Significance
Phosphorus is scarce in many subtropical ocean regions, and phytoplankton in these regions adjust their biochemical composition such that they require less of it. We show here that phytoplankton in the ultra–low-phosphorus Sargasso Sea are enriched in polyphosphate (polyP), a phosphorus molecule hitherto thought of primarily as a luxury storage product in marine phytoplankton. We further show that polyP appears to be more readily recycled in the surface ocean than other phosphorus-containing biochemicals. Thus, the high relative levels, and fast cycling, of polyP in low-phosphorus environments may form a feedback loop that contributes bioavailable phosphorus for primary production, potentially reducing the likelihood of growth limitation by phosphorus.
Keywords: nutrient limitation, phosphorus cycling, marine phytoplankton, lipids
Abstract
Phytoplankton alter their biochemical composition according to nutrient availability, such that their bulk elemental composition varies across oceanic provinces. However, the links between plankton biochemical composition and variation in biogeochemical cycling of nutrients remain largely unknown. In a survey of phytoplankton phosphorus stress in the western North Atlantic, we found that phytoplankton in the phosphorus-depleted subtropical Sargasso Sea were enriched in the biochemical polyphosphate (polyP) compared with nutrient-rich temperate waters, contradicting the canonical oceanographic view of polyP as a luxury phosphorus storage molecule. The enrichment in polyP coincided with enhanced alkaline phosphatase activity and substitution of sulfolipids for phospholipids, which are both indicators of phosphorus stress. Further, polyP appeared to be liberated preferentially over bulk phosphorus from sinking particles in the Sargasso Sea, thereby retaining phosphorus in shallow waters. Thus, polyP cycling may form a feedback loop that attenuates the export of phosphorus when it becomes scarce, contributes bioavailable P for primary production, and supports the export of carbon and nitrogen via sinking particles.
Phosphorus (P) is an essential element for all living organisms. However, P can be extremely scarce in open-ocean surface waters such as in the subtropical western North Atlantic (the Sargasso Sea), where soluble reactive P (SRP) concentrations are routinely <10 nmol⋅L−1 and turnover rates are on the order of hours (1). Despite this scarcity, primary production by phytoplankton in the Sargasso Sea does not appear to be limited primarily by P (2, 3), reflecting the intensity of P recycling by the microbial community (4, 5) and the exquisite adaptations of marine phytoplankton to low P conditions, which remain to be fully characterized.
Phytoplankton respond to low P by producing enzymes such as alkaline phosphatase to hydrolyze extracellular dissolved organic P molecules (4, 6, 7), increasing the affinity and rate of P uptake (8, 9) and reducing their inventory of P-containing biochemicals (1, 10). In contrast, when P is abundant, phytoplankton take up excess P and store it as a luxury reserve that is generally thought to be composed of polyphosphate (polyP) (10–12). This modulation of P-containing biochemicals results in basin-scale relationships between P availability and biomass carbon-to-phosphorus (C:P) ratios (13). Presently, the only class of molecules known to consistently contribute to these gradients in cellular P are lipids because P stress in phytoplankton triggers substitution of non–P-membrane lipids for phospholipids, such as the sulfolipid sulfoquinovosyldiacylglycerol (SQDG) for the phospholipid phosphatidylglycerol (PG) (1, 14). However, lipid substitution alone probably cannot account for the full range of C:P observed in the ocean, and yet an understanding of other biochemical drivers of the C:P gradient remains elusive. Further, it is unknown how changes in plankton biochemical composition influence the recycling of nutrients.
Polyphosphate (polyP), a ubiquitous inorganic P polymer of three to hundreds of residues, has diverse physiological roles and complex dynamics in microbes. It is critical for surviving nutritional stress and stationary phase (15–17) but is also important for P homeostasis: microbes produce polyP when P is more abundant than required for growth, so-called luxury uptake, and break down this polyP store upon P stress (18). Moreover, if P-stressed cells experience a spike in P availability, they overproduce polyP in excess of luxury uptake levels, the so-called “overplus” response (19). Given these complex dynamics, it has been hypothesized that polyP might be either virtually absent, or particularly abundant, in oligotrophic marine systems (20).
Results and Discussion
Sectional Measurements of P Molecules and Phytoplankton Physiology.
We measured polyP in particles collected on glass fiber GF/F filters (0.7-µm nominal pore size) along a transect from the P-replete temperate western North Atlantic (TWNA) to the P-depleted Sargasso Sea. Most surface ocean particulate C, N, and P are in living biomass, with heterotrophic bacteria contributing ∼30% of particulate P in the subtropical North Atlantic (13). However, GF/F filters probably capture only ∼50% of heterotrophic bacteria (21, 22). Although bacteria may have contributed to the trends we describe below, the data are likely dominated by phytoplankton community signals. This view is supported by observed changes in SQDG:PG (see following paragraph) as marine heterotrophic bacteria are PG-rich and do not substitute lipids under P stress (1). PolyP was extracted using an enzyme-based method, and fluorometric measurements were calibrated against synthetic polyP (23), which were corrected for background sample fluorescence and matrix effects, giving a relative measure of polyP concentration that is expressed as nanoequivalents per liter (neq⋅L−1) of the standard. We present the ratio of polyP to total particulate P (polyP:TPP) although it is not a quantitative measure of the absolute molar quantity of TPP that is composed of polyP because polyP:TPP > 1 in some instances (detailed discussion of the enzymatic polyP extraction method and comparison with the more-established NaOH-based extraction are discussed in ref. 23 and SI Materials and Methods). SRP, TPP, APase activity, and membrane lipids were measured to examine how patterns in polyP related to biogeochemical P pools and known metrics of P stress.
