Significance
Elevated CO2 combined with globally warm temperatures in the Eocene make its climate ideal for understanding modern global warming and its biotic consequences. Globally low temperature seasonality—the relationship between winter and mean annual temperatures—has been proposed as key to differential Eocene biodiversity and community patterns. Palms are important winter temperature indicators by their sensitivity to frost; however, their presence in paleocommunities may be masked by taphonomic constraints and identification difficulties. We used fossil obligate palm-feeding beetles to establish the presence of palms in a cool upland in midlatitude western North America. In this way, we provide a more precise characterization of climate during an important interval of the emergence of modern ecosystems.
Keywords: palm bruchines, paleoclimate, Okanagan Highlands, palm pollen
Abstract
Eocene climate and associated biotic patterns provide an analog system to understand their modern interactions. The relationship between mean annual temperatures and winter temperatures—temperature seasonality—may be an important factor in this dynamic. Fossils of frost-intolerant palms imply low Eocene temperature seasonality into high latitudes, constraining average winter temperatures there to >8 °C. However, their presence in a paleocommunity may be obscured by taphonomic and identification factors for macrofossils and pollen. We circumvented these problems by establishing the presence of obligate palm-feeding beetles (Chrysomelidae: Pachymerina) at three localities (a fourth, tentatively) in microthermal to lower mesothermal Early Eocene upland communities in Washington and British Columbia. This provides support for warmer winter Eocene climates extending northward into cooler Canadian uplands.
Eocene climates have received intense scrutiny in recent decades, providing an increasingly detailed analog system with which to assess the dynamics of modern global climate change, thereby allowing prediction of its long-term trends and biotic consequences. It was a time of increased global temperatures associated with elevated atmospheric carbon levels, particularly during episodic hyperthermal events such as the Early Eocene Climatic Optimum (EECO) (1, 2). The Eocene was also notable for greater climatic homogeneity, with low latitudinal temperature gradients coupled with low temperature seasonality extending to the poles, creating a global climate type unknown today outside of restricted regions. This is thought to have had far-ranging biological consequences, e.g., for intercontinental dispersal, global biodiversity patterns, community structures, and evolutionary trajectories (e.g., refs. 2–6). Here, we use the presence of insects that have obligate associations with floral climatic indicators to refine characterization of winter climate in a cool higher midlatitude upland during the EECO, and therefore the environmental context of these biotic patterns during an interval of global warmth.
A key factor in these dynamics may have been warmer winter temperatures [coldest month mean temperature (CMMT)] relative to mean annual temperature (MAT) even in mid- and higher latitude regions of cooler MAT (3). Consequently—as in the modern tropics and some southern hemisphere midlatitude coastal areas—Eocene organisms across the globe would not have been burdened by the necessity to allocate resources to endure hostile extreme winter climates with its costs for metabolic and insulation adaptations, or escape its effects by an annual period of dormancy or expenditure of large amounts of energy in migration. This would allow the potential of near year-round reproduction, feeding, and growth, providing an explanatory context for observed differential Eocene diversity patterns. In the geologically brief time scale of our modern interval of global climate change, the partial release of such constraints by warming winters is seen to have had a large influence on natural communities, with resulting high economic and social impact, e.g., the current dramatic mountain pine beetle infestations of western North America (7, 8).
Palms as Indicators of Winter Temperatures
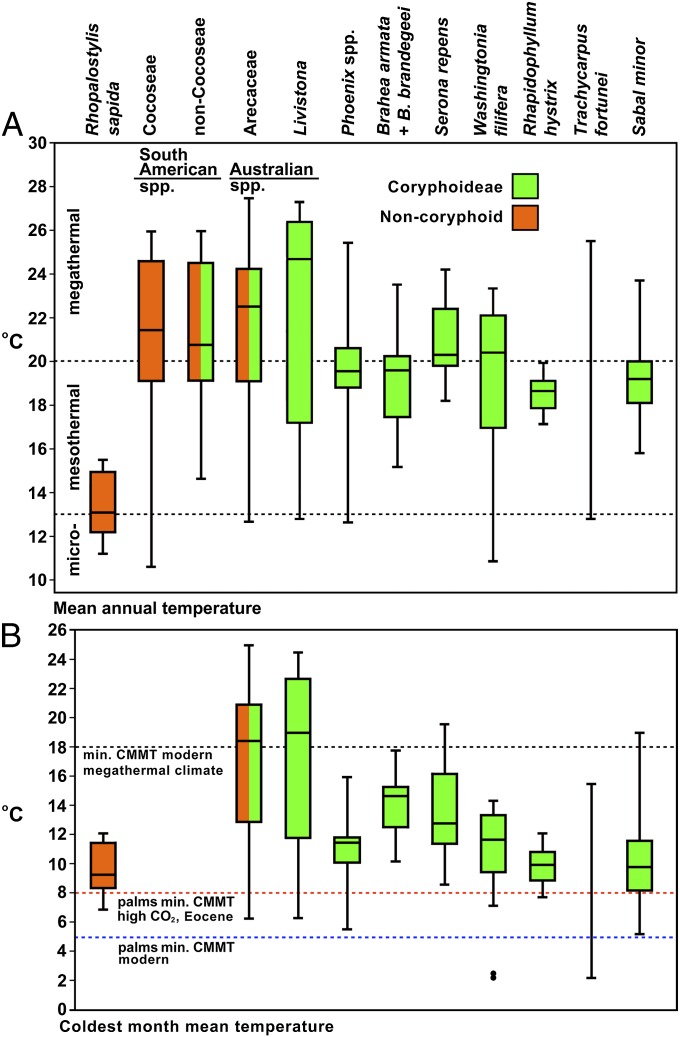
Eocene temperature seasonality has been characterized using proxy thermometers such as palms (Arecaceae), whose seeds and seedlings cannot survive sustained freezing, today limiting their natural distribution to regions of CMMT >5 °C (3, 9), although some palms may tolerate CMMT ∼2.5 °C (Fig. 1). Freezing tolerance is reduced under high atmospheric CO2, so Eocene records of palms may indicate CMMT >8 °C (10, 11).
