Significance
The ability of the worm Caenorhabditis elegans to taste or smell can influence its lifespan, and the effect of odors on lifespan has also been shown to exist in the fruit fly Drosophila. Now we provide evidence that fly lifespan is also affected by its ability to taste: some taste inputs shorten lifespan, whereas others increase it. In flies, the lifespan-shortening taste inputs act via an insulin-like signaling pathway and its downstream transcription factor dFOXO, whereas the lifespan-lengthening taste inputs can act independently of this pathway. The taste influence on lifespan is also unlikely linked to changes in food intake levels. Thus, different taste inputs will affect lifespan through more than one mechanism.
Keywords: sensory system, insulin signaling, physiology, gustatory receptors, aging
Abstract
In Caenorhabditis elegans, a subset of gustatory neurons, as well as olfactory neurons, shortens lifespan, whereas a different subset of gustatory neurons lengthens it. Recently, the lifespan-shortening effect of olfactory neurons has been reported to be conserved in Drosophila. Here we show that the Drosophila gustatory system also affects lifespan in a bidirectional manner. We find that taste inputs shorten lifespan through inhibition of the insulin pathway effector dFOXO, whereas other taste inputs lengthen lifespan in parallel to this pathway. We also note that the gustatory influence on lifespan does not necessarily depend on food intake levels. Finally, we identify the nature of some of the taste inputs that could shorten versus lengthen lifespan. Together our data suggest that different gustatory cues can modulate the activities of distinct signaling pathways, including different insulin-like peptides, to promote physiological changes that ultimately affect lifespan.
Aging is a universal process that causes deterioration in the biological functions of an organism over the progression of its lifetime. This process is affected by genetic and environmental factors, whose interaction could be mediated by the sensory system, which perceives and transmits environmental information to modulate the signaling activities of downstream target tissues. Accordingly, external sensory cues and sensory neuron activities have been shown to alter the lifespan of both Caenorhabditis elegans and Drosophila melanogaster (1–6).
In C. elegans, the laser ablation of a specific subset of gustatory or olfactory neurons extends lifespan, whereas ablation of a different subset of gustatory neurons shortens it (1). Interestingly, at least part of this sensory influence on lifespan has also been observed in other animals. In Drosophila, impairment of olfaction through a mutation in Or83b, which encodes a broadly expressed atypical odorant receptor (7), increases lifespan (3). In addition, exposure of dietary-restricted flies to food odors, like live yeast, can partly suppress their long-life phenotype (3). The conservation of the olfactory influence on lifespan is thus consistent with the possibility that gustatory inputs will also bidirectionally alter the lifespan of both C. elegans and D. melanogaster.
The effects of sensory neurons on C. elegans lifespan have been shown to be partly mediated by insulin/IGF signaling (1, 2, 8). The insulin/IGF pathway also affects fly lifespan: down-regulation of the activities of the insulin receptor InR and the receptor substrate, CHICO, extends lifespan (9, 10). Moreover, an increase in activity of the downstream transcription factor dFOXO, which is negatively regulated by both InR and CHICO, increases fly lifespan (11, 12). Consistent with these observations, mutations in several of the Drosophila insulin-like peptide (dilp) genes (13), which are expressed in the median neurosecretory cells (mNSCs) in the fly brain (14–17), or ablation of the mNSCs (18) also extends lifespan. Because these mNSCs send projections to the subesophageal ganglion (SOG) (14, 17), a group of interneurons involved in processing gustatory information in the fly brain (19, 20), it raises the intriguing possibility that, like in worms, the effects of the insulin/IGF pathway on fly lifespan are also subject to gustatory cues.
Thus, in this study, we tested whether the gustatory influence on lifespan is present in flies, and whether its effects are mediated by insulin/IGF signaling. Drosophila has on its labellum (mouthpart), legs, and wings many taste sensilla that have bristle-like structures, which are innervated by two to four gustatory neurons and a mechanosensory neuron (refs. 21 and 22; reviewed in ref. 23). Using genetic tools that eliminate a subset or most of the fly’s taste bristles and the corresponding gustatory neurons that innervate them, we demonstrate that, like in C. elegans, there are taste inputs that lengthen Drosophila lifespan and other taste inputs that shorten it. We also show that the gustatory influence on fly lifespan is partly dependent on the activity of the dFOXO transcription factor, which acts downstream of insulin signaling. Through a screen of taste receptor mutants, we additionally uncover the possible nature of the gustatory cues that can lengthen versus shorten lifespan.
Results
Taste Inputs Affect Drosophila Lifespan.
To test the hypothesis that taste inputs affect fly lifespan, we first compared two classes of taste-impaired flies to control flies that have wild-type taste perception. Accordingly, we used the Pox neuro (Poxn) null mutant PoxnΔM22-B5, whose taste bristles are either missing or are transformed into bristles that lack gustatory innervations but retain the mechanosensory innervation (24). However, Poxn is a gene with pleiotropic activities, which also include functions in the central nervous system and the development of antenna, legs, and male genitalia (24–28). Hence, for our studies, we compared PoxnΔM22-B5 mutants that carry the complete rescue construct with PoxnΔM22-B5 mutants that carry rescue constructs that lack enhancer elements required for the formation of either a subset of (labellar) or most taste bristles (Fig. S1) (27, 29).