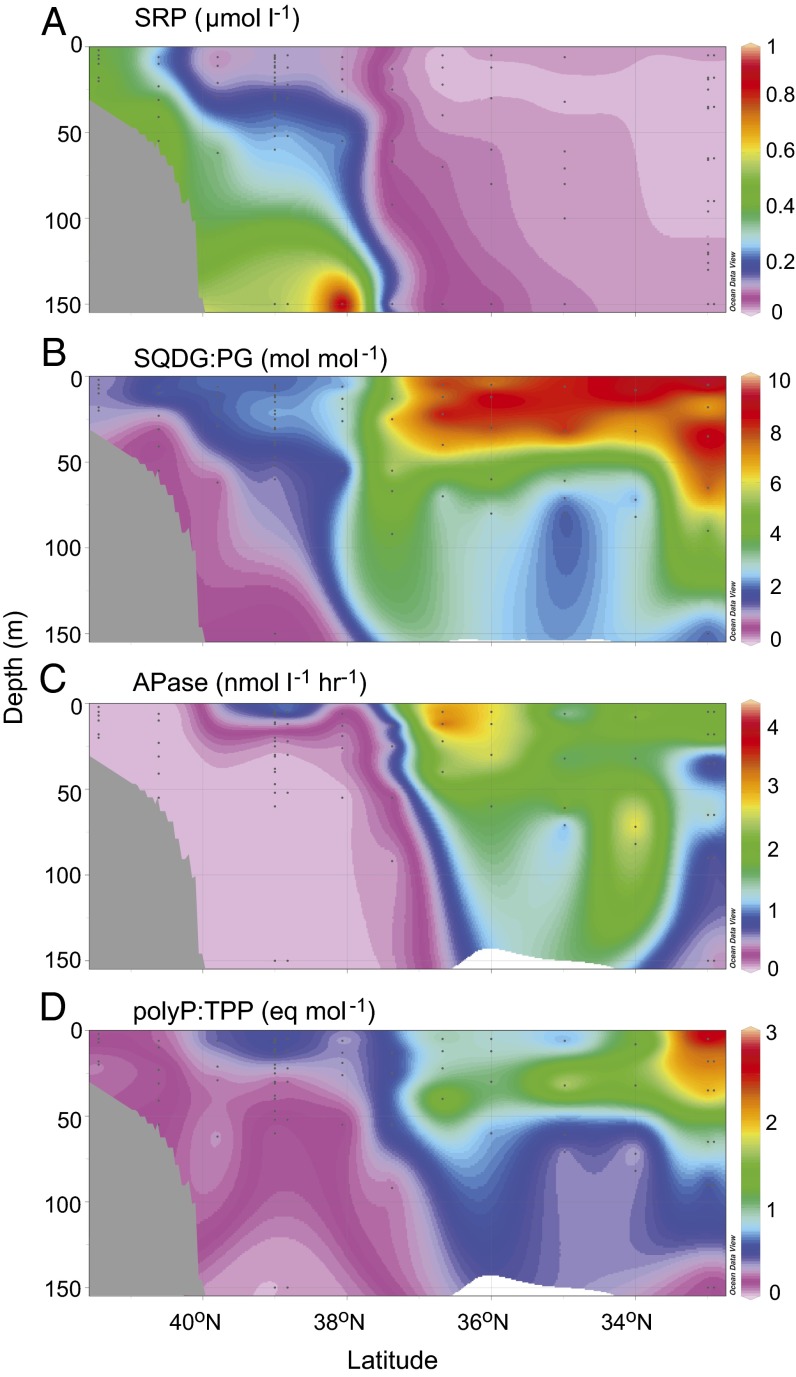
The transect crossed the Gulf Stream at 37.4°N, which separated the TWNA from the Sargasso Sea. In the TWNA, surface mixed layer SRP concentrations were 100–400 nmol⋅L−1, alkaline phosphatase (APase) activity was detected in just 3 out of 46 samples (1.4–2.2 nmol P⋅L−1⋅h−1), and the molar sulfolipid to phospholipid ratio (SQDG:PG) was 0.3–2.3. In the Sargasso Sea, SRP concentrations were 1–25 nmol⋅L−1, APase was always detected (1.3–4.3 nmol P⋅L−1⋅h−1), and SQDG:PG was 4–10 (Fig. 1). These data clearly indicate phytoplankton P stress in the Sargasso Sea coincident with the low SRP; very similar observations along a similar western North Atlantic transect in 2008 suggest that these patterns are representative of the region in general (Fig. S1).
Fig. 1.
Sectional data obtained during a cruise from Woods Hole, MA to St. George’s, Bermuda in April of 2012. Gray shading indicates bathymetry. The Gulf Stream, separating productive northern waters from the oligotrophic subtropics, was crossed at ∼37.4°N. (A) Soluble reactive phosphorus (SRP) decreased sharply across the shelf-break and then fell to mostly <10 nmol⋅L−1 in the Sargasso Sea. (B) Sulfolipid (SQDG) to phospholipid (PG) molar ratios and (C) total alkaline phosphatase activity increased concomitantly from north to south. (D) The ratio of polyphosphate (polyP) to total particulate phosphorus (TPP) also increased; note that this ratio is a relative measure expressed as nanoequivalents of a synthetic polyP standard. An identical latitudinal trend with fivefold increase in polyP:TPP from the offshore TWNA to the Sargasso Sea was found using NaOH instead of enzymatic polyP extraction, although with lower absolute polyP values. Data from the enzymatic polyP method are presented for consistency to Fig. 3 and because the method is more sensitive, which allowed greater coverage of the transect (SI Materials and Methods).
