of the pandemic HA by a heterologous H1 HA offers a simple resolution to the long-standing conundrum that antibodies to the swine/pandemic virus HA suddenly disappeared in those born after ∼1922 (16, 17, 20, 30, 31), even though seasonal H1N1 obviously continued to circulate (SI Appendix, SI Text). However, less parsimonious scenarios, such as a separate cross-species introduction of the seasonal H1 HA from birds or even from other mammalian hosts such as horses, in or shortly after 1918, cannot be formally excluded (SI Appendix, SI Text and Fig. S12). Recovery of archival IAV genomes from ∼1907–1917 and the 1920s might definitively resolve these questions.
Model to Explain the 1918 Mortality Patterns.
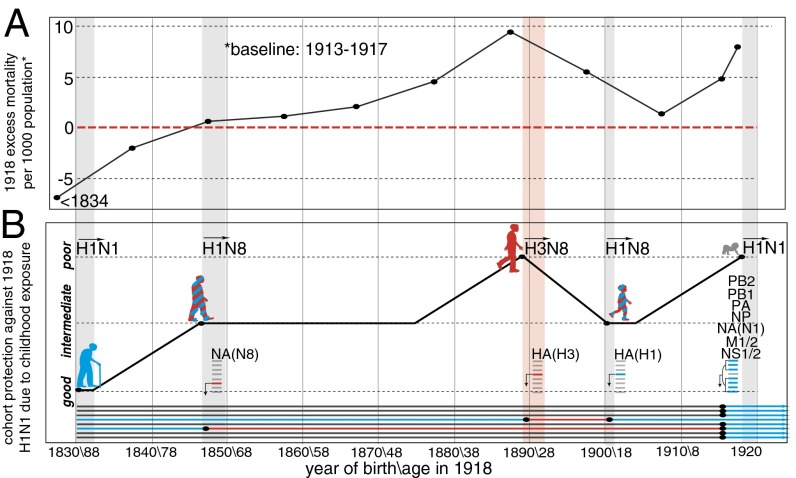
Elderly individuals may have been protected from the 1918 virus by childhood exposure to an H1N1-like virus (5). We estimate that H1 and the H2 + H5 lineage diverged from a common ancestor near the time of the 1830 pandemic (SI Appendix, SI Text and Figs. S13 and S14). Moreover, protection was clearly greatest in those born before 1834 (5) (Fig. 3A), implicating the 1830–1833 pandemic virus, which would have primed the majority of that age group. If an H1-like virus emerged in 1830, it would likely have been positioned near one of the orange stars close to the root of the tree in SI Appendix, Fig. S13. Those primed as children between 1830 and 1889 by this HA lineage would likely have had considerable protection against the 1918 HA, comparable to that exhibited during the 2009 H1N1 pandemic by those born before 1957 (32), based on the similar genetic distances separating the childhood and pandemic virus HA in each case (SI Appendix, Figs. S13 and S14).
Fig. 3.
Excess mortality in 1918 and the childhood exposure/cohort immunity model. (A) Age group-specific annual excess mortality due to pneumonia and influenza in 1918 (data from ref. 5). (Year of birth and age in 1918 are indicated below B.) (B) Expected cohort protection due to childhood exposure. Each segment of the 1918 H1N1 genome is shown in blue. Putative H1- and N1-like genes in the 1830, 1847, and 1900 genomes are also shown in blue, whereas putative heterosubtypic genes (H3 and N8) in the 1847, 1889, and 1900 viruses are shown in red. The human silhouettes are colored by putative childhood exposure to HA and NA antigens matched or mismatched to the 1918 H1N1 (+/+, blue; +/−, blue/red; −/−, red), whereas the newborn is colored gray, indicating no prior exposure. The pandemics of 1847, 1889 (red), 1900, and 1918 are indicated with vertical bars.
It is likely that the majority of those born worldwide shortly before and during 1889–1893 were infected by the 1889 pandemic H3N8 virus (12). A progressively larger percentage of those born either earlier or later, however, would have experienced initial childhood exposure to the putative H1 viruses that immediately preceded (1830–1889) or followed (∼1900–1918) the 1889 virus. Based on observations of the 1893 cohort, about half of whom were primed with H3 (14), we assume in Fig. 3B that ∼0% of newborns, ∼50% of 7-y-olds, and ∼100% of 14-y-olds would already have been exposed to prepandemic seasonal strains in 1847, 1889, 1900, and 1918. Although these numbers are undoubtedly inexact, we believe they are reasonable for developing a qualitative model as described below.