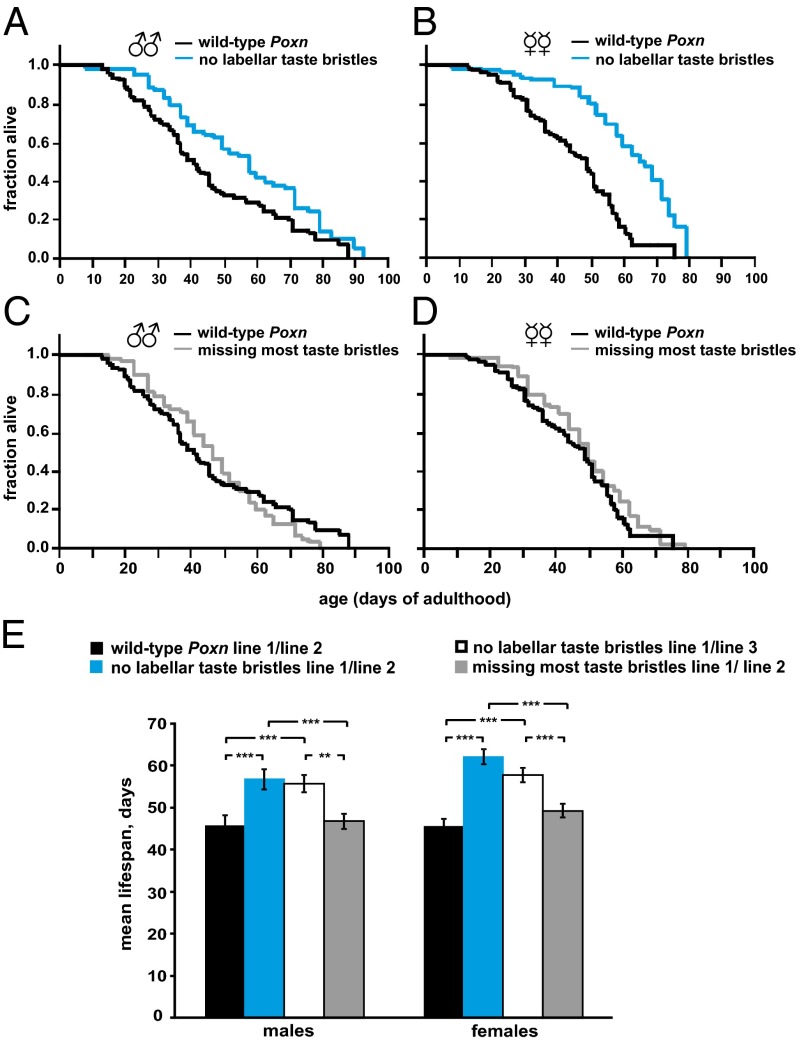
We analyzed different combinations of independent transgenic lines that were extensively backcrossed to the same background and found that flies missing a subset of taste inputs, i.e., labellar taste bristles, live longer than flies with wild-type taste inputs (Fig. 1 A, B, and E and Table S1). We observed that this effect is more robust in females than in males (Fig. 1 A, B, and E and Table S1). Interestingly, we also found that the removal of additional taste bristles from the legs and wings suppresses the long-life phenotype of both labellar taste-impaired male and female flies (Fig. 1 C–E and Table S1). Because the difference between the two classes of taste-impaired flies resides in the number and location of the missing taste bristles (Fig. S2) (27, 29), this suggests that the suppression was due to other taste deficiencies rather than general deleterious effects elsewhere. Notably, this suppression could also be complete (Fig. 1 C–E) or partial (see Fig. 3 A and B), which may arise from the variability of gustatory cues present in the environment. Accordingly, taste sensory organs that perceive a specific set of cues would presumably affect lifespan only in the presence of such cues, an idea that has been illustrated in C. elegans by sensory mutants that exhibit lifespan phenotypes only on certain food sources (30). Thus, the complete or partial antagonism between labellar versus leg and wing taste inputs could be explained, for example, by the variable quality of the yeast present in the Drosophila’s diet from experiment to experiment. Together these studies suggest that both the positive and negative influences of taste inputs on lifespan are conserved in Drosophila.
Fig. 1.
Taste inputs affect fly lifespan bidirectionally. (A and B) Unmated males and females lacking labellar taste bristles (blue curve) live longer than wild-type Poxn control flies (black curve). The detailed statistical data on the survival analyses in this figure (trial 1) are shown in Table S1. (C and D) Loss of additional taste bristles (gray curve; trial 1 in Table S1) suppresses the long-life phenotype of labellar taste-impaired flies. (E) For comparison of the different genotypes, the mean lifespans are shown as a bar graph. All error bars represent ±SEM. **P ≤ 0.01 and ***P ≤ 0.001, according to the Wilcoxon test.