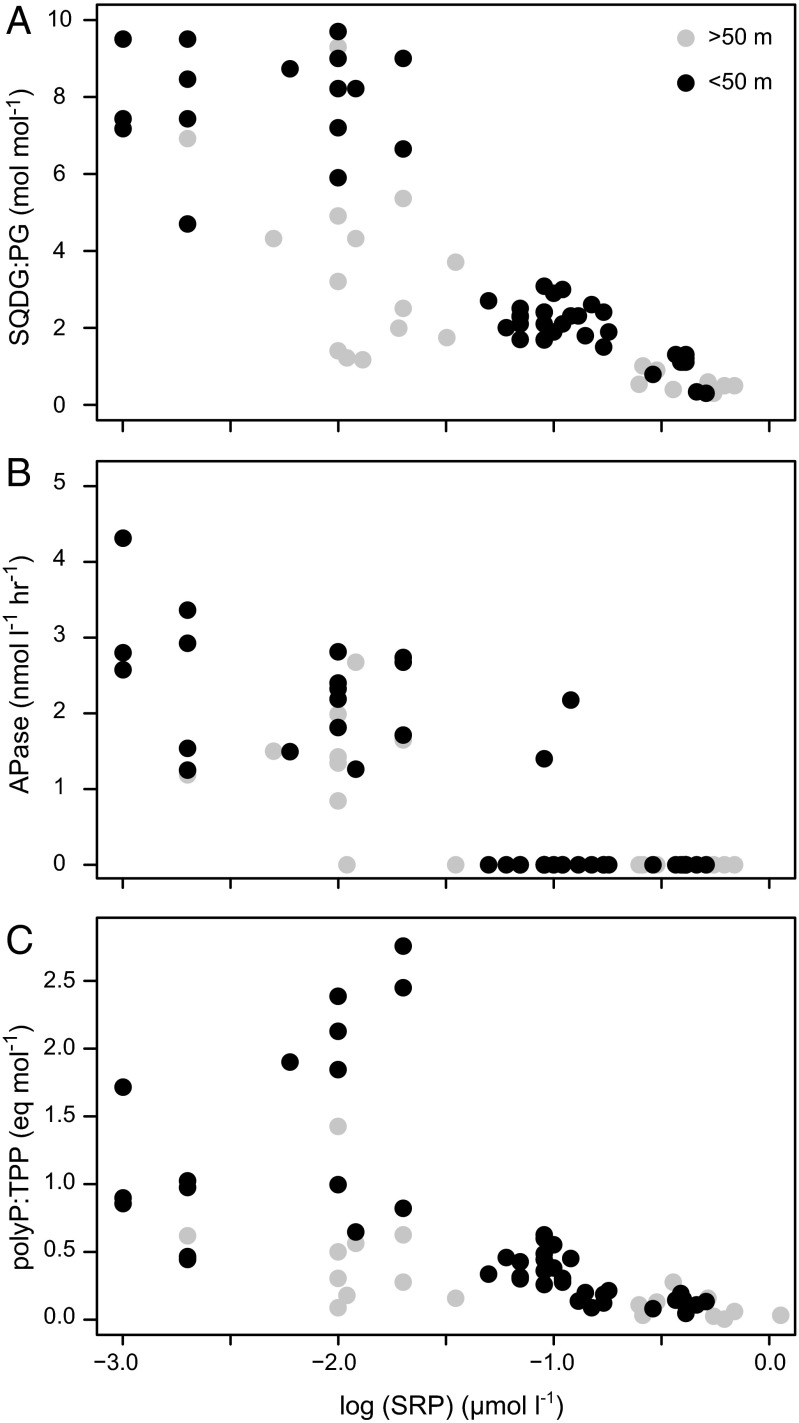
The polyP:TPP ratio was approximately fivefold higher in the Sargasso Sea than in the TWNA (Fig. 1 and Table S1). In the upper 50 m across the entire transect, polyP:TPP was inversely correlated with SRP concentration, increasing sharply below ∼25 nmol⋅L−1 SRP (Fig. 2) (Spearman’s rho ≥0.80, P < 0.001). Similar correlations with SRP were observed for SQDG:PG and APase on both cruises (Fig. 2 and Fig. S1) (Spearman’s rho ≥0.80, P < 0.001). The polyP:TPP therefore covaries with known responses to P stress, such that the latitudinal gradients in polyP:TPP, SQDG:PG, APase, and SRP divide the western North Atlantic into two biogeochemical provinces, characterized by distinct regimes of phytoplankton physiology and P cycling.
Fig. 2.
Cruise data from Fig. 1 plotted against soluble reactive phosphorus (SRP) on a log scale. (A) The molar sulfolipid-to-phospholipid ratio (SQDG:PG), (B) alkaline phosphatase activity (APase), and (C) polyP-to-total particulate P ratio (polyP:TPP) all increased sharply below ∼25 nmol⋅L−1 SRP. Note that the trends were most pronounced in the upper 50 m; for all three panels, Spearman’s rho ≥0.80, P < 0.001.
The latitudinal gradient in polyP:TPP was driven by ninefold lower TPP concentrations in the Sargasso Sea versus the TWNA, consistent with recent reports (13) whereas polyP concentrations were less than twofold lower (Fig. S2 and Table S1).This gradient indicates that phytoplankton experiencing P stress in the Sargasso Sea preferentially maintain polyP over other cellular P reservoirs. In contrast, lipid P was 10-fold lower, 15 ± 1 nmol⋅L−1 versus 1.3 ± 0.17 nmol⋅L−1, effecting a shift from 11% of the TPP to 8.4% of TPP (Table S1). However, based on our measured C:P ratios (TWNA, 106 ± 11; Sargasso Sea, 200 ± 33) and TPP concentrations, we estimate that P-sparing strategies collectively save 13 nmol⋅L−1 TPP, but that phospholipid substitution accounted for only 14% of this saving (Table S1 and SI Materials and Methods). Therefore, predominant strategies for reducing cellular P probably involve DNA (e.g., “genome streamlining”) (24), RNA (e.g., slower growth) (25), or other P-containing biochemicals whereas polyP cannot be spared beyond a certain extent. Indeed, polyP is intimately linked to primary metabolism in both eukaryotes and prokaryotes such that the inability to synthesize polyP results in a diverse range of cellular defects (26, 27).
