SUMMARY
Objective
Tuberculosis (TB) causes nearly 1.5 million deaths annually worldwide. Active TB disease can destroy lung parenchyma leading to cavities. Immune responses that predispose or protect individuals from lung damage during tuberculosis are poorly defined.
Design
Enrolled subjects (N=73) had bilateral infiltrates and underwent bronchoalveolar lavage (BAL) to sample lung immune cells and assay BAL cell cytokine production.
Results
All had sputum culture demonstrating Mycobacterium tuberculosis and 22/73 (30%) had cavities on their chest radiograph. Those with cavities at presentation had higher percent PMN in BAL as well as lower IP-10 (p<0.01) and IL-6 (p=0.013) in BAL cell supernatants, compared to those without cavities. There was no correlation between cavities and other BAL or serum cytokines. IP-10 was negatively associated with BAL PMN. IP-10 and IL-6 expression above median decrease the odds of cavities by 79% and 78% in logistic regression models. IP-10 and IL-6 clustered with IFN-γ and TNF-α in a principal component analysis while IL-4 clustered with PMN.
Conclusion
Increasing IP-10 and IL-6 production by BAL cells is associated with non-cavitary tuberculosis in patients who present with radiographically advanced TB. IP-10 and IL-6 may reflect an effective Th-1 immune control pathway for TB, attenuating tuberculous lung destruction.
Keywords: biomarkers, cavitary tuberculosis, Th-1, innate immunity
INTRODUCTION
Tuberculosis is one of the top ten causes of mortality in low and middle-income countries. In 2010, there were 8.8 million new cases of TB diagnosed and 1.45 million deaths (1). The disease is transmitted through the air and once inhaled, Mycobacterium tuberculosis (Mtb) lodges in the lower respiratory tract and replicates slowly. It induces a Mtb specific Th-1 immune response over several months that controls but does not eliminate the bacteria producing latent TB infection (LTBI). Approximately 10% of HIV negative individuals with LTBI develop active TB over the course of their lives. With HIV co-infection, there is a marked deficiency in Th-1 immunity and a large majority of those with LTBI will develop active disease if not on antiretroviral therapy (2). In high TB burden countries like South Africa, approximately 40-50% of the population has LTBI; 1% of the population of South Africa developed active TB in 2009 producing an annual incidence of 490,000 cases (1).
The immune response to Mtb can destroy lung tissue, producing defects in the lung that appear as cavities on chest radiograph. In the absence of effective treatment, 50% of immune competent patients with tuberculosis will die of lung dysfunction. Even with curative anti-tuberculous chemotherapy, those with cavities will be left with pulmonary scars that can produce lifelong respiratory disability and premature mortality (3-6). This is especially important given the emergence of multi-drug resistant (MDR) and extended-drug resistant (XDR) tuberculosis (7).
The CD4+ Th-1 lymphocyte responses are required for effective acquired immunity to Mtb (8). Adding the Th-1 cytokine interferon (IFN) gamma by aerosol improves response to therapy by more rapidly converting the sputum smear from positive to negative and reducing respiratory symptoms (9). IFN gamma also induces the chemokine inducible 10-kDa protein (IP-10 also named CXCL10) in the human lung (10, 11). Peripheral blood of patients with LTBI or active tuberculosis strongly induce IP-10 upon stimulation with TB antigens (12, 13). Increased lung IP-10 is associated with lack of cavities in HIV infected patients with tuberculosis (14). In model systems IP-10 recruits T cells to sites of inflammation and may play a role in generation and function of effector T cells (15). In the absence of IFN-γ there is excessive PMN infiltration into the lung injury during tuberculosis leading to increased mortality (16).
To better understand the relation of immune state to cavity formation in HIV negative patients who presented with culture confirmed TB and bilateral infiltrates/cavities, we studied immune cells from the lower respiratory tract using bronchoalveolar lavage (BAL). We performed a cross sectional study of an urban cohort in Cape Town presenting with bilateral infiltrates and many with at least one cavity. We confirmed prior observations that IP-10 in the lung reduced the risk of having cavities on chest radiograph. We now report that increasing release of IL-6 by BAL cells is also a biomarker for protection from cavities. These two biomarkers are co-regulated and likely are part of an effective Th-1 immune response that protects against tissue destruction in patients with advanced tuberculosis on presentation.