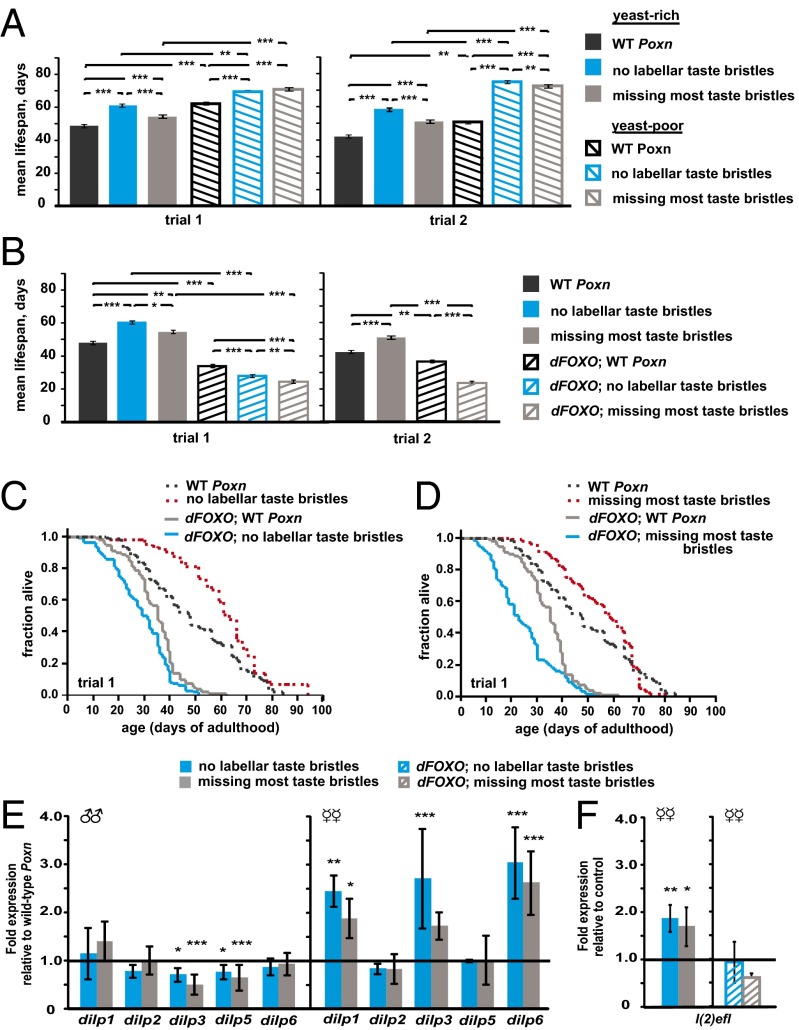
Fig. 3.
The effects of yeast and dFOXO on the taste influence on lifespan. (A) The mean lifespan (±SEM) of control and taste-mutant flies on yeast-enriched versus nonenriched Zurich fly food are depicted as bar graphs. (B) The mean lifespan (±SEM) of control and taste mutants that have wild-type (WT) dFOXO versus those of flies that carry mutations in dFOXO are shown as bar graphs. (C and D) Survival curves of taste-mutant (red) and control (black) flies that have wild-type dFOXO versus those of the corresponding taste-mutant (blue) and control (gray) flies that carry mutations in dFOXO. (E and F) The transcript levels of dilps in the heads (E) and of l(2)efl in the bodies (F) of adult male and/or female taste mutants (blue and gray bars) are shown normalized to control levels (horizontal line across the graph). The relative expression levels in the heads of controls for males are 0.72 ± 0.14 (dilp1), 1.26 ± 0.41 (dilp2), 1.96 ± 0.71 (dilp3), 1.63 ± 0.48 (dilp5), and 1.32 ± 0.29 (dilp6); and females 0.45 ± 0.08 (dilp1), 1.12 ± 0.08 (dilp2), 0.83 ± 0.10 (dilp3), 1.15 ± 0.09 (dilp5), and 0.57 ± 0.03 (dilp6). The relative expression levels of l(2)efl in the bodies of control female flies are 0.76 ± 0.25 for wild-type dFOXO and 0.32 ± 0.08 for mutant dFOXO. Each mean value represents three biological replicates of 30 pooled flies. All flies in A–F again carry transheterozygous insertions of the relevant transgenes in their genomes. All error bars represent ±SEM. *P ≤ 0.01, **P ≤ 0.01, and ***P ≤ 0.001 (Wilcoxon test for A, and B; randomized complete block design ANOVA for E and F). The detailed statistical data on the above lifespan analyses can be found in Table S1.
The Physiology of Taste-Impaired Flies Does Not Resemble That of Food Level-Restricted Flies.