Deep chlorophyll maxima (DCM) were found in both the TWNA (25–40 m) and the Sargasso Sea (60–100 m) (Fig. S2). In both regions, the upper part of the DCM (TWNA, 30 m; Sargasso Sea, 65 m) contained levels of polyP, TPP, and polyP:TPP ratios similar to surface values whereas the base of the DCM (TWNA, 40 m; Sargasso Sea, 90 m) contained severalfold less polyP, TPP, and much lower polyP:TPP ratios (Fig. 1 and Fig. S2). This difference might indicate a shift away from autotrophic biomass in the total particle pool at the deepest extent of the euphotic zone. In the Sargasso Sea, the higher polyP:TPP at the base of the DCM might also reflect the physiological response of phytoplankton to a supply of SRP via diapycnal mixing across the phosphocline; SRP was indeed slightly elevated at depth in this region (usually ≥10 nmol⋅L−1). Thus, the vertical gradient in polyP:TPP and SRP is similar to the latitudinal gradient in surface waters.
Physiological Shifts in polyP:TPP in Field and Cultured Populations of Synechococcus.
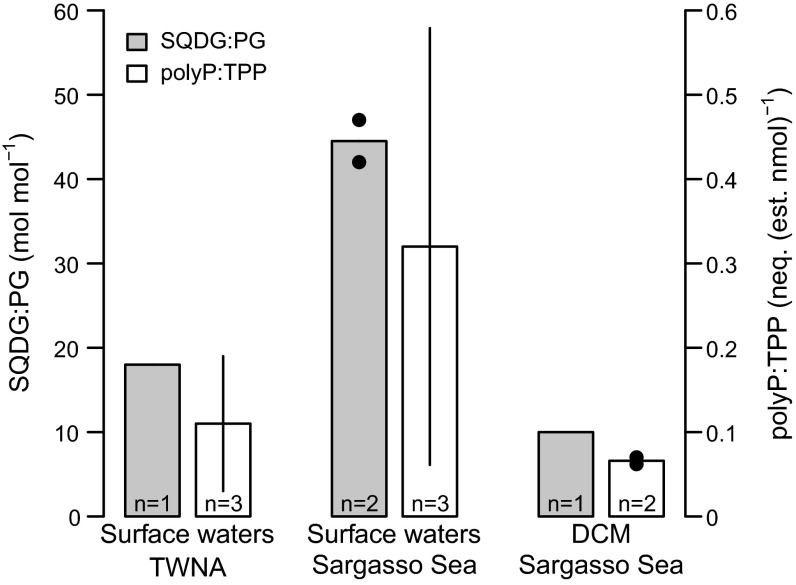
The polyP:TPP gradient in surface waters is not solely due to taxonomic shifts. Using cell-sorting flow cytometry, natural populations of Synechococcus, one of the dominant taxa throughout the transect, were isolated for biochemical analysis. SQDG:PG was greater than twofold higher in cells from surface waters of the Sargasso Sea relative to surface TWNA waters, but SQDG:PG was low in cells in the Sargasso Sea DCM (Fig. 3). Although absolute concentrations of polyP in Synechococcus did not differ latitudinally, we estimate, using Synechococcus P quotas reported from the region, that polyP:TPP was approximately threefold higher in cells sorted from the Sargasso Sea (28) (Fig. 3). This difference in polyP:TPP cannot be statistically resolved because of the low sample sizes and high variability inherent to isolating a single genus from a mixed community. Nevertheless, the trend of higher polyP:TPP in Synechococcus mimics the latitudinal gradient, underscoring the physiological underpinnings of the two biogeochemical provinces.
Fig. 3.
Sulfolipid-to-phospholipid ratio (SQDG:PG) and estimated polyP-to-total particulate P ratio (polyP:TPP) in Synechococcus cells isolated by cell-sorting flow cytometry. Mean ± SD are shown for cases where n = 3, and mean and range are shown for cases where n = 2. To estimate polyP:TPP, we used the Synechococcus cellular P quotas reported from the subtropical western North Atlantic on a previous cruise (28); for polyP measurements in the Sargasso Sea, we used the average cellular P quotas from inside an anticyclonic and a cyclonic eddy (26.5 amol P⋅cell−1) whereas, for TWNA data, we used the higher quota reported from inside a mode water eddy (50.8 amol P⋅cell−1). A twofold difference in cellular P quota between P-starved and P-replete cells is consistent with results obtained in laboratory Synechococcus cultures (50).
Elevated polyP:TPP ratios may be common in planktonic microbes from the Sargasso Sea: whole-community copy numbers of polyP-related genes detected in marine metagenomic surveys were higher in low SRP settings (29). Using 31P NMR, the cyanobacterium Trichodesmium was shown to increase polyP:TPP from 0.2% in P-replete cultures to 16% in P-starved cultures, and polyP:TPP ratios in Trichodesmium colonies collected from the Sargasso Sea were similarly high (8.0–25%) (30). Furthermore, polyP:TPP also increased sevenfold in P-stressed diatom batch cultures (31), consistent with the fivefold difference in polyP:TPP of bulk particles we report here between the TWNA and the Sargasso Sea.