Fig. 1.
Climatic profile of palms (median, quartile, and maximum and minimum values), with an emphasis on cold tolerant clades (principally Coryphoids). (A) MAT profiles of selected extant palm taxa. (B) CMMT profiles of selected extant palm taxa with key thresholds for megathermal (tropical) climates and for palms: CMMT >5 °C for modern palms (with few exceptions: Two outlier occurrences shown as dots are for W. filifera) (3); CMMT >8 °C for palms under Eocene levels of atmospheric CO2 (10). Systematics follows Baker et al. (43).
Consequently, the overwhelming greatest diversity of genera and species of palms is now restricted to the tropics, with only a few genera occurring outside of it and the subtropics (12). The most cold-tolerant of all living palms, inhabiting the limits of this range, are almost exclusively members of the tribes Trachycarpeae (e.g., Rhapidophyllum hystrix, Serenoa repens, Trachycarpus fortunei, and Washingtonia filifera), Cryosophileae (e.g., Trithrinax spp.), and Sabaleae (i.e., Sabal minor), all in the subfamily Coryphoideae, with only the New Zealand arecoid palm Rhopalostylis sapida matching their cold-tolerance (Fig. 1).
In the Eocene, the range of palms extended into regions of cooler MAT well outside their modern latitudinal limits (3, 11, 13, 14), supporting the reconstruction of globally more equable climates, i.e., milder winters in cooler regions. For example, in the Early Eocene Okanagan Highlands series of montane localities of far-western North America (Fig. 2), the vegetative organs of the extinct coryphoid palm Uhlia allenbyensis are common in the Princeton Chert (Allenby Formation, BC, Canada), with their anatomy indicating a close affinity to the modern Coryphoideae genera Brahea, Rhapidophyllum, and Serenoa of the tribe Trachycarpeae (15). Palm macrofossils have not been reported from any of the other Okanagan Highlands Eocene floras (16, 17).
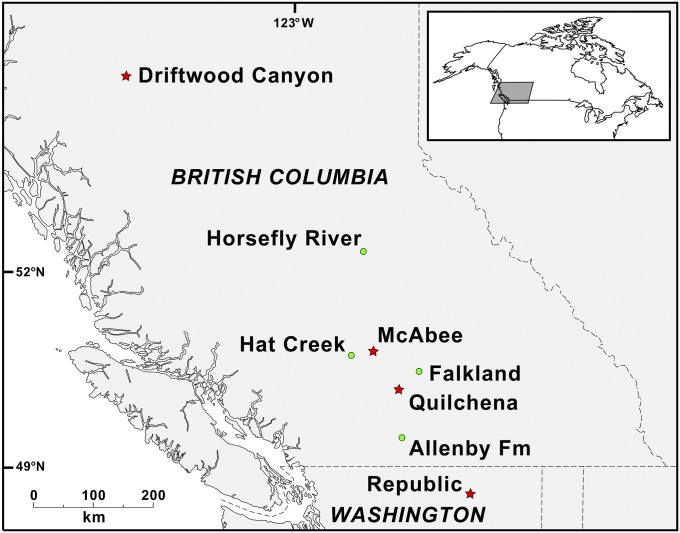
Fig. 2.
Map of Early Eocene Okanagan Highland fossil sites. Red stars represent beetle sites: Republic, Quilchena, and McAbee, with confirmed Pachymerina; Republic and McAbee, including beetles assigned to Caryobruchus + Speciomerus; and Driftwood Canyon with likely Pachymerina. Green dots represent other Okanagan Highlands sites: Allenby Formation with palm macrofossils in chert, Uhlia allenbyensis (15); putative palm pollen reported from the Allenby Formation, Quilchena, Falkland, McAbee, Hat Creek, and Driftwood Canyon (for Quilchena, see Palms as Indicators of Winter Temperatures; for others, see ref. 18 and references therein). No evidence of palms has been reported from Okanagan Highlands localities at Republic or Horsefly River.