We propose that putative 1830–1847 H1N1, 1847–1889 H1N8, 1889–1900 H3N8, and 1900–1918 H1N8 IAV lineages determined the childhood exposure patterns of the various age groups alive in 1918 (Fig. 3B, Lower). In Fig. 3B (Upper), we depict the protection expected against the 1918 H1N1 virus for each birth-year cohort from 1830 to 1918 under this scenario. All else being equal, the 1830 cohort (who were 88 y old in 1918) would have had the best protection against the 1918 H1N1 virus due to childhood exposure to a doubly homosubtypic virus. The 1847 cohort (71-y-olds) would have had intermediate protection due to the presence of H1 HA antigens in their childhood virus, but a heterosubtypic NA. The 1889 cohort (29-y-olds) would have had the least protective immunity from childhood, almost all having been primed with a heterosubtypic H3 HA and a heterosubtypic N8 NA. The 1900 cohort (18-y-olds) would have had intermediate protection, having been exposed to a homosubtypic HA and heterosubtypic NA. Finally, most infants, with the proportion increasing to ∼100% with declining age, would have lacked protection because they would have had no prior exposure whatsoever to IAV antigens.
The expected protection of the cohorts born in the years between the pandemics would follow the curve shown in Fig. 3B, given the assumptions detailed above. Half of those aged 7 y in 1889, for example, would already have been primed by the 1847–1889 virus before the 1889 pandemic; the remainder would have been primed by the 1889–1900 virus, leaving the 1882 birth year cohort (36-y-olds) with protection in 1918 midway between the 1847–1875 and 1889 birth-year cohorts (Fig. 3B).
The excess mortality patterns in 1918 (Fig. 3A) closely match the expected protection curve based on this cohort immunity model (Fig. 3B). Indeed, the actual peak in mortality among young adults occurred precisely in those born from 1889 to 1893 (1) (25- to 29-y-olds; Fig. 1), the only age group in 1918 whose childhood exposure would have been almost exclusively to a fully heterosubtypic virus (H3N8) (also ref. 33). Note that the peak in excess mortality in 1918 (Fig. 3A) closely overlaps the peaks in both N8 and H3 antibody responses in birth years near 1889 (Fig. 1 A and B). In particular, the percentage of deaths due to pandemic influenza-related causes in various age groups in several Canadian cities, from the most thorough analysis to date of mortality data from 1918 (33), shows a striking congruence with the presence of H3 antibody titers (Fig. 1B), one that is difficult to dismiss as random coincidence.
Importantly, no single event, including the 1889 pandemic, can explain the overall pattern. We propose that it was the aggregate exposure of the various cohorts to different pandemic viruses, and crucially also to the seasonal influenza lineages in interpandemic periods, that set the stage for what unfolded in 1918. This scenario provides a straightforward potential explanation for the inflection points in the mortality-by-age curve in 1918, including the observation that the most elderly cohort suffered lower mortality in 1918 than in 1911–1917. The switch to H1N1 may have resulted in a virus to which their childhood exposure provided better protection than it did to the putative H1N8 strain immediately preceding the pandemic. The same switch would have had the opposite effect in young adults exposed in childhood to H3N8, limiting the usefulness of their N8 NA-directed antibody responses and leaving them with comparatively ineffective antibody protection against both major antigenic glycoproteins of the pandemic H1N1 virus. The age groups on either side of the “H3N8” cohort would have enjoyed considerable protection against H1N1 due to their childhood exposure to homosubtypic HA antigens (Fig. 3B), whereas the youngest individuals lacked any prior exposure and associated protection in 1918.
It is also possible that an absence of prior immunity to the new avian M2 and NP proteins of the 1918 virus contributed to the severity of the 1918 pandemic. M2 immunity is associated with decreased viral replication in the lungs and less severe disease (34), and preexisting immunity to NP decreases susceptibility to secondary bacterial pneumococcal infection (35). The M2 and NP proteins of the 1918 virus may have been considerably divergent from the previous human variant, which likely predated the homogenization of IAV internal genes after the selective sweep in these genes that occurred in the late 1800s (10).