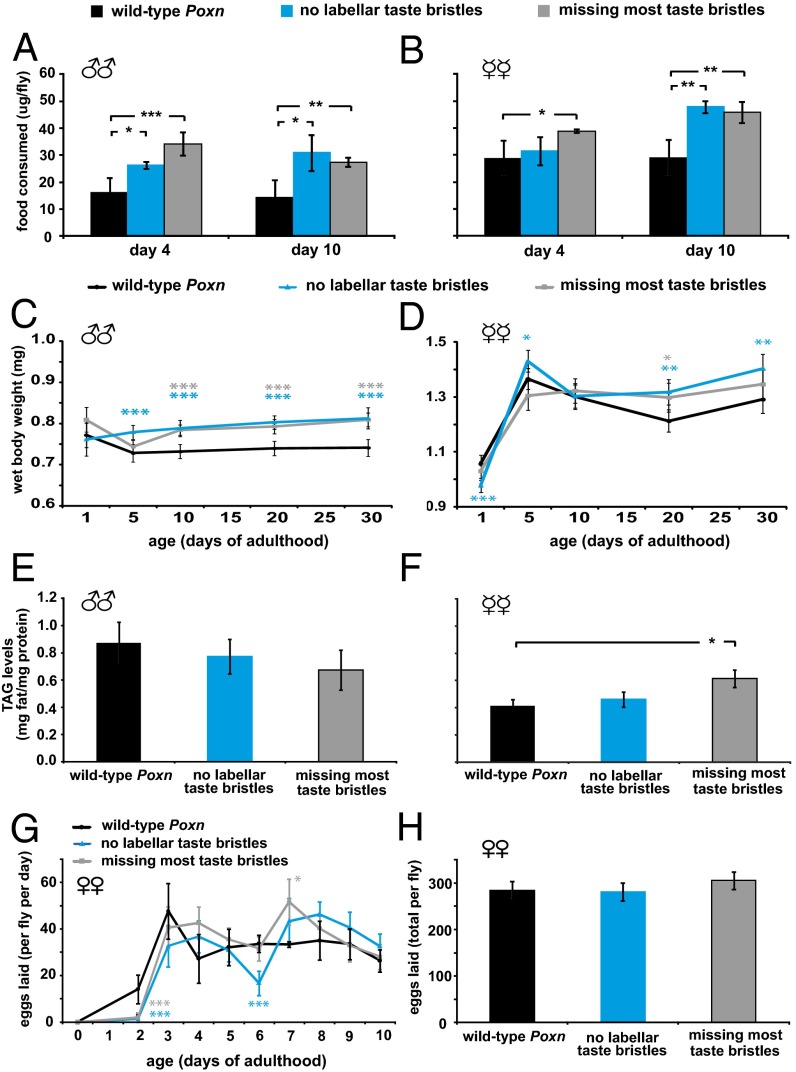
It is possible that loss of taste inputs might lead to decreases in feeding rates, which in turn would alter Drosophila lifespan. Indeed, a reduction in the level of food intake that does not result in malnutrition, which is commonly known as dietary restriction (DR), extends lifespan, whereas a further reduction in feeding, which presumably leads to a state of starvation, causes a shorter lifespan (31). Surprisingly, however, we observed a lack of correlation between food intake and lifespan of control and labellar taste-impaired flies (Fig. S2A and Table S1). Indeed, in most cases, both classes of taste-impaired flies exhibit increased food intake compared with control flies (Fig. 2 A and B and Fig. S2A), which shows that the lifespan phenotypes of these flies (Table S1) are not necessarily due to a restriction in food intake. Moreover, we found that taste-impaired flies weigh heavier as they get older (Fig. 2 C and D), which is again unlike the lower body weights observed in flies with restricted food intake levels (32).
Fig. 2.
Taste-impaired flies do not resemble food level-restricted flies. (A and B) Taste-impaired adult mutant (blue and gray bars) males and females are compared with controls (black bars) at two different ages after a 24-h feeding regimen. The food consumption values are normalized per fly and each mean is derived from 3 to 4 biological replicates of 7 to 10 pooled flies. The error bars in A–H represent 95% confidence intervals. (C and D) The body weight of taste mutants (blue and gray lines) are compared with controls (black line) at different ages. Each data point represents the mean from at least three measurements of 10 individual flies. (E and F) TAG levels of 10-d-old adult taste-mutant and control flies are compared. Each mean represents five biological replicates of 9 to 10 pooled flies. (G and H) The fecundity of taste-impaired flies is compared with that of controls. The number of eggs laid per fly per day (G) or the total number of eggs laid per fly over a period of 10 d (H) is shown. Each time point represents data from 6 to 10 adult females. All flies in A–H carry transheterozygous insertions of the relevant transgenes in their genomes. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
To show further that taste-impaired flies are not eating less food due to the lack of hedonic stimuli from food components, we measured their triacylglyceride (TAG) levels. Again, we saw no correlation between the lifespan and TAG levels of these flies: taste-impaired flies have similar TAG content as control flies (Fig. 2 E and F and Fig. S2B).
Because reduced reproductive output is also a hallmark of DR (33), we compared the rates of reproduction and total fecundity in control and taste-mutant flies. Although we detected some differences between these flies in the number of eggs laid per day (Fig. 2G), we observed that the various groups of flies laid a similar cumulative number of eggs within a period of 10 d (Fig. 2H). Thus, our data together suggest that the lifespan alterations we observe in flies lacking taste bristles are not simply due to the general restriction of food levels.
Besides food levels, the nature of the food source has also been shown to influence an animal’s lifespan (30, 34–37). Because yeast is a component of fly food that can shorten fly lifespan (36) partly through an olfaction-mediated mechanism (3), we asked whether the effect of yeast on lifespan also acts via taste inputs. However, we found that the absence of a yeast supplement in the diet (Materials and Methods) can still extend the lifespan of all taste-impaired and control flies, and that the taste-impaired female flies can still live longer than control flies on the non–yeast-enriched diet (Fig. 3A and Table S1).
Some Taste Inputs Require the Activity of the Insulin Pathway Effector dFOXO.
In worms, the taste influence on lifespan can act in parallel to DR but is mediated by the insulin signaling pathway (1). In flies, reduction-of-function mutations in the insulin receptor InR (10) or the receptor substrate chico (9) have been shown similarly to extend lifespan, which require the activity of the transcription factor dFOXO (11, 12). In contrast, dFOXO activity is not required for the lifespan increase caused by DR (38). Thus, these observations led us to ask whether dFOXO also mediates the lifespan extension observed in flies lacking a subset of taste inputs.