Palm pollen (as Sabal granopollenites) has been reported in the Allenby Formation and other Okanagan Highlands sites at Driftwood Canyon, Falkland, Hat Creek, and McAbee (18, 19), although not at others of this series, e.g., Quilchena or Republic. Searches of Quilchena slides prepared by R.W.M. have so far identified only one possible palm pollen grain (Fig. 3) of the Sabal type; it is, however, larger (length = 40 µm) than the size range defined by Rouse (20) (28–32 µm) for S. granopollenites, and should be referred to Liliacidites (21). Harley and Baker (22, 23) reviewed the record of fossil palm-like pollen and express caution in identification, based on its similarity to nonpalm monosulcate monocots and some fossil form genera of nonangiosperm origin. Evidence other than pollen is needed to confirm that palms were distributed widely in the Eocene Okanagan Highlands.
Fig. 3.
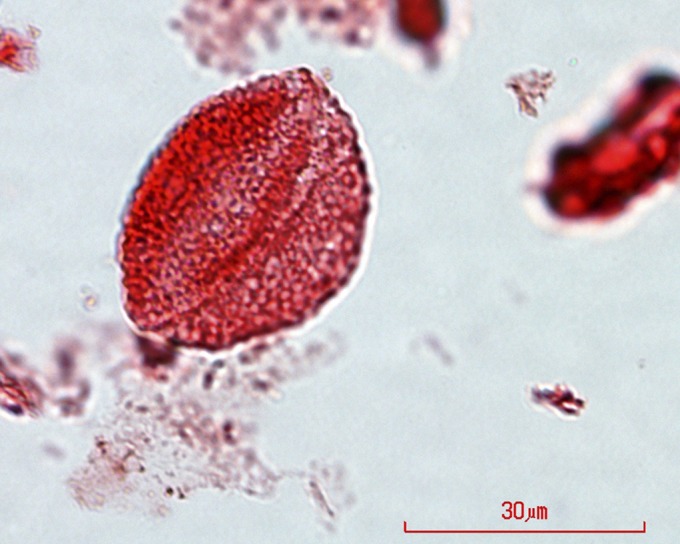
Fossil palm-like pollen. Early Eocene pollen from the Quilchena (BC, Canada) locality of the Okanagan Highlands resembling palm pollen, but assigned to Liliacidites.
The lack of palm macrofossils from any Okanagan Highlands locality other than the Princeton Chert may reflect that the others are all lacustrine shale localities (except Hat Creek: coal), which might at least in part explain this absence by taphonomic bias. The Chert preserves an in situ aquatic community, and all of the vegetative organs of U. allenbyensis are preserved. In lake sediments, however, the persistence of leaves on the trunk after death in many low-statured monocot plants, including coryphoid palms such as most S. minor, S. repens, and to a limited extent Trachycarpus spp., greatly restrict leaf fossilization (24). U. allenbyensis is reconstructed as a low-statured rhizomatous plant similar in ecology to S. repens (15) (Fig. S1). Palms were only recently confirmed at the Florissant locality (Colorado: late Eocene, lacustrine shale) by a single coryphoid palm leaf; long-standing fruit and pollen evidence had been considered controversial (25).
Palm Beetles
Here, we evaluate the Eocene upland winter temperature in the Okanagan Highlands using fossil palm bruchines, beetles of the obligate palm-feeding chrysomelid subtribe Pachymerina as an indicator for the presence of palms where direct macrofossil evidence may be obscured by these factors. In the Eocene, the tribe Pachymerini (bruchines, which includes legume as well as palm feeders) is known from the Early Eocene Okanagan Highlands locality at Quilchena, BC (26), the late Eocene Florissant (27), and an apparently coeval locality in Primorye, Pacific coastal Russia (28); all tentatively assumed to either belong to legume feeding taxa or not associated with host plants. Here, we reexamine the Quilchena beetle and eight new fossils from Okanagan Highlands localities at Driftwood Canyon and McAbee, BC; and Republic, WA (Figs. 2, 4, and 5).
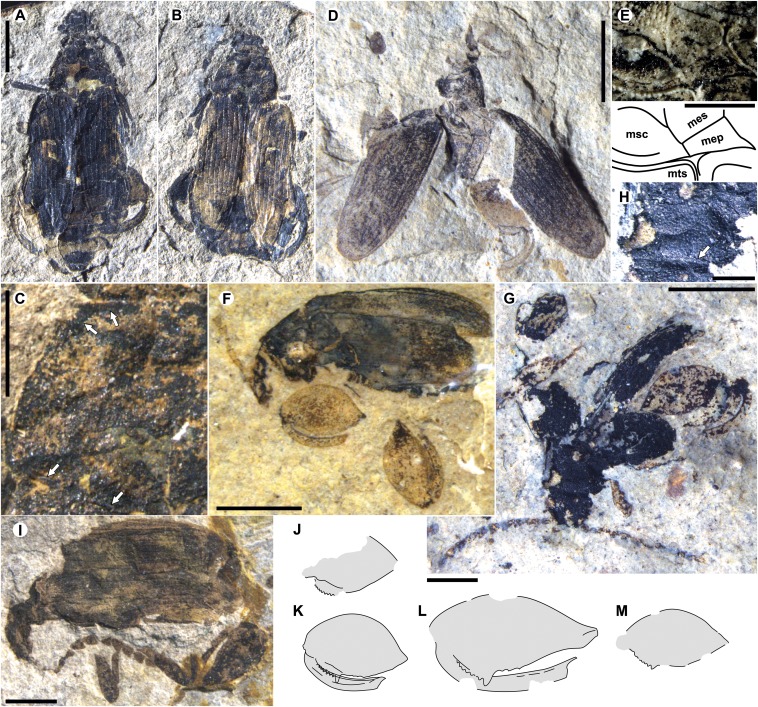
Fig. 4.