We can conceive of two mechanisms whereby the childhood exposure of different age groups could have shaped the mortality patterns in 1918. First, a mechanism akin to original antigenic sin (OAS) (36) may have interfered with immune responses in some of those infected in 1918 (33, 37), peaking in those exposed to the 1889 virus. Although OAS has been traditionally considered a within-subtype phenomenon (36, 38–40), it is plausible that interactions between heterosubtypic viruses could also occur (41). Indeed, Masurel (42) reported that when immunized with an H3N2 vaccine, about 5% of individuals primed in childhood by H1N1 yielded strong HA inhibition antibody responses to H1N1, without any appearance of antibody responses to H3N2 virus. We also speculate that exposure to H1 HA stalk antigens could have resulted in unprotective (OAS-mediated) recall of antibodies to H3 HA stalk epitopes in some H3N8-primed individuals. Such misdirected immune responses could have had dire consequences in 1918 for those initially infected by H3N8. Even if most or all 20- to 40-y-olds in 1918 had already been exposed to the putative H1 virus circulating between ∼1900 and 1918, we speculate that their initial exposure to an H3 virus might nevertheless have interfered with their immune responses to the 1918 HA. This process, combined with weak or nonexistent immunity to the newly emerging NA, M2, and NP proteins in the 1918 virus, may have rendered this subgroup at particularly high risk for severe disease and more vulnerable than young adults in any pandemic since 1918. These other pandemics have occurred against a backdrop of widespread prior exposure of the human population to a homosubtypic NA (1968, 2009) and/or HA (1977, 2009), or to a heterosubtypic but phylogenetically closely related HA (SI Appendix, Fig. S13) with a very similar HA stalk (1957) (33).
The alternative is that an unlucky subset of individuals had been exposed only to heterosubtypic H3 HA antigens before 1918 (i.e., they had escaped infection by the putative H1N8 virus that circulated from ∼1900–1918). Unlike virtually all older and younger age cohorts (except infants), such an H1-naive subset of 20- to 40-y-olds would have been unable to benefit from anamnestic immune responses to H1 antigens. Importantly, immunopathology is not invoked in this scenario: Heterosubtypic prior infection would provide positive but limited protection, better than being completely immunologically naive but worse than prior exposure to N1 and/or H1 antigens. There is ample evidence from animal models that this effect might be expected: Prior exposure to a heterosubtypic influenza A virus (but not to influenza B virus) is associated with lower death rates compared with completely naive hosts (35, 43). However, it is also associated with higher viral lung titers and enhanced pneumonia and death rates compared with prior exposure to a homosubtypic virus. The observation that even higher mortality than in 20- to 40-y-olds was observed in 1918 in groups that were largely immunologically naive, young infants (Fig. 1B) and isolated populations (e.g., a staggering 22% mortality in Samoa) (44), suggests that exposure to H3N8, although hardly optimal, may nevertheless have been considerably better than no prior IAV exposure at all.
It is important to note that unlike antigenic imprinting this “H1-naive” hypothesis requires that a sizeable proportion of 20- to 40-y-olds remained unexposed to H1 before 1918, although most younger individuals (e.g., the excess mortality trough in those ∼15 y of age in 1918) were exposed. Although this idea might be unrealistic, we note that if an H1 virus indeed emerged in the early 20th century, it was mild enough to go unnoticed at the time (11–13); in addition, although seasonal influenza virus attack rates peak at the age of 2–3 y and reach an average of 20.3% per year among children under 5 y of age, they are much lower for adults of working age: just 6.6% on average, including influenza B virus infections (45). Thus, it is plausible that a quarter or more of the 20- to 40-y-olds alive in 1918 could have remained unexposed even if an H1N8 virus had been circulating seasonally for 10–15 y before 1918.
Discussion
We hypothesize that childhood exposure to an H3N8 virus may have made some young adults in 1918 a sort of temporal counterpart to highly vulnerable geographically isolated populations, inducing suboptimal immunity that tilted the odds in favor of secondary infection with the wide range of bacterial pathogens that cause most influenza-related mortality. This small wedge of the population may have had ineffective immunity not only to some antigens that are currently targets for “universal” vaccines (M2 and NP, newly emerged from avian influenza in 1918) but, uniquely, also against both major antigenic glycoproteins and the conserved HA stalk domain. Antibodies targeting the HA stalk provide powerful protection against severe disease, but there is little or no cross-protection between phylogenetically divergent group 1 HA subtypes (e.g., H1 in 1918) and group 2 subtypes (e.g., H3 in 1889) (46). If this model is correct, then current medical interventions, especially antibiotics and vaccines against several pneumonia-causing bacteria, could be expected to reduce mortality dramatically if we were faced today with an otherwise similar set of pandemic ingredients.