We found that removal of dFOXO suppressed the long-life phenotype of labellar taste-impaired flies (Fig. 3 B and C and Table S1), which suggests that the labella receive taste inputs that shorten lifespan in a dFOXO-dependent manner. Moreover, we observed that loss of additional taste bristles from the legs and wings of female dFOXO mutants further shortened their lifespan (Fig. 3 B and D and Table S1), which suggests that leg and wing taste inputs also affect lifespan independent of dFOXO.
Because the taste influence on lifespan appears to partially require dFOXO activity, we measured the steady-state transcript levels of several dilps, some of which are known dFOXO targets—e.g., dilp3 and dilp6 (13, 39–41)—in both classes of taste mutants. We detected significant changes in only some of the dilps of the taste-impaired flies (Fig. 3E). In females, dilp1, dilp3, and dilp6 are elevated in both classes of taste mutants (Fig. 3E), and these taste-dependent increases are suppressed by loss of dFOXO activity (Fig. S3). To determine whether dFOXO targets other than dilp3 and dilp6 are also affected by taste inputs, we compared the levels of l(2)efl, which encodes a small heat shock protein whose expression requires dFOXO (42), in the bodies of taste-mutant and control flies. Similar to dilp1, dilp3, and dilp6, we found that l(2)efl is increased in both taste mutants in the presence of dFOXO, but not in the absence of dFOXO (Fig. 3F). Together our findings suggest that loss of labellar taste inputs increase dFOXO activity to lengthen lifespan (Fig. 3 B, C, E, and F and Table S1). Interestingly, because we observed that the dilp changes are similar in both classes of taste-impaired flies (Fig. 3E and Fig. S3), these data also suggest that the dFOXO-independent leg and wing taste inputs antagonize, and/or act completely parallel to, DILP function.
The sexual dimorphism of the taste influence on lifespan, i.e., its more robust effects in females than in males (Fig. 1 and Table S1), was also apparent at the level of dilp expression (Fig. 3E). In contrast to the changes in dilp expression in females, we found a decrease in dilp3 and dilp5 transcripts of male taste-impaired flies (Fig. 3E). These differences in dilp expression patterns in response to taste inputs may reflect the differences in the perceived nutrient requirements of unmated male and female flies.
Together these data show that the taste influence on lifespan involves distinct mechanisms that act in parallel: a dFOXO-dependent versus a dFOXO-independent pathway (Fig. 3 B–D and Table S1). This is also reflected in the differences in the taste influence on the expression of specific dilps (Fig. 3E), each of which may promote a particular set of physiological changes that ultimately affect lifespan (13, 40).
Different Gustatory Receptors Have Distinct Effects on Lifespan.
To confirm the bidirectional effects of taste on lifespan, we assayed the lifespan of some known gustatory receptor mutants. We found that some taste receptor mutants have a short lifespan, e.g., the sweet taste receptor Gr5a (43), whereas other taste receptor mutants have a long lifespan (Table 1) (44), like the water receptor ppk28 (45). Interestingly, mutations that disrupt the Gr5a locus have previously been shown to decrease lifespan (46), consistent with our observation with a mutation that specifically deletes this gene [ΔGr5a (43); Table 1]. Moreover, the companion article (44) in PNAS details the mechanisms through which the water receptor ppk28 affects lifespan. Thus, our data together suggest the nature of some taste inputs that can modulate Drosophila longevity.
Table 1.
Specific gustatory receptor mutants exhibit short- versus long-lived phenotypes
| Genotype |
Sample size |
Lifespan, d |
||||||
| Mutant | Control [G] | Food | Nmut | Nctrl | Meanmut | Meanctrl | Δ lifespan, % | P value |
| Male | ||||||||
| w; dpr1 | w1118 [6] | ZRH | 92 | 94 | 43.62 | 47.76 | −8.67 | 0.0615 |
| w; ΔGr5a | w1118 [8] | SY10 | 246 | 248 | 60.38 | 64.21 | −5.96 | 0.0285 |
| w; ΔGr5a | w1118 [8] | SY10 | 294 | 284 | 57.79 | 66.60 | −13.23 | 0 |
| w; ΔGr33a | w1118 [6] | ZRH | 92 | 84 | 49.88 | 55.23 | −9.69 | 0.0038 |
| w; ΔGr33a | w1118 [7] | SY10 | 241 | 250 | 65.04 | 62.32 | −4.36 | 0.4320 |
| w; ΔGr66a | w1118 [4] | ZRH | 78 | 94 | 62.00 | 47.76 | 29.82 | 0 |
| w; ΔGr66a | w1118 [6] | SY10 | 237 | 250 | 69.11 | 62.32 | 10.90 | 0.0060 |
| w; ΔGr93a | w1118 [6] | ZRH | 77 | 94 | 46.52 | 47.76 | −2.60 | 0.3384 |
| w; Δppk28 | w1118 [6] | ZRH | 91 | 84 | 61.58 | 55.23 | 11.50 | 0.0165 |
| w; Δppk28 | w1118 [8] | SY10 | 245 | 243 | 72.09 | 61.72 | 16.80 | 2.45 × 10−5 |
| Female | ||||||||
| w; dpr1 | w1118 [6] | ZRH | 188 | 91 | 41.18 | 47.28 | −12.90 | 0.0002 |
| w; ΔGr5a | w1118 [8] | SY10 | 225 | 251 | 52.74 | 64.93 | −18.93 | 0 |
| w; ΔGr5a | w1118 [8] | SY10 | 297 | 280 | 51.55 | 70.52 | −26.90 | 0 |
| w; ΔGr33a | w1118 [6] | ZRH | 93 | 96 | 51.42 | 48.05 | 7.01 | 0.0741 |
| w; ΔGr33a | w1118 [7] | SY10 | 247 | 250 | 69.11 | 58.23 | 18.71 | 0 |
| w; ΔGr64 | w1118 [8] | S30Y5 | 295 | 272 | 36.72 | 45.90 | −20.00 | 0 |
| w; ΔGr66a | w1118 [4] | ZRH | 86 | 91 | 58.90 | 47.28 | 24.58 | 0 |
| w; ΔGr66a | w1118 [6] | SY10 | 251 | 250 | 75.78 | 58.23 | 30.14 | 0 |
| w; ΔGr93a | w1118 [6] | ZRH | 98 | 91 | 44.95 | 47.28 | 4.93 | 0.0090 |