Quilchena, Republic, and Driftwood Canyon Pachymerina. Specimens from (A–C) Quilchena, (D–H, J, K, and M) Republic, and (I and L) Driftwood Canyon. (A) Q-0061a part and (B) counterpart. (C) Q-0061b, showing impressed marginal line (arrows) surrounding pronotum; (top) anterior margin. (D) SR 05-03-10. (E) Detail (photograph and drawing) of SR 05-03-10 showing mesepimeron, extending and broad mesally. mep, mesepimeron; mes, mesopleuron; msc, mesocoxal cavity; mts, metasternum. (F) SR 99-78-71. (G) SRUI 00-94-80, (H) Detail showing impressed line on anterior margin (arrow). (I) RBCM.EH2013.031.0001.001. (J–M) Drawings of metafemora of SR 05-03-10, SR 99-78-71, RBCM.EH2013.031.0001.001, and SRUI 00-94-80 respectively. Metatibiae also on K and L. (Scale bars: 2 mm in A, D, F, G, and I; B is the same scale as in A; and 1 mm in C and J–M; 500 μm in E and H.) (E–G) Wetted with ethanol.
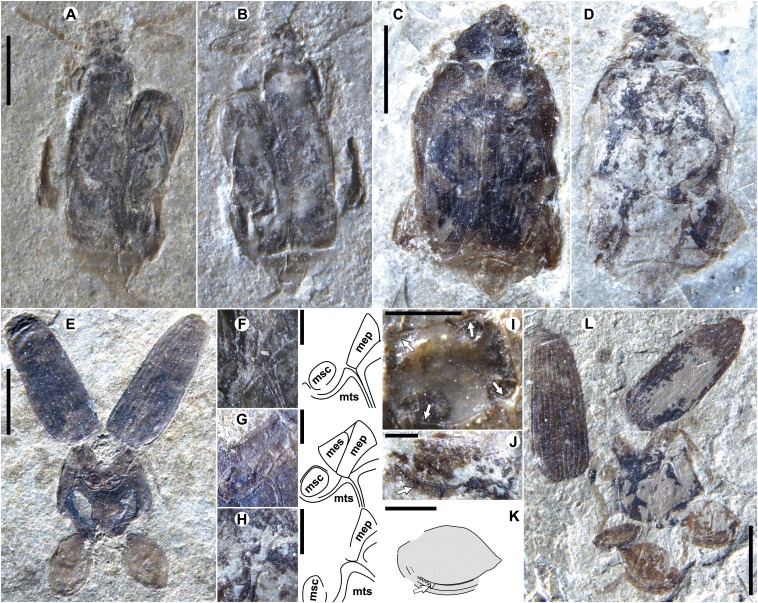
Fig. 5.
McAbee Pachymerina. (A) F-1542. (B) F-1543, counterpart of F-1542. (C) F-1540, showing dorsal morphology. (D) F-1541, counterpart of F-1540, showing ventral morphology. (E) F-939, ventral, pleural portions of the meso- and metathoracic segments, elytra (top) and hind legs (bottom) preserved. (F–H) Details (photographs and drawings) showing mesepimeron broad mesally (Fig. 4E and abbreviations therein) of F-1542, F-939, and F-1541 respectively. (I) F-1543 showing impressed marginal line (arrows) surrounding pronotum; (top) anterior margin. (J) Hind leg of F-1541, arrow shows position of denticle 1, distad midfemur. (K) Drawing of femur and tibia of F-1544, arrow indicating denticle 1; orientation as in J (distal femur to the left). (L) F-1544, preserved portions mostly as for E. (Scale bars: 2 mm in A, C, E, and L; B is the same scale as A and B, and D as C; 1 mm in I and K; and 500 μm in F–H and J.)
We assign these beetles to the Pachymerini by the following (see terminology in Materials and Methods; Figs. S2–S4): mesepimeral plate broadly joining the mesocoxa (plesiomorphic for bruchines) combined with metafemora incrassate, with pecten; metatibiae arcuate and mucronate, mucro wedge-shaped; and metacoxa about half-width of metafemur and first sternite (29, 30). We further determined them as palm bruchines, Pachymerina (Table 1 and Figs. 4 and 5), by the presence of either an impressed marginal line extending to the lateral/anterior margins of the pronotum, or broadly triangular tarsomeres one and two on the fore- and midlegs (27, 29, 30).
Table 1.