If childhood exposure of different age groups is indeed a key predictor of outcome to a pandemic strain (i.e., if antigenic imprinting and not just the intrinsic virulence of the virus shapes mortality patterns), then current approaches to studying influenza pathogenesis might need to be rethought. For instance, using immunologically naive animals to characterize the pathogenicity of the reconstructed 1918 virus may be methodologically problematic, because the pathogenesis of the actual pandemic virus appears to have been profoundly affected by the prior immunity experienced by different age cohorts, a potentially crucial factor not reflected in such an experimental design. This idea could also help explain why genomic analyses of large datasets, such as for the 2009 pandemic H1N1 virus, have not yet identified “virulence factors” that can explain why the virus causes a life-threatening infection in some people but is asymptomatic in others. In short, it may be useful to frame questions about pandemic IAV pathogenicity in terms of how well (or poorly) prior exposure protects different age groups from the >20% general mortality that can occur in “virgin soil” populations (44, 47).
Finally, our findings suggest that childhood exposure of different age groups to distinct influenza virus variants may also strongly influence age-related mortality patterns during seasonal epidemics, as well as to H5N1 and H7N9 viruses. We hypothesize that the current severity of seasonal H3N2, which was remarkably mild among the elderly cohort of 1968 (22) but now kills ∼18-fold more patients aged >65 y than H1N1 (48, 49), may be, in part, a consequence of antigenic imprinting rather than the intrinsic virulence of the virus. The current elderly cohort (unlike the elderly cohort in 1968, who had been primed in ∼1889–1900 by an H3 virus) was primed exclusively by H1N1 (SI Appendix, SI Text). We predict that as the 1968 H3N2-primed cohort begins to replace the H1N1- and H2N2-primed cohorts among those >65 y of age, H3N2-dominated epidemics may diminish in frequency and severity and H1N1-dominated epidemics may increase (assuming these subtypes are still cocirculating in future decades).
Prior exposure of some age cohorts to H7N9 and H5N1 clearly cannot explain the age-specific mortality patterns seen with these viruses, because they are not thought to have circulated previously in humans; all age groups have had prior exposure only to heterosubtypic HA. However, childhood exposure to group 1 (H1, H2) vs. group 2 (H3) HA antigens, ones that are either shared or not shared with the group 1 HA of H5N1 or the group 2 HA of H7N9, is remarkably predictive of disease severity: We find that virtually all fatalities from H5N1 (group 1) have occurred in younger patients initially exposed to H3N2 (group 2) and, conversely, that almost all H7N9 (group 2) mortality has occurred in older individuals initially infected by H1N1 or H2N2 (group 1). The opposing patterns of age-specific mortality/protection with H5N1 and H7N9 are highly statistically significant and are almost perfect mirror images on either side of the group 1-to-group 2 HA transition that occurred with the emergence of H3N2 in 1968 (SI Appendix, SI Text and Fig. S15). We hypothesize that anamnestic recall of immune responses to the HA stalk antigens of initial childhood exposure may underlie this pattern by predisposing patients to severe disease when they encounter H5N1 or H7N9 viruses with group-mismatched HA but strongly protecting them when these viruses have group-matched HA proteins.
Immunization strategies that mimic the apparently powerful lifetime protection afforded by initial childhood exposure might dramatically reduce mortality due to both seasonal and novel IAV strains. Better understanding of cohort immunity effects, which may be more pervasive and powerful than previously appreciated, might thus lead to improved understanding of IAV pathogenesis and to better prediction, prevention, and control of both seasonal and pandemic influenza.
Materials and Methods
IAV Sequence Data Preparation.