| w; Δppk28 | w1118 [6] | ZRH | 96 | 96 | 54.31 | 48.05 | 13.03 | 0.0003 |
| w; Δppk28 | w1118 [8] | SY10 | 241 | 246 | 74.79 | 55.97 | 33.63 | 0 |
We measured the lifespan of control (ctrl) and mutant (mut) flies in parallel in independent trials. The number of backcrosses to w1118 (w) per taste mutant is indicated in column 2 by “[G].” Each assay was carried out according to the Zurich (ZRH) protocol and diet (supplemented with yeast paste) or to the Ann Arbor protocol and diet (SY10 or S30Y5). See Materials and Methods for the exact genotype of each receptor mutant assayed. P values are according to the log-rank test.
Discussion
Many biological processes are conserved between C. elegans, Drosophila, and higher organisms. For example, the insulin/IGF1 pathway regulates the physiology, and consequently the longevity, of worms, flies, and mice (9, 10, 47–49). Similarly, the sensory influence on lifespan is conserved in both worms (1) and flies at the level of olfaction (3, 5). This study, as well as that of Waterson et al. (44), shows that the conserved sensory effects on lifespan can also be extended to gustation.
As in C. elegans (1), our study illustrates that the gustatory system can also affect Drosophila lifespan bidirectionally (Figs. 1 and 3 A and B, Table 1, and Table S1), which shows no correlation with and is thus likely independent of the animal’s level of food intake (Fig. 2 A and B and Fig. S2A). Indeed, both longer- and shorter-lived taste-impaired flies usually have higher food intake levels and body weights (Fig. 2 A–D and Fig. S2A), which is reminiscent of human studies that demonstrated a negative correlation between food intake (or body mass index) and taste sensitivity to certain food components (50, 51).
Although it is independent of food intake levels, it remains possible that the gustatory influence on fly lifespan could depend on the type of food source. In C. elegans, the sensory influence depends on the recognition of food types, which can have different effects on lifespan (30, 34–37). In Drosophila, the food-type effect on lifespan has been demonstrated through alterations in the protein composition of its food source. For example, yeast restriction or an imbalance in dietary amino acids can extend lifespan (36, 52, 53). However, yeast restriction alone does not always increase fly lifespan under all conditions that do not cause malnutrition (52), which suggests that other lifespan-influencing food-derived factors are also involved. In fact, the perception of water is one of the cues, among many, that can affect lifespan (Table 1) (44, 54). Thus, because the gustatory system senses many different types of food-derived cues, which could elicit different physiological outcomes, it should not be surprising that the taste influence on lifespan will involve more than one mechanism.
Consistent with this idea, we show that this influence requires both dFOXO-dependent and -independent pathways that act in parallel (Fig. 3 B–D and Table S1). Labellar taste inputs inhibit longevity through the dFOXO pathway (Fig. 3 B and C and Table S1), an effect that is either completely (Fig. 1) or partly (Fig. 3 A and B and Table S1) antagonized by parallel taste inputs from the legs and wings. Because flies missing most taste inputs show a similar change in the expression of dilps and the dFOXO target l(2)efl compared with flies that are only impaired in labellar taste (Fig. 3 E and F), this suggests that (i) the dFOXO-dependent lifespan-lengthening signal is present in both classes of taste mutants and (ii) the dFOXO-independent taste inputs from the legs and wings counteract this signal. However, the degree of antagonism between these two pathways presumably depends on the presence or absence of specific gustatory cues in the animal’s environment. This would be analogous to C. elegans sensory mutants that exhibit food source-dependent lifespan phenotypes (30).
Our findings that only some dilps are altered in taste-impaired flies (Fig. 3E) suggest that specific gustatory cues modulate the activities of discrete sets of dilps, which are expressed either in the mNSCs or the fat bodies of the fly (13–17, 40, 55). This could occur through the SOG interneurons that can act as a relay center between gustatory neurons and the dilp-expressing mNSCs (14, 19, 20) or fat bodies (13, 40). Moreover, the nature of the dilps that have altered expression in female versus male taste-impaired flies could yield insight into the sexual dimorphism of the taste influence on lifespan. In females, dilp1, dilp3, and dilp6 are highly expressed in the heads of taste-impaired flies (Fig. 3E). Although high dilp1 or dilp3 expression has not been shown to affect lifespan, increased expression of dilp6 in the head fat body through overexpression of dFOXO has previously been shown to extend lifespan (41). Thus, it is tempting to speculate that labellar taste-impaired female flies live longer due to dilp6 overexpression. In males, on the other hand, taste impairment does not change dilp6 levels, but slightly down-regulates dilp3 and dilp5 (Fig. 3E). Loss of these dilps, together with that of dilp2, has been found to mediate the DR effects on lifespan (13). However, it is currently unclear how down-regulation of dilp3 and dilp5 would promote longevity in male taste-mutant flies.