Phytoplankton might either depend on permanently high relative polyP quotas for critical physiological functions, perhaps linked to the P-stress response, analogous to bacterial cultures (15–17). Alternatively, if SRP fluctuates rapidly in the Sargasso Sea, cells might be in a perpetual overplus state. To test these two ideas, surface water from the Sargasso Sea was incubated in bottles spiked with 200 nmol⋅L−1 SRP. With the SRP addition, Synechococcus cells increased their polyP content approximately twofold (22 and 33 attoequivalents polyP⋅cell−1 versus 8.5 and 17 attoequivalents⋅cell−1 in control incubations; both n = 2), which is consistent with an overplus response. This response would be unlikely if polyP were already at overplus levels and thus is suggestive of P stress, not chronic overplus in situ. Culture experiments with Synechococcus WH8102 confirmed that cells are capable of the overplus response (Fig. S3), but polyP:TPP actually decreased upon addition of P, unlike the high values found in the Sargasso Sea. In the experimental cultures, polyP per cell decreased by 20% upon P stress, consistent with some of the polyP acting as a luxury store. However, the elevated polyP:TPP and elevated SQDG:PG in P-stressed cultures matched patterns observed in the Sargasso Sea whereas the patterns from replete and overplus cultures did not (32) (Fig. 4 and Fig. S3). Thus, both field and culture observations from Synechococcus appear to discount a polyP overplus response. The modest net polyP breakdown upon P-stress might mask substantial intracellular recycling: cells have multiple polyP pools with distinct physiological functions and dynamics (33, 34), and enzymes to synthesize and degrade polyP are both up-regulated in P-stressed cultures (31). Moreover, P-stressed Trichodesmium cultures have polyP:TPP ratios indistinguishable from those of natural Sargasso Sea Trichodesmium populations (30), which is consistent with our Synechococcus observations.
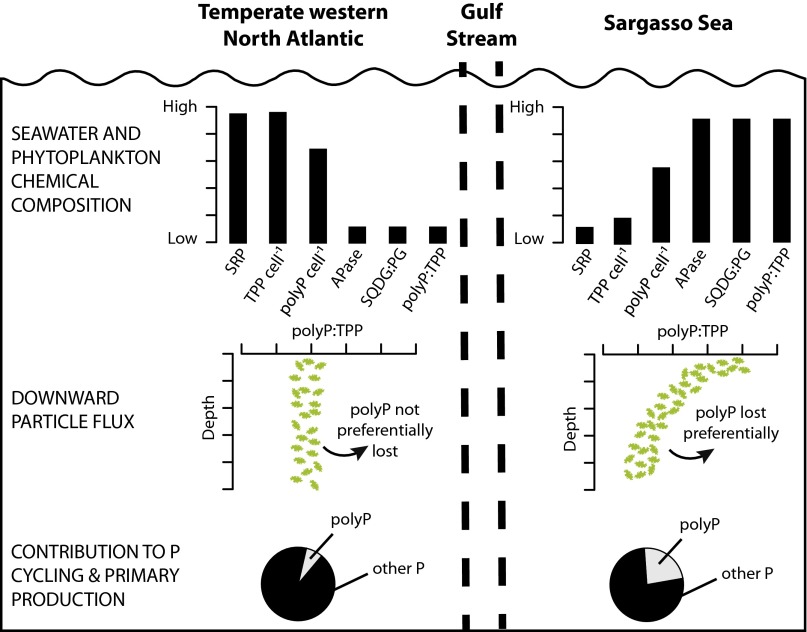
Fig. 4.
Conceptual model highlighting the biogeochemical differences between the two North Atlantic provinces in phytoplankton phosphorus physiology. In the temperate western North Atlantic, high external P concentrations provide enough P for cells to synthesize phospholipids, carry high total intracellular P quotas, and high polyphosphate (polyP) levels due to luxury storage. However, polyP is overall a modest component of total cellular P. As dead particles sink out of the euphotic zone and are degraded and reprocessed by microbes and zooplankton, polyP is lost in proportion to total P, so the polyP:TPP ratio does not change with depth. Consequently, the contribution of polyP to overall P cycling and primary production is minor. In the subtropical western North Atlantic (Sargasso Sea), ultra-low ambient P concentrations induce a cellular P-stress response, leading to substitution of non-P lipids for phospholipids, production of the alkaline phosphatase enzyme, a strong reduction in total cellular P, but retention of a significant polyP pool. Sinking particles preferentially lose polyP relative to total P, possibly due to preferential remineralization, allowing P from polyP to be preferentially retained in the upper ocean. Consequently, polyP is a significant, previously unrecognized pool of bioavailable P in this oligotrophic system, which likely supports a larger fraction of primary production and carbon export than in nutrient-replete waters.
PolyP has many functions in addition to its role in P homeostasis, including metal detoxification and energy storage. PolyP forms complexes with metal cations and was implicated in luxury iron storage in a diatom (35). However, although polyP can help detoxify metals, in which case polyP is broken down and metal–phosphate complexes transported out of the cell (36), a role in metal storage remains entirely speculative, and it seems unlikely that microbes in the dust-rich Sargasso Sea would use the very scarce P for the primary purpose of maintaining a luxury store of metals. PolyP can be used by some microbes as an energy store in sub- and anoxic environments (37, 38), but, in these settings, >10% of the organism’s dry weight is in the form of solid polyP granules (39, 40), unlike along our transect. The far more modest polyP levels seen during P stress or stationary phase could only cover a cell’s energy needs for timescales of seconds (41). Although it may be difficult to disentangle the effects of P stress and stationary-phase physiology on polyP accumulation in laboratory batch cultures, stationary phase is unlikely to be involved in polyP accumulation in the field because phytoplankton growth rates are not markedly reduced in the Sargasso Sea (42). Overall, the polyP:TPP gradient appears to be more strongly linked to the SRP gradient than other potential controlling factors.