We collected all IAV full-length sequences from humans, birds, and pigs encoding the H1, H2, and H5 subtypes of HA and the N1 subtype of NA. Identical sequences and apparent recombinants and other problematic sequences were excluded. For each gene, a subset of sequences of a size amenable to molecular clock analyses (∼300 sequences) was sampled, preserving the most basal sequences in the major clades and reducing the number of overrepresented recent sequences so that sampling across different years was fairly even. Because the effective sampling time of post-1977 to pre-2009 human H1N1 is 27 y earlier than the actual sampling date, we shifted the dates accordingly (10). The full-length swine and human IAV sequences from the alignments of the PB2, PB1, PA, NP, M1/2, and N1/2 genes from a study by Worobey et al. (10) were used for the analyses summarized in SI Appendix, Figs. S9 and S10.
Phylogenetic Analyses.
We analyzed these IAV alignments with the HSLC model as described (10) using a Gaussian Markov random field Bayesian skyride coalescent tree prior and a general time reversible + gamma substitution model. Each major host group was allowed its own rate in the HSLC model. For the analysis of the H1, H2, and H5 subtypes, all of the avian sequences were assumed to evolve at the same rate or were allowed independent rates, with similar results in each case (SI Appendix, Fig. S14); the human H2 and human H1 clades were allowed their own rates. We ran analyses for 50 million steps in most cases and used Tracer v1.5 to ensure effective sample size values >200. We used TreeAnnotator to infer and annotate MCC trees. To test the robustness of the deep, pre-1918 divergence time of the human H1 lineage, as well as the clustering of the 1918 sequences with the classic swine influenza lineage rather than with the postpandemic seasonal human H1N1 lineage, we conducted several additional analyses of H1 datasets. These analyses included (i) exclusion of the 189-nt HA fragments from 1918, as well as laboratory strains of IAV from both humans and swine from the 1930s; (ii) subsampling at most one sequence per host lineage per year; (iii) subsampling only sequences sampled before the extinction of H1N1 in 1957; (iv) separate analysis of the HA stalk domain (sites 1–150 and sites 921–1,698); and (v) analysis including only the 565 third-position sites and the subset of 503 silent third-position sites. [We used MacClade v4.08a (50) to visualize all amino acids substitutions along the MCC tree and then determined which were due to substitutions at the third codon position by referring to the genetic code and the nucleotide alignment. One hundred thirteen of 4,107 third-position substitutions along the MCC tree were nonsynonymous.]
U Content Analyses.
We compared the U content of the 1918 HA and NA sequences with the range observed in avian viruses (SI Appendix, Fig. S7). We estimated an upper bound on when the 1918 HA sequence emerged in a mammalian host using the approach described by Worobey et al. (10), calculating how long a sequence starting at the average U content among avian strains would take to increase to the U content value observed in the 1918 sequence, assuming the rate of U content increase in human H3 HA (because it appears that the H1 lineage was approaching an asymptote between 1918 and 1957). The overall H3 substitution rate (10) is slightly higher than that of H1 (Fig. 2), so this assumption likely provides a conservative estimate of the upper bound (i.e., if the rate of U content increase in H1 were slightly lower than in H3, this discrepancy would suggest the entry into humans was slightly earlier than our estimate of ∼1905). The upper and lower range estimates were determined using the upper and lower 95% confidence interval values for the avian U content distribution. A P value for a test of the hypothesis that the avian-to-human jump predated 1918, based on U content, was calculated as the proportion, out of 10,000 replicates, in which the year drawn from the above-mentioned distribution was greater than (i.e., postdated) 1918.
Tests for Adaptive Evolution.
We used the random effects branch-site model (51) for detecting episodic diversifying selection (EDS) in the H1 HA phylogeny. We included representative sequences from each host lineage to permit a search for evidence of EDS on the branch between each host, and within each host after putative host jumps (SI Appendix, Fig. S5).
Tests of Whether Within-Human H1 HA Diversity Predates Between-Host Diversity in Other Genes.
A P value for a test of the hypothesis that the within-human diversity of the H1 subtype of HA predates the human + swine + avian N1 NA diversity was calculated by drawing a date from the human H1 TMRCA posterior density and a date from the multihost N1 TMRCA posterior density, and then determining the proportion of 10,000 replicates for which the N1 date was earlier than the H1 date. The same approach was used for tests of whether the within-human H1 diversity predates the human + swine diversity within N1 and each of the internal genes (SI Appendix, Fig. S10) and for a test of whether the within-human H1 diversity predates the human + swine + avian PB1 diversity (SI Appendix, Fig. S9).