Finally, the observation that the gustatory and olfactory systems influence the lifespan of both worms and flies (this study and refs. 1, 3, 5, 44, and 54) raises the intriguing possibility that the sensory system also affects mammalian lifespan. In mammals, both gustatory and olfactory information are relayed to the hypothalamus, a region in the brain that controls behavior and physiology (reviewed in ref. 56). Thus, it is conceivable that the processing of such sensory information by the hypothalamus may lead to physiological changes, which in turn may have bidirectional effects on mammalian lifespan.
Materials and Methods
Fly Stocks.
All transgenic rescue constructs (SuperA-158, SuperA-207-1, Full1, Full115, Full152, ΔXBs, and ΔPBs) were as formerly described (27, 29). The dFOXO null alleles (dFOXO21 and dFOXO25) (57), and the taste receptor mutants [dpr1 (58), ΔGr5a (43), Gr33a1 (ΔGr33a) (59), ΔGr64 (60), Gr66aex83 (ΔGr66a) (61), Gr93a2 (ΔGr93a) (62), and Δppk28 (45)] were also as described previously. All flies were backcrossed at least seven times to the w1118 background (SI Materials and Methods), with the exception of some of the taste receptor mutants, which were backcrossed four or six times to w1118 before the lifespan screen. To further minimize the effect of the genetic background on the experimental results, transheterozygous combinations of the relevant Poxn rescue constructs and dFOXO alleles were compared, which should also ensure that the disruption of insertion sites is heterozygous in the transgenic animals analyzed. The full genotypes of the transgenic flies used in this study are listed in SI Materials and Methods. All flies were maintained at 25 °C.
Lifespan Assays.
Zurich.
The lifespan of the progeny of 3- to 5-d-old male and female wild-type Poxn and taste bristle mutant flies in the presence or absence of dFOXO, as well as some of the taste receptor mutants, were measured at 25 °C under constant humidity (60%) with a 12:12 h light:dark cycle. To minimize stress-induced mortality in very young adults, freshly eclosed flies (within a 2-h time window) were transferred to new bottles, where they were aged for 2 h. These flies were then collected under mild CO2 anesthesia for the lifespan assays, in which adult virgin males and virgin females were separated. The lifespan measurements, which were started on the first day of adulthood, were done by placing 8–12 flies per vial, which contained standard Zurich fly food [10% (wt/vol) yeast, 7.5% (wt/vol) dextrose, 5.5% (wt/vol) corn meal, 1% (wt/vol) flour, 0.8% agar, 0.1% nipasol, and 0.05% nipagin] supplemented with a drop of yeast paste on top of the food, unless stated otherwise. Flies were transferred to fresh tubes and scored for survival thrice a week. The JMP 5.1 (SAS) software was used to determine the Kaplan–Meier survival probabilities, mean lifespan, and statistical comparisons among the different assay conditions by applying the log-rank or Wilcoxon test where appropriate. If the ratio of hazard functions (ratio of mortality rates) between two groups of animals stays approximately constant over time, the log-rank test serves as the appropriate test; otherwise, the Wilcoxon test is more appropriate (63).
Ann Arbor.
The lifespan of some of the taste receptor mutants were measured as described in ref. 44, where flies were fed a 10% (wt/vol) sucrose and yeast diet (SY10) or a 30% (wt/vol) sucrose–5% (wt/vol) yeast diet (S30Y5).
Feeding Assays, Body Weight, and TAG Measurements and Quantitative Measurement of mRNA Levels.
Control and mutant flies were collected as described above (Zurich protocol and diet) and transferred regularly to fresh food until the specified days of adulthood, upon which food intake, body weight, TAG levels, and dilp and l(2)efl expression were measured. See SI Materials and Methods for a description of the different assays.
Fecundity Assays.
See SI Materials and Methods for a description of the assays.
Supplementary Material
Acknowledgments
We thank M. Noll for most of the reagents used in this study, and we thank M. Noll, D. Brunner, J. Pielage, L. Pile, and S. Todi for supporting this work; E. Hafen for dFOXO21 and dFOXO25 flies; M. Steinmann-Zwicky for w1118 flies; J. Yanrui for ΔPBs flies; H. Amrein, C. Montell, K. Scott, and the Bloomington Stock Center for the taste receptor mutants; O. Georgiev for technical advice on molecular biology; M. Moser and N. Saha for help on the quantitative PCR experiments; S. Tiefenböck for advice on the TAG measurements; and S. Kalvakuri, M. Daube, S. Chilaka, G. Ristic, and the J.A. Laboratory at Wayne State University for help on fly husbandry. We also thank the Noll Laboratory for discussions about this study and M. Noll, I. Katic, and W. Maier for critical comments on the manuscript. This work was supported by the Novartis Research Foundation (I.O. and J.A.) and Wayne State University (R.C. and J.A.); University of Zurich (W.B.); National Institutes of Health (NIH) Grants 5-T-32-GM007315 and T-32-AG000114 (to M.J.W.); NIH Grants R-01-AG030593, R-01-AG043972, and R-01-AG023166; and the Glenn Foundation, the American Federation for Aging Research, and the Ellison Medical Foundation (S.D.P.). A part of this work also used the Drosophila Aging Core of the Nathan Shock Center of Excellence in the Biology of Aging (National Institute on Aging Grant P30-AG-013283).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315466111/-/DCSupplemental.