Fossil beetle diagnostic characters, localities, ages, and MAT values
| Locality | Specimen | Age, Ma | MAT, °C | MW | MP | IL | TM | PS | PT |
| Republic | 49.4 ± 0.5* | 13.5 ± 2.2† | |||||||
| SR 99-94-80 | X | X | |||||||
| SR 05-03-10 | X | X | |||||||
| SRUI 99-78-71 | X | X | X | ||||||
| Quilchena | 51.5 ± 0.4‡ | 14.8 ± 2.0†–15.0 ± 0.6† | |||||||
| Q-0061 | X | X | |||||||
| McAbee | 52.90 ± 0.83* | 9.5§–13.5 ± 2.5† | |||||||
| F-1540/1 | X | X | X | X | X | ||||
| F-1542/3 | X | X | X | ||||||
| F-1544 | X | X | |||||||
| F-939 | X | X | |||||||
| Driftwood Canyon | 51.77 ± 0.34* | 6.2 ± 4.7†–12.3 ± 1.9† | |||||||
| RBCM.031 | X | X |
MAT values are from both leaf physiognomy and nearest living relative analyses, which provide the lower and upper values for each site, respectively. MW and MP are diagnostic of Pachymerini (MP within Buchinae); IL and TM of Pachymerina; PS of Caryobruchus and Speciomerus (within Pachymerina), and PT of Caryobruchus, Speciomerus, and Caryoborus (when these character states are found within Pachymerina) (28, 29). IL, impressed marginal line extending to anterior lateral and anterior margins of the pronotum; MW, metacoxae about half width of metafemorae and first sternite; MP, mesepimeral plate broad mesally, meets mesocoxa and/or metasternum; PS, pecten short, distad midfemur; PT, pecten mesad tibia on flexed hind leg; RBCM.031, RBCM.EH2013.031.0001.001; TM, broadly triangular tarsomeres 1 and 2 on the fore- and midleg.
Moss et al. (18).
Greenwood et al. (16).
Villeneuve and Mathewes (46).
Dillhoff et al. (47).
Results
We found all but one (from Driftwood Canyon) to be palm bruchids, including the Quilchena beetle; its previous tentative association with the Caryopemina (26) was incorrect. In Republic specimens SR 99-94-80 and SRUI 99-78-71 (Fig. 4), and McAbee specimens F-1540/1 and F-1544 (Fig. 5), the pecten positioned mesad the metatibia in the flexed hind leg excludes Pachymerus, and the pecten placed in the distal portion of the metafemur further excludes Pachymerus and also Caryoborus, placing these beetles in the Caryobruchus–Speciomerus group. Caryobruchus and Speciomerus are separated by fine details of morphology not preserved in these specimens. Modern species of Caryobruchus show a marked preference for palm seeds of the coryphoid tribes Coryphaea, Phoeniceae, and Hyphorbeae, whereas recorded hosts of Speciomerus are found within the Arecoid Cocoaceae (31).
Assignment of the single Driftwood Canyon beetle RBCM.EH2013.031.0001.001 (Fig. 4 I and L) to subtribe level is problematic by preservation, as it bears a damaged and incomplete pronotum and lacks the distal portions of its fore- and middle legs. Its pecten morphology and positioning in the flexed hind leg is, as above, distinctive of Caryobruchus and Speciomerus when these character states are found within the Pachymerina; however, these are also characteristic of some Old World Caryedontina, a possibility that cannot be excluded here. Within the Caryedontina, Afroredon is clearly excluded by its distinctively squat shape; Exoctenophorus by the pecten mesad the tibia in the flexed hind leg; and Caryotrypes by antennal morphology (segments slender, 5–10 feebly serrate in Caryotrypes). It is possible that it belongs to the tropical Mimocaryedon, which is only known from Lake Manyara, Tanzania (3.75° S latitude, 955 m elevation) (32), or to the genus Caryedon, which today ranges widely through Africa (best known in the tropics), the eastern Mediterranean, Arabian Peninsula, southern Black Sea to Caspian Sea region, and tropical and subtropical South and Southeast Asia (33).
Discussion
These findings provide robust support for the presence of palms at Quilchena and McAbee previously suggested by tentative pollen evidence, and further at Republic, where evidence of palms has not been recovered. The Driftwood Canyon beetle as Pachymerina would provide support for pollen evidence of palms there; but even a Caryedontina affinity would place it in a group with a marked modern preference for tropical to subtropical climates. We also examined beetles from the late Eocene Florissant Formation, confirming their association with the Pachymerina (27), consistent with the recent confirmation of palms there (25). We did not examine the Russian beetle; however, Zherikhin assigned it to the same genus as those from Florissant (28).
All of the Okanagan Highlands localities considered here have estimated microthermal climates (i.e., MAT ≤13 °C) except Quilchena, which is thought to have been lower mesothermal (Table 1).
Peak regional tectonic uplift during Okanagan Highlands times resulted in a mountainous topography comparable to the modern British Columbian Coast and Selkirk ranges (34, 35). Floral proxies indicate a considerably warmer climate in coeval nearby coastal lowland formations, with a substantial elevational MAT gradient upslope to the cooler Okanagan Highlands (16, 17, 19, 36–39). It is highly unlikely that all localities bearing Pachymerina were situated at the precise elevation of the coldest limits of palm tolerance. Any deviation would have to be downslope, i.e., warming winters relative to the established MAT estimates, not cooling them, and Okanagan Highland palms would then have been growing in a climate of even narrower temperature seasonality than is suggested by their minimum winter temperature tolerance.