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for insightful criticisms and comments. This work was supported by a grant from the David and Lucile Packard Foundation (to M.W.) and Grant 092807 from the Wellcome Trust (to A.R.). The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 278433-PREDEMICS and European Research Council Grant Agreement 260864.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.M.F. is a guest editor invited by the Editorial Board.
See Commentary on page 7892.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324197111/-/DCSupplemental.
References
- 1.Frost WH. The epidemiology of influenza. JAMA. 1919;73(5):313–318. [Google Scholar]
- 2.Taubenberger JK, Morens DM. 1918 Influenza: The mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Taubenberger JK. 1918 influenza, a puzzle with missing pieces. Emerg Infect Dis. 2012;18(2):332–335. doi: 10.3201/eid1802.111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens DM, Fauci AS. The 1918 influenza pandemic: Insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 5.Luk J, Gross P, Thompson WW. Observations on mortality during the 1918 influenza pandemic. Clin Infect Dis. 2001;33(8):1375–1378. doi: 10.1086/322662. [DOI] [PubMed] [Google Scholar]
- 6.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng ZM, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci USA. 2011;108(39):16416–16421. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taubenberger JK, et al. Molecular virology: Was the 1918 pandemic caused by a bird flu? Was the 1918 flu avian in origin? (Reply) Nature. 2006;440(7088):E9–E10. doi: 10.1038/nature04823. [DOI] [PubMed] [Google Scholar]
- 9.Smith GJ, et al. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci USA. 2009;106(28):11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worobey M, Han GZ, Rambaut A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature. 2014;508(7495):254–257. doi: 10.1038/nature13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendal AP, Minuse E, Maassab HF, Hennessy AV, Davenport FM. Influenza neuraminidase antibody patterns of man. Am J Epidemiol. 1973;98(2):96–103. doi: 10.1093/oxfordjournals.aje.a121543. [DOI] [PubMed] [Google Scholar]
- 12.Patterson KD. Pandemic Influenza, 1700-1900: A Study in Historical Epidemiology. Totowa, NJ: Rowan & Littlefield; 1986. [Google Scholar]
- 13.Davenport FM, Minuse E, Hennessy AV, Francis T., Jr Interpretations of influenza antibody patterns of man. Bull World Health Organ. 1969;41(3):453–460. [PMC free article] [PubMed] [Google Scholar]
- 14.Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ. 1999;77(10):820–828. [PMC free article] [PubMed] [Google Scholar]
- 15.Rabadan R, Levine AJ, Robins H. Comparison of avian and human influenza A viruses reveals a mutational bias on the viral genomes. J Virol. 2006;80(23):11887–11891. doi: 10.1128/JVI.01414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shope RE. The incidence of neutralizing antibodies for swine influenza virus in the sera of human beings of different ages. J Exp Med. 1936;63(5):669–684. doi: 10.1084/jem.63.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis T, Magill TP. The incidence of neutralizing antibodies for human influenza virus in the serum of human individuals of different ages. J Exp Med. 1936;63(5):655–668. doi: 10.1084/jem.63.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masurel N, Mulder J. Studies on the content of antibodies for equine influenza viruses in human sera. Bull World Health Organ. 1966;34(6):885–893. [PMC free article] [PubMed] [Google Scholar]
- 19.Masurel N. Relation between Hong Kong virus and former human A2 isolates and the A-EQU12 virus in human sera collected before 1957. Lancet. 1969;1(7601):907–910. doi: 10.1016/s0140-6736(69)92544-6. [DOI] [PubMed] [Google Scholar]
- 20.Masurel N, Heijtink RA. Recycling of H1N1 influenza A virus in man—A haemagglutinin antibody study. J Hyg (Lond) 1983;90(3):397–402. doi: 10.1017/s0022172400029028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rekart M, Rupnik K, Cesario TC, Tilles JG. Prevalence of hemagglutination inhibition antibody to current strains of the H3N2 and H1N1 subtypes of influenza A virus in sera collected from the elderly in 1976. Am J Epidemiol. 1982;115(4):587–597. doi: 10.1093/oxfordjournals.aje.a113340. [DOI] [PubMed] [Google Scholar]
- 22.Bodewes R, et al. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol. 2011;18(3):469–476. doi: 10.1128/CVI.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsen L, Reichert TA, Miller C. The virtues of antigenic sin: Consequences of pandemic recycling on influenza-associated mortality. Int Congr Ser. 2004;1263:791–794. [Google Scholar]