Fate of PolyP and Significance for P and N Cycling.
To assess the biogeochemical fate of polyP in the western North Atlantic, TPP and polyP were measured in sinking particles collected in sediment traps deployed at 150 m for three successive 24-h periods in both the TWNA and the Sargasso Sea. In the TWNA, polyP:TPP values for sinking and for bulk surface particles were indistinguishable (0.34 ± 0.05 versus 0.39 ± 0.17 neq⋅nmol−1, t = 0.85, df = 11.6, P = 0.412). In contrast, in the Sargasso Sea, polyP:TPP was significantly lower in sinking particles than in bulk surface particles (0.69 ± 0.21 versus 2.0 ± 0.68 neq⋅nmol−1, t = 4.52, df = 7.8, P = 0.002). Although our sediment-trap observations are limited in time and space, the data indicate that polyP produced by P-stressed Sargasso Sea phytoplankton is discriminated against during the biogeochemical processing of living biomass into sinking particles captured by our sediment traps at 150 m, and thus P from polyP is preferentially retained in shallower waters in the Sargasso Sea (Fig. 4). The exact mechanisms behind this discrimination are not known. However, because the majority of phytoplankton biomass is remineralized before it escapes surface waters as sinking particles, we speculate that regulation of hydrolytic enzyme activities, such as APase, by external dissolved P concentrations might effectively prime the microbial community for preferential polyP recycling in low-P surface waters.
The flux of TPP in sediment traps in the Sargasso Sea has increased markedly over the last decade (43), which may potentially indicate changing annual patterns in P export relative to other systems. Consistent with this observation, our sediment-trap data suggest that a greater fraction of the surface pools of TPP and polyP were exported in the Sargasso Sea than in the TWNA (Table S1). However, we estimate that the reduction in polyP:TPP between the surface and the sediment traps in the Sargasso Sea reduced by a factor of three the percentage of the polyP stock exported (SI Materials and Methods); thus, preferential polyP recycling mitigates TPP export in the Sargasso Sea to a significant degree. Preferential retention of polyP in surface water provides an additional potential explanation for why relative nitrate-to-phosphate ratios (the so-called N* tracer) increase with depth in the Sargasso Sea, which cannot be accounted for solely by nitrogen fixation (44).
The sediment trap results also suggest considerable variation across different ocean provinces in the extent to which polyP is either recycled in surface waters or exported to depth. Further work is required to assess preferential polyP remineralization in the deep-sea and in marine sediments, which, if confirmed, has the potential to impact water-column phosphorus inventories and nutrient stoichiometries on global scales, particularly because export of polyP to sediments can promote long-term P burial by promoting apatite formation (11).
Dissolved polyP is an inorganic polymer but would nonetheless compose part of the operationally defined dissolved organic P (DOP) pool (45). If intact polyP is preferentially released from sinking particles into surface waters, then polyP may contribute more to DOP in the Sargasso Sea than the ∼10% reported from P-replete regions (11, 12, 46). Dissolved polyP, but not all DOP, is readily bioavailable to phytoplankton (12, 47), and most primary production in the Sargasso Sea is based on nutrient recycling and DOP utilization (4, 43). As polyP is apparently both required for surviving chronic P-stress and rapidly recycled in P-depleted surface waters, we speculate that polyP forms the basis for a feedback loop that retains P in surface waters, rendering growth limitation by P less likely and maintaining high fluxes of nitrogen and carbon export per unit P via the biological pump. Our data are beginning to shed light on the long-standing question (20, 48) of whether polyP plays a biogeochemically significant role in oligotrophic ocean regions.
Materials and Methods
Full methods are described in SI Materials and Methods. Particles were collected with Niskin bottles and wide-aperture net sediment traps between Woods Hole and Bermuda and filtered onto GF/F filters. Lipids were analyzed by liquid chromatography mass spectrometry using authentic standards after solvent extraction (49); we report the ratio of sulfoquinovosyldiacylglycerol (SQDG) to phosphatidylglycerol (PG). Total particulate phosphorus was quantified using the molybdate blue method after K2S2O8 wet oxidation. Particulate organic carbon was determined on a stable isotope ratio mass spectrometer after fuming the samples with concentrated hydrochloric acid. PolyP was determined fluorometrically after boiling and enzymatic digestion, accounting for background fluorescence and matrix effects (23). Comparison with a synthetic polyP standard allows relative quantification expressed as nanoequivalents of P in the standard. A different enzymatic digestion based on snake-venom phosphodiesterase yielded identical polyP values (Fig. S4) whereas an NaOH leach gave lower absolute polyP values but the same latitudinal trend in polyP:TPP (Fig. S5). Alkaline phosphatase activity was measured in unfiltered seawater using the substrate 4-methylumbelliferyl phosphate (2012 cruise) and on filtered particles using the substrate 6,8-difluoro-4-methylumbelliferyl phosphate (2008 cruise). Soluble reactive phosphorus was measured using standard methods. Samples for cell-sorting flow cytometry were filtered, formaldehyde-fixed, and flash-frozen (32); after sorting, Synechococcus were filtered and flash-frozen. Additional particle samples for lipid analysis were collected on 0.2 μm PVDF filters; SQDG:PG ratios in PVDF-filtered samples were converted to GF/F equivalent values using the relationship in Fig. S6 (SI Materials and Methods). Synechococcus WH8102 was grown in SN medium using standard methods. Samples for chemical analyses and cell counts (flow cytometry) were taken daily once fluorescence in P-limited cultures stopped increasing. P was resupplied to Refeed cultures to 45 µmol⋅L−1 24 h before final sampling.