The climates and communities of numerous hot lowland fossil localities deposited in the EECO have been examined, such as the megathermal Green River Formation in the midcontinental United States (for a review of Green River climates, see refs. 5 and 40). The establishment of warmed winters by the presence of palms in such communities does not, however, indicate reduced temperature seasonality as would their presence in the cooler Okanagan Highlands communities, where they constrain winter temperatures closer to microthermal and lower mesothermal MAT values established independently by bioclimatic and leaf physiognomy analyses (16, 40). Previous analysis based on paleobotanical proxies indicates that McAbee summers were not excessively hot (3). High EECO summer temperatures cannot be excluded, however (e.g., see isotopic analyses for the Bighorn Basin) (41), as the physiological limits of warmer summer temperatures on plants and therefore plant-based proxies, is not well constrained (40). General patterns of Okanagan Highlands summer temperatures would, however, follow from the relationship between CMMT and MAT by definition.
The presence of Pachymerina implies mild winters (Figs. S5 and S6) across this cooler montane setting from northern Washington into midlatitude Canadian localities, all a few degrees more northerly in the Eocene (42). This strengthens the supposition of equable Eocene extratropical climates, with the consequent implications for a globally nonmodern climatic context impacting the emergence of modern ecosystems in the late greenhouse world.
Materials and Methods
We examined nine fossil beetles from four localities of the Okanagan Highlands series of Early Eocene fossil localities: three from Republic, WA, on loan from the Stonerose Interpretive Center (SR 05-03-10; SR99-78-71a, b; SRUI 00-94-80); one from Quilchena, BC, in the Simon Fraser University collection (Q-0061a, b); four from McAbee, BC, on loan from Thompson Rivers University (UCCIPR L-18 F-1540/1; F-1542/3; and F-1544); and one from Driftwood Canyon, BC, on loan from BC Parks (RBCM.EH2013.031.0001.001). We follow the palm systematics of Baker et al. (43) and the beetle systematics of Nilsson and Johnson (30). Subtribe Pachymerina (palm bruchines) was previously considered by many authors as the tribe Pachymerini sensu Nilsson and Johnson (30), with Bruchinae the family Bruchidae; we treat the Bruchinae as a subfamily of the Chrysomelidae and adjust references to previously published ranks accordingly.
Analyses.
Climate range analyses for both the beetles (Tables S1–S4) and palms (Fig. 1) used existing data sources (references in SI Text) or were done using the DIVA-GIS software (www.diva-gis.org) with the WorldClim dataset (www.worldclim.org) to generate estimates of MAT, winter temperature [coldest quarter mean temperature ∼1–2 °C warmer than CMMT, which palm climate ranges are reported in] and mean annual precipitation for modern localities to a 1-km square resolution (44). Climate analyses for palms further used ANUCLIM 6.1 (45) for the Australian records.
Terminology (Figs. S2–S4).
Metafemora incrassate: femora of the hind leg thickened, swollen.
Pecten: comb-like structure on the posterior–ventral portion of metafemora.
Denticles: projections from pecten forming “teeth of the comb.”
Metatibia arcuate: tibia of the hind leg curved.
Metatibia mucronate: projection (mucro) extending from distal surface of the hind tibiae.
Metatibia carinate: with grooves (carinae) running lengthwise along the hind tibiae.
Mesocoxa: basal segment of the middle leg, attaching the leg to the thorax.
Metacoxa: basal segment of the hind leg.
Tarsomeres: subdivisions of the tarsus, portion of the leg distad the tibia.
Mesepimeron: posterior division of the middle thoracic pleuron (exterior, side).
Impressed marginal line on pronotum: a groove on the dorsal surface of the pronotum, just inside its margin.
Supplementary Material
Acknowledgments
We thank BC Parks and J. Howard (BC Parks, Smithers) for facilitating fieldwork at the Driftwood Canyon Provincial Park; G. Rose for facilitating fieldwork at Quilchena; G. Wilson, G. Bacon, and K. Volkman for the donation of Republic specimens (to Stonerose Interpretive Center) and C. Brown for their loan; D. Langevin for the donation of one of the McAbee specimens [to Thompson Rivers University (TRU)] and N. Van Wagoner for TRU specimen loans; D. Langevin, R. Campbell, and R. Drachuck for facilitating fieldwork at McAbee; C. Labandeira, D. Levin, and F. Marsh (Smithsonian Institution) and B. Farrell and P. Perkins (Museum of Comparative Zoology) for Florissant specimen loans; V. Makarkin for Russian papers and translations; and M. Pellatt (Parks Canada) for the use of digital microphotography equipment. This work was funded by a Natural Sciences and Engineering Research Council (NSERC Canada) fellowship (to S.B.A.) and NSERC Discovery Grants 311934 (to D.R.G.) and 3835 (to R.W.M.). We thank an anonymous donor for travel funding and imaging equipment in San Diego.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Fossils are deposited in the collections of Simon Fraser University, The Royal British Columbia Museum, The Stonerose Interpretive Center (Republic, Washington), and Thompson Rivers University.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323269111/-/DCSupplemental.