References
- 1.Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41(1):45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 2.Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402(6763):804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- 3.Libert S, et al. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315(5815):1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19(9):715–722. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon PC, Kuo T-H, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8(4):e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao R, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152(4):806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 9.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 10.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 11.Giannakou ME, et al. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305(5682):361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 12.Hwangbo DS, Gershman B, Tu M-P, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429(6991):562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 13.Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6(2):e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science. 2002;296(5570):1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 15.Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001;304(2):317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- 16.Brogiolo W, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11(4):213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 17.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12(15):1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 18.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott K, et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104(5):661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 20.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3(9):e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nottebohm E, et al. The gene poxn controls different steps of the formation of chemosensory organs in Drosophila. Neuron. 1994;12(1):25–34. doi: 10.1016/0896-6273(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 22.Nayak SV, Singh SN. Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster meigen (Diptera: Drosophilidae) Int J Insect Morphol Embryol. 1983;12:273–291. [Google Scholar]
- 23.Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009;47:139–185. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- 24.Awasaki T, Kimura K. pox-neuro is required for development of chemosensory bristles in Drosophila. J Neurobiol. 1997;32(7):707–721. doi: 10.1002/(sici)1097-4695(19970620)32:7<707::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Awasaki T, Kimura K. Multiple function of poxn gene in larval PNS development and in adult appendage formation of Drosophila. Dev Genes Evol. 2001;211(1):20–29. doi: 10.1007/s004270000119. [DOI] [PubMed] [Google Scholar]
- 26.Dambly-Chaudière C, et al. The paired box gene pox neuro: A determinant of poly-innervated sense organs in Drosophila. Cell. 1992;69(1):159–172. doi: 10.1016/0092-8674(92)90127-x. [DOI] [PubMed] [Google Scholar]
- 27.Boll W, Noll M. The Drosophila Pox neuro gene: Control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129(24):5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- 28.Bopp D, Jamet E, Baumgartner S, Burri M, Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989;8(11):3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krstic D, Boll W, Noll M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE. 2009;4(2):e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maier W, Adilov B, Regenass M, Alcedo J. A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 2010;8(5):e1000376. doi: 10.1371/journal.pbio.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCay CM, Cromwell MF, Maynard LA. The effect of retarded growth upon the length of life span and ultimate body size. J Nutr. 1935;10(1):63–79. [PubMed] [Google Scholar]
- 32.Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4(6):309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 33.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263(1371):755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 34.Garsin DA, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300(5627):1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 35.MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJ. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell. 2013;153(1):240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3(7):e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23(4):496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: Not required, but its activity modulates the response. Aging Cell. 2008;7(2):187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 39.Broughton S, et al. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3(11):e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17(6):874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai H, Kang P, Tatar M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell. 2012;11(6):978–985. doi: 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flatt T, et al. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA. 2008;105(17):6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4(12):1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 44.Waterson MJ, et al. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc Natl Acad Sci USA. 111:8137–8142. doi: 10.1073/pnas.1315461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465(7294):91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rollmann SM, et al. Pleiotropic fitness effects of the Tre1-Gr5a region in Drosophila melanogaster. Nat Genet. 2006;38(7):824–829. doi: 10.1038/ng1823. [DOI] [PubMed] [Google Scholar]
- 47.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 48.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 49.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 50.Simchen U, Koebnick C, Hoyer S, Issanchou S, Zunft HJF. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur J Clin Nutr. 2006;60(6):698–705. doi: 10.1038/sj.ejcn.1602371. [DOI] [PubMed] [Google Scholar]
- 51.Stewart JE, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104(1):145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 52.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7(4):478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462(7276):1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gendron CM, et al. Drosophila life span and physiology are modulated by sexual perception and reward. Science. 2014;343(6170):544–548. doi: 10.1126/science.1243339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okamoto N, et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell. 2009;17(6):885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alcedo J, Maier W, Ch'ng Q. Sensory influence on homeostasis and lifespan: Molecules and circuits. In: Tavernarakis N, editor. Protein Metabolism and Homeostasis in Aging, Advances in Experimental Medicine and Biology. Vol 694. Austin, TX: Landes Bioscience; 2010. pp. 197–210. [PubMed] [Google Scholar]
- 57.Jünger MA, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2(3):20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura M, Baldwin D, Hannaford S, Palka J, Montell C. Defective proboscis extension response (DPR), a member of the Ig superfamily required for the gustatory response to salt. J Neurosci. 2002;22(9):3463–3472. doi: 10.1523/JNEUROSCI.22-09-03463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19(19):1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17(20):1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16(18):1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 62.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106(11):4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee ET, Go OT. Survival analysis in public health research. Annu Rev Public Health. 1997;18:105–134. doi: 10.1146/annurev.publhealth.18.1.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.