METHODS
Study Subjects
Pulmonary tuberculosis subjects were recruited from April 2005 to December 2006 in the Division of Pulmonology at the University of Cape Town with the following inclusion criteria(9). All subjects had Mtb cultured from their sputum, were drug-sensitive and had bilateral infiltrates. Age, gender, race/ethnicity, fever, respiratory symptoms, and smoking status were obtained at the screening evaluation. BMIs were calculated from height and weight measured at screening. All subjects had posterior-anterior and lateral chest x-rays and chest CT.. The cavities were suggested on standardized PA chest radiographs during screening for that study and confirmed by chest CT at the time of study enrolment. (9). Chest radiographs and CT were scored for cavities by a pulmonologist. Sputum smear was performed at the time of bronchoscopy two weeks after screening.
Ethics Statement
This study received approval from both the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee and the New York University Medical Center Institutional Review Board; all study subjects signed informed consent in their native language(9).
Bronchoalveolar lavage (BAL)
BAL was performed using lidocaine as a local anesthetic. The bronchoscope was inserted via the nasal passage to the lower respiratory tract, wedged, and a lavage was performed using 5, 20-ml aliquots of normal saline in each of three involved lung segments (total of 300 mL). Recovered fluid was pooled and filtered over sterile gauze, and a total cell count was performed. A cytospin slide was prepared and stained with Diff-Quik. Five hundred cells were counted to determine the cell differential. BAL cells were cultured in RPMI for 24 hours at 106 BAL cells/ml. BAL cell supernatants were aliquoted and frozen at −80oC. Samples were thawed once at 4°C and assayed using 13-Plex Human Pro-inflammatory Panel according to manufacturer's recommendations (Millipore, Billerica, MA). The supernatants were analyzed on a Luminex 200IS (Luminex Corporation, Austin, TX) using MasterPlex TM QT software (Ver. 1·2; MiraiBio, Inc).
Statistical Analysis
Database management and statistics were performed using SPSS 20 (IBM, USA). Normally distributed data were expressed as mean and standard deviation. Data not distributed normally and multi-analyte comparisons were evaluated using a Mann Whitney U-test. Significance was assessed at p <0.05. Analytes that were significantly different between those with and without cavities were used to construct logistic regression models with cavities as the dichotomous outcome variable. The models were adjusted for the potential confounders of BMI, gender and age. Principal component analysis (PCA) an unsupervised method of data set reduction was used to assess the major sources of variation attributable to cytokine, cavity and BAL cell variables. Kaiser stopping criterion of Eigenvalues greater 1 and a Varimax rotation was chosen allowing an orthogonal solution.
RESULTS
By design, all 73 patients in this cohort had culture positive pulmonary tuberculosis with bilateral infiltrates on their chest x-rays. The group was young with a median age of 34 years and 75% were male. Black Africans accounted for 41% of the cohort while patients that reported being of mixed race accounted for 59%.
They were underweight with a median BMI of 18.7. A large proportion (77%) had a history of current smoking. They uniformly had reactivity to tuberculin skin testing (median 25 mm). A majority (80%) were AFB smear positive while minority (41%) had fever on presentation (Table 1).
Table 1.
Demographics of the study patients
| Cavitary disease | ||||
|---|---|---|---|---|
| Yes (n = 22) n (%) or mean [SD] | No (n = 51) n (%) or mean [SD] | P value | ||
| n | 22 | 51 | ||
| Age, years | 35 [10] | 33 [11] | 0.338* | |
| Male sex | 15 (68.2) | 40 (78.4) | 0.351 | |
| BMI, kg/m2 | 18.58 [2.91] | 19.12 [2.37] | 0.460* | |
| Smoking (ever) | 17 (77.3) | 39 (76.5) | 0.941 | |
| Race | Black | 8 (36.4) | 22 (43.1) | 0.589 |
| Mixed | 14 (63.6) | 29 (56.9) | ||
| TST, mm | 34 [16] | 30 [13] | 0.348* | |
| AFB Smear-positive | 17 (77.3) | 42 (82.4) | 0.613 | |
| Fever | 9 (40.9) | 21 (41.2) | 0.983 | |
Unpaired t-test with Welch's correction; all other significance assessed using x2
SD = standard