References
- 1.Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451(7176):279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- 2.Secord R, et al. Evolution of the earliest horses driven by climate change in the Paleocene-Eocene Thermal Maximum. Science. 2012;335(6071):959–962. doi: 10.1126/science.1213859. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood DR, Wing SL. Eocene continental climates and latitudinal temperature gradients. Geology. 1995;23(11):1044–1048. [Google Scholar]
- 4.Archibald SB, Bossert WH, Greenwood DR, Farrell BD. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology. 2010;36(3):374–398. [Google Scholar]
- 5.Archibald SB, Johnson KR, Mathewes RW, Greenwood DR. Intercontinental dispersal of giant thermophilic ants across the Arctic during early Eocene hyperthermals. Proc Biol Sci. 2011;278(1725):3679–3686. doi: 10.1098/rspb.2011.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archibald SB, Greenwood DR, Mathewes RW. Seasonality, montane beta diversity, and Eocene insects: Testing Janzen's dispersal hypothesis in an equable world. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;371:1–8. [Google Scholar]
- 7.Powell JA, Logan JA. Insect seasonality: Circle map analysis of temperature-driven life cycles. Theor Popul Biol. 2005;67(3):161–179. doi: 10.1016/j.tpb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Raffa KF, Powell EN, Townsend PA. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc Natl Acad Sci USA. 2013;110(6):2193–2198. doi: 10.1073/pnas.1216666110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larcher W, Winter A. Frost susceptibility of palms: Experimental data and their interpretation. Principes. 1981;25:143–152. [Google Scholar]
- 10.Royer DL, Osborne CP, Beerling D. High CO2 increases the freezing sensitivity of plants: Implications for paleoclimatic reconstructions from fossil floras. Geology. 2002;30(11):963–966. [Google Scholar]
- 11.Sluijs A, et al. Warm and wet conditions in the Arctic region during Eocene Thermal Maximum 2. Nat Geosci. 2009;2(11):777–780. [Google Scholar]
- 12.Eiserhardt WL, Svenning J-C, Kissling WD, Balslev H. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Ann Bot (Lond) 2011;108:1391–1416. [Google Scholar]
- 13.Eldrett JS, Greenwood DR, Harding IC, Huber M. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature. 2009;459(7249):969–973. doi: 10.1038/nature08069. [DOI] [PubMed] [Google Scholar]
- 14.Pross J, et al. Integrated Ocean Drilling Program Expedition 318 Scientists Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature. 2012;488(7409):73–77. doi: 10.1038/nature11300. [DOI] [PubMed] [Google Scholar]
- 15.Erwin DW, Stockey RA. Silicified monocotyledons from the Middle Eocene Princeton chert (Allenby Formation) of British Columbia, Canada. Rev Palaeobot Palynol. 1991;70:147–162. [Google Scholar]
- 16.Greenwood DR, Archibald SB, Mathewes RW, Moss PT. Fossil biotas from the Okanagan Highlands, southern British Columbia and northern Washington State: Climates and ecosystems across an Eocene landscape. Can J Earth Sci. 2005;42(2):167–185. [Google Scholar]
- 17.Smith RY, Basinger JF, Greenwood DR. Early Eocene plant diversity and dynamics in the Falkland flora, Okanagan Highlands, British Columbia, Canada. Palaeobiodivers Palaeoenviron. 2012;92:309–328. [Google Scholar]
- 18.Moss PT, Greenwood DR, Archibald SB. Regional and local vegetation community dynamics of the Eocene Okanagan Highlands (British Columbia – Washington State) from palynology. Can J Earth Sci. 2005;42(2):187–204. [Google Scholar]
- 19.Dillhoff RM, Dillhoff TA, Greenwood DR, DeVore ML, Pigg KB. The Eocene Thomas Ranch flora, Allenby Formation, Princeton, British Columbia, Canada. Botany. 2013;91(8):514–529. [Google Scholar]
- 20.Rouse GE. Plant microfossils from the Burrard Formation of western British Columbia. Micropaleontology. 1962;8(2):187–218. [Google Scholar]
- 21.Nichols J, Ames HT, Traverse A. On Arecipites Wodehouse, Monocolpopollenites Thomson & Pflug, and the species “Monocolpopollenites tranquillus”. Taxon. 1973;22(2-3):241–256. [Google Scholar]
- 22.Harley MM, Baker WJ. Pollen aperture morphology in Arecaceae: Application within phylogenetic analyses, and a summary of record of palm-like pollen the fossil. Grana. 2001;40(1-2):45–77. [Google Scholar]
- 23.Harley MM. A summary of fossil records for Arecaceae. Bot J Linn Soc. 2006;151(1):39–67. [Google Scholar]
- 24.Smith SY. 2013. The fossil record of non-commelinid monocots. Early Events in Monocot Evolution, eds Wilkin P, Mayo SJ (Cambridge Univ Press, Cambridge, UK), Systematics Association Special Vol 83, pp 29–59.