Supplementary Material
Acknowledgments
Constructive criticism by two anonymous reviewers is gratefully acknowledged. Justin Ossolinski, Suni Shah, James Collins, Louie Wurch, and Emily Peacock helped with sample collection and analyses at sea; Kimberly Popendorf, Helen Fredricks, Jamey Fulton, Jeremy Tagliaferre, Abby Heithoff, and Bethanie Edwards assisted with laboratory analyses; and Monica Rouco-Molina and Athena Aitcher helped with Synechococcus cultures. Kathy Krogslund conducted nutrient analyses and Carl Johnson measured particulate organic carbon. We thank Julia Diaz for discussions about polyP. This work was supported by a Doherty Postdoctoral Scholarship (to P.M.) and by National Science Foundation Grants OCE-1031143 (to B.A.S.V.M.), OCE-1059582 (to S.T.D.), and OCE-1045966 (to M.W.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7890.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321719111/-/DCSupplemental.
References
- 1.Van Mooy BAS, et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature. 2009;458(7234):69–72. doi: 10.1038/nature07659. [DOI] [PubMed] [Google Scholar]
- 2.Moore CM, et al. Processes and patterns of oceanic nutrient limitation. Nat Geosci. 2013;6:701–710. [Google Scholar]
- 3.Mills MM, Ridame C, Davey M, La Roche J, Geider RJ. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature. 2004;429(6989):292–294. doi: 10.1038/nature02550. [DOI] [PubMed] [Google Scholar]
- 4.Mather RL, et al. Phosphorus cycling in the North and South Atlantic Ocean subtropical gyres. Nat Geosci. 2008;1:439–443. [Google Scholar]
- 5.Lomas MW, et al. Sargasso Sea phosphorus biogeochemistry: An important role for dissolved organic phosphorus (DOP) Biogeosciences. 2010;7:695–710. [Google Scholar]
- 6.Duhamel S, Dyhrman ST, Karl DM. Alkaline phosphatase activity and regulation in the North Pacific Subtropical Gyre. Limnol Oceanogr. 2010;55(3):1414–1425. [Google Scholar]
- 7.Lomas MW, Swain A, Shelton R, Ammerman JW. Taxonomic variability of phosphorus stress in Sargasso Sea phytoplankton. Limnol Oceanogr. 2004;49(6):2303–2310. [Google Scholar]
- 8.Mazard S, Wilson WH, Scanlan DJ. Dissecting the physiological response to phosphorus stress in marine Synechococcus isolates (Cyanophyceae) J Phycol. 2012;48:94–105. doi: 10.1111/j.1529-8817.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 9.Riegman R, Stolte W, Noordeloos AAM, Slezak D. Nutrient uptake and alkaline phosphatase (EC 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. J Phycol. 2000;36:87–96. [Google Scholar]
- 10.Geider RJ, La Roche J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol. 2002;37:1–17. [Google Scholar]
- 11.Diaz JM, et al. Marine polyphosphate: A key player in geologic phosphorus sequestration. Science. 2008;320(5876):652–655. doi: 10.1126/science.1151751. [DOI] [PubMed] [Google Scholar]
- 12.Solórzano L, Strickland JDH. Polyphosphate in seawater. Limnol Oceanogr. 1968;13(3):515–518. [Google Scholar]
- 13.Martiny AC, et al. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat Geosci. 2013;6:279–283. [Google Scholar]
- 14.Martin P, Van Mooy BAS, Heithoff A, Dyhrman ST. Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J. 2011;5(6):1057–1060. doi: 10.1038/ismej.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ault-Riché D, Fraley CD, Tzeng CM, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180(7):1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao NN, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J Bacteriol. 1996;178(5):1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiba T, et al. Inorganic polyphosphate and the induction of rpoS expression. Proc Natl Acad Sci USA. 1997;94(21):11210–11215. doi: 10.1073/pnas.94.21.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee G-Y. A continuous culture study of phosphate uptake, growth rate and polyphosphate in Scenedesmus sp. J Phycol. 1973;9:495–506. [Google Scholar]
- 19.Werner TP, Amrhein N, Freimoser FM. Novel method for the quantification of inorganic polyphosphate (iPoP) in Saccharomyces cerevisiae shows dependence of iPoP content on the growth phase. Arch Microbiol. 2005;184(2):129–136. doi: 10.1007/s00203-005-0031-2. [DOI] [PubMed] [Google Scholar]
- 20.Karl DM, Björkman KM. Dynamics of DOP. In: Hansell DA, Carlson CA, editors. Biogeochemistry of Marine Dissolved Organic Matter. New York: Academic Press; 2002. pp. 249–366. [Google Scholar]
- 21.Kirchman DL, Keil RG, Wheeler PA. The effect of amino acids on ammonium utilization and regeneration by heterotrophic bacteria in the subarctic Pacific. Deep-Sea Res. 1989;36(11):1763–1776. [Google Scholar]
- 22.Lee S, Fuhrman JA. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol. 1987;53(6):1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin P, Van Mooy BAS. Fluorometric quantification of polyphosphate in environmental plankton samples: Extraction protocols, matrix effects, and nucleic acid interference. Appl Environ Microbiol. 2013;79(1):273–281. doi: 10.1128/AEM.02592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannoni SJ, et al. Genome streamlining in a cosmopolitan oceanic bacterium. Science. 2005;309(5738):1242–1245. doi: 10.1126/science.1114057. [DOI] [PubMed] [Google Scholar]