- 24.Schoenbaum SC, Coleman MT, Dowdle WR, Mostow SR. Epidemiology of influenza in the elderly: Evidence of virus recycling. Am J Epidemiol. 1976;103(2):166–173. doi: 10.1093/oxfordjournals.aje.a112214. [DOI] [PubMed] [Google Scholar]
- 25.Mulder J, Masurel N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet. 1958;1(7025):810–814. doi: 10.1016/s0140-6736(58)91738-0. [DOI] [PubMed] [Google Scholar]
- 26.Webster RG, Laver WG. The origin of pandemic influenza. Bull World Health Organ. 1972;47(4):449–452. [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989;63(11):4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koen JS. A practical method for the field diagnoses of swine diseases. Am J Vet Med. 1919;14(9):468–470. [Google Scholar]
- 29.Nelson MI, et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog. 2008;4(2):e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrewes CH, Laidlaw PP, Smith W. Influenza: Observations on the recovery of virus from man and on the antibody content of human sera. Br J Exp Pathol. 1935;16(6):566–582. [Google Scholar]
- 31.Davenport FM, Hennessy AV, Drescher J, Mulder J, Francis T., Jr Further observations on the relevance of serologic recapitulations of human infection with influenza viruses. J Exp Med. 1964;120(6):1087–1097. doi: 10.1084/jem.120.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adalja AA, Henderson DA. Original antigenic sin and pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16(6):1028–1029. doi: 10.3201/eid1606.091563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnon A, et al. Age-specific mortality during the 1918 influenza pandemic: Unravelling the mystery of high young adult mortality. PLoS ONE. 2013;8(8):e69586. doi: 10.1371/journal.pone.0069586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22(23-24):2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Haynes L, et al. Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections. J Immunol. 2012;189(10):4921–4929. doi: 10.4049/jimmunol.1201916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104(6):572–578. [Google Scholar]
- 37.Shanks GD, Brundage JF. Pathogenic responses among young adults during the 1918 influenza pandemic. Emerg Infect Dis. 2012;18(2):201–207. doi: 10.3201/eid1802.102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenport FM, Hennessy AV. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J Exp Med. 1956;104(1):85–97. doi: 10.1084/jem.104.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster RG, Kasel JA, Couch RB, Laver WG. Influenza virus subunit vaccines. II. Immunogenicity and original antigenic sin in humans. J Infect Dis. 1976;134(1):48–58. doi: 10.1093/infdis/134.1.48. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183(5):3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waffarn EE, Baumgarth N. Protective B cell responses to flu—No fluke! J Immunol. 2011;186(7):3823–3829. doi: 10.4049/jimmunol.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masurel N. Swine influenza virus and the recycling of influenza-A viruses in man. Lancet. 1976;2(7979):244–247. doi: 10.1016/s0140-6736(76)91038-2. [DOI] [PubMed] [Google Scholar]
- 43.Schulman JL, Kilbourne ED. Induction of partial specific heterotypic immunity in mice by a single infection with influenza A virus. J Bacteriol. 1965;89(1):170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanks GD, Hussell T, Brundage JF. Epidemiological isolation causing variable mortality in Island populations during the 1918-1920 influenza pandemic. Influenza Other Respi Viruses. 2012;6(6):417–423. doi: 10.1111/j.1750-2659.2011.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molinari N-AM, et al. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Steel J, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio. 2010;1(1) doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe RJ. Alaska’s great sickness, 1900: An epidemic of measles and influenza in a virgin soil population. Proc Am Philos Soc. 1982;126(2):91–121. [PubMed] [Google Scholar]
- 48.Thompson WW, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 49.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 50.Maddison DR, Maddison WP. 2005. MacClade 4: Analysis of Phylogeny and Character Evolution, Version 4.08a. Available at http://macclade.org. Accessed April 28, 2011.
- 51.Kosakovsky Pond SL, et al. A random effects branch-site model for detecting episodic diversifying selection. Mol Biol Evol. 2011;28(11):3033–3043. doi: 10.1093/molbev/msr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.