- 25.Manchester SR. 2001. Update on the Megafossil flora of Florissant, Colorado. Fossil Flora and Stratigraphy of the Florissant Formation, Colorado. Proceedings of the Denver Museum of Nature and Science, eds Evanoff E, Gregory-Wodzicki K, Johnson K. Series 4, No 1, pp 137–161.
- 26.Archibald SB, Mathewes RW. Early Eocene insects from Quilchena, British Columbia and their paleoclimatic implications. Can J Zool. 2000;78(8):1441–1462. [Google Scholar]
- 27.Kingsolver JM. A new fossil bruchid genus and its relationship to modern genera (Coleoptera: Bruchidae: Pachymerinae) Col Bull. 1965;19(1):25–30. [Google Scholar]
- 28.Zherikhin VV. In: Kainozoi Dal'nego Vostoka. Krasilov VA, Klimova RS, editors. Vladivostok, Russia: Far Eastern Branch of Academy of Sciences of the USSR; 1989. pp. 1–250. [Google Scholar]
- 29.Bridwell JC. A preliminary generic arrangement of the palm bruchids and allies (Coleoptera) with descriptions of new species. Proc Entomol Soc Wash. 1929;31(8):141–160. [Google Scholar]
- 30.Nilsson JA, Johnson CD. A taxonomic revision of the palm bruchids (Pachymerini) and a description of the world genera of Pachymerinae (Coleoptera: Bruchidae) Mem Am Entomol Soc. 1993;41:1–104. [Google Scholar]
- 31.Johnson CD, Zona S, Nilsson JA. Bruchid beetles and palm seeds: Recorded relationships. Principes. 1995;39(1):25–35. [Google Scholar]
- 32.Decelle J. Nouveaux genres et especes de Caryedontini (Col. Bruchidae, Pachymerinae) d'Afrique et de Madagascar. Bull Ann Soc R Entomol Belg Entomol. 1968;104:413–426. [Google Scholar]
- 33.Borowiec L. The genera of seed-beetles (Coleoptera Bruchidae) Pol Pismo Entomol. 1987;57:3–206. [Google Scholar]
- 34.Ewing TE. Paleogene tectonic evolution of the Pacific Northwest. J Geol. 1980;88(6):619–638. [Google Scholar]
- 35.Tribe S. Eocene paleo-physiography and drainage directions, southern Interior Plateau, British Columbia. Can J Earth Sci. 2005;42(2):215–230. [Google Scholar]
- 36.Smith RY, Basinger JF, Greenwood DR. Depositional setting, floristics and paleoenvironment of the Early Eocene Falkland site, a new fossil flora locality from the Okanagan Highlands, British Columbia. Can J Earth Sci. 2009;46(11):811–822. [Google Scholar]
- 37.Rouse GE, Hopkins WS, Jr, Piel KM. Palynology of some Late Cretaceous and Early Tertiary deposits in British Columbia and adjacent Alberta. 1971 Symposium on Palynology of the Late Cretaceous and Early Tertiary, eds Kosanke RM, Cross AT. Geol S Am S 127:213–246. [Google Scholar]
- 38.Mustoe GE, Gannaway WL. Paleogeography and paleontology of the Early Tertiary Chuckanut Formation, Northwest Washington. Wash Geol. 1997;25(3):3–18. [Google Scholar]
- 39.Wolfe JA, Forest CE, Molnar P. Paleobotanical evidence of Eocene and Oligocene paleoaltitudes in midlatitude western North America. Geol Soc Am Bull. 1998;110(5):664–678. [Google Scholar]
- 40.Huber M, Caballero R. The early Eocene equable climate problem revisited. Clim Past. 2011;7:603–633. [Google Scholar]
- 41.Snell KE, et al. Hot summers in the Bighorn Basin during the early Paleogene. Geology. 2013;41(1):55–58. [Google Scholar]
- 42.Andrews JA. True polar wander: An analysis of Cenozoic and Mesozoic paleomagnetic poles. J Geophys Res. 1985;90(B9):7737–7750. [Google Scholar]
- 43.Baker WJ, et al. Complete generic-level phylogenetic analyses of palms (Arecaceae) with comparisons of supertree and supermatrix approaches. Syst Biol. 2009;58(2):240–256. doi: 10.1093/sysbio/syp021. [DOI] [PubMed] [Google Scholar]
- 44.Hijmans RJ, et al. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 45.Xu T, Hutchinson M. New developments and applications in the ANUCLIM spatial climatic and bioclimatic modelling package. Environ Model Softw. 2013;40:267–279. [Google Scholar]
- 46.Villeneuve M, Mathewes R. 2005. An Early Eocene Age for the Quilchena Fossil Locality, Southern British Columbia (Geol Sur Can Curr Res, Ottawa), 2005-A4, pp 1–7.
- 47.Dillhoff RM, Leopold EB, Manchester SR. The McAbee flora of British Columbia and its relation to the Early-Middle Eocene Okanagan Highlands flora of the Pacific Northwest. Can J Earth Sci. 2005;42(2):155–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.