- 25.Elser JJ, et al. Growth rate–stoichiometry couplings in diverse biota. Ecol Lett. 2003;6:936–943. [Google Scholar]
- 26.Fraley CD, et al. A polyphosphate kinase 1 (ppk1) mutant of Pseudomonas aeruginosa exhibits multiple ultrastructural and functional defects. Proc Natl Acad Sci USA. 2007;104(9):3526–3531. doi: 10.1073/pnas.0609733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freimoser FM, Hürlimann HC, Jakob CA, Werner TP, Amrhein N. Systematic screening of polyphosphate (poly P) levels in yeast mutant cells reveals strong interdependence with primary metabolism. Genome Biol. 2006;7(11):R109. doi: 10.1186/gb-2006-7-11-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twining BS, Nunez-Milland D, Vogt S, Johnson RS, Sedwick PN. Variations in Synechococcus cell quotas of phosphorus, sulfur, manganese, iron, nickel, and zinc within mesoscale eddies in the Sargasso Sea. Limnol Oceanogr. 2010;55(2):492–506. [Google Scholar]
- 29.Temperton B, Gilbert JA, Quinn JP, McGrath JW. Novel analysis of oceanic surface water metagenomes suggests importance of polyphosphate metabolism in oligotrophic environments. PLoS ONE. 2011;6(1):e16499. doi: 10.1371/journal.pone.0016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orchard ED, Benitez-Nelson CR, Pellechia PJ, Lomas MW, Dyhrman ST. Polyphosphate in Trichodesmium from the low-phosphorus Sargasso Sea. Limnol Oceanogr. 2010;55(5):2161–2169. [Google Scholar]
- 31.Dyhrman ST, et al. The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS ONE. 2012;7(3):e33768. doi: 10.1371/journal.pone.0033768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popendorf KJ, Lomas MW, Van Mooy BAS. Microbial sources of intact polar diacylglycerolipids in the Western North Atlantic Ocean. Org Geochem. 2011;42(7):803–811. [Google Scholar]
- 33.Kulaev I, Vagabov V, Kulakovskaya T. New aspects of inorganic polyphosphate metabolism and function. J Biosci Bioeng. 1999;88(2):111–129. doi: 10.1016/s1389-1723(99)80189-3. [DOI] [PubMed] [Google Scholar]
- 34.Kulaev IS, Vagabov VM, Kulakovskaya TV. The Biochemistry of Inorganic Polyphosphates. 2nd Ed. New York: Wiley; 2004. p. 277. [Google Scholar]
- 35.Nuester J, Vogt S, Twining BS. Localization of iron within centric diatoms of the genus Thalassiosira. J Phycol. 2012;48(3):626–634. doi: 10.1111/j.1529-8817.2012.01165.x. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez S, Jerez CA. Copper ions stimulate polyphosphate degradation and phosphate efflux in Acidithiobacillus ferrooxidans. Appl Environ Microbiol. 2004;70(9):5177–5182. doi: 10.1128/AEM.70.9.5177-5182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comeau Y, Hall KJ, Hancock REW, Oldham WK. Biochemical model for enhanced biological phosphorus removal. Water Res. 1986;20(12):1511–1521. [Google Scholar]
- 38.Schulz HN, Schulz HD. Large sulfur bacteria and the formation of phosphorite. Science. 2005;307(5708):416–418. doi: 10.1126/science.1103096. [DOI] [PubMed] [Google Scholar]
- 39.Kortstee GJ, Appeldoorn KJ, Bonting CF, van Niel EW, van Veen HW. Recent developments in the biochemistry and ecology of enhanced biological phosphorus removal. Biochemistry (Mosc) 2000;65(3):332–340. [PubMed] [Google Scholar]
- 40.Mullan A, Quinn JP, McGrath JW. Enhanced phosphate uptake and polyphosphate accumulation in Burkholderia cepacia grown under low pH conditions. Microb Ecol. 2002;44(1):69–77. doi: 10.1007/s00248-002-3004-x. [DOI] [PubMed] [Google Scholar]
- 41.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: A molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 42.Marañón E. Phytoplankton growth rates in the Atlantic subtropical gyres. Limnol Oceanogr. 2005;50(1):299–310. [Google Scholar]
- 43.Lomas MW, et al. Two decades and counting: 24-years of sustained open ocean biogeochemical measurements in the Sargasso Sea. Deep Sea Res Part II Top Stud Oceanogr. 2013;93:16–32. [Google Scholar]
- 44.Singh A, Lomas MW, Bates NR. Revisiting N2 fixation in the North Atlantic Ocean: Significance of deviations from the Redfield Ratio, atmospheric deposition and climate variability. Deep Sea Res Part II Top Stud Oceanogr. 2013;93:148–158. [Google Scholar]
- 45.Karl DM, Tien G. MAGIC: A sensitive and precise method for measuring dissolved phosphorus in aquatic environments. Limnol Oceanogr. 1992;37(1):105–116. [Google Scholar]
- 46.Young CL, Ingall ED. Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis. Aquat Geochem. 2010;16:563–574. [Google Scholar]
- 47.Moore LR, Ostrowski M, Scanlan DJ, Feren K, Sweetsir T. Ecotypic variation in phosphorus-acquisition mechanisms within marine picocyanobacteria. Aquat Microb Ecol. 2005;39:257–269. [Google Scholar]
- 48.Karl DM. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu Rev Mar Sci. 2014;6(1):279–337. doi: 10.1146/annurev-marine-010213-135046. [DOI] [PubMed] [Google Scholar]
- 49.Popendorf KJ, Fredricks HF, Van Mooy BAS. Molecular ion-independent quantification of polar glycerolipid classes in marine plankton using triple quadrupole MS. Lipids. 2013;48(2):185–195. doi: 10.1007/s11745-012-3748-0. [DOI] [PubMed] [Google Scholar]
- 50.Bertilsson S, Berglund O, Karl DM, Chisholm SW. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol Oceanogr. 2003;48:1721–1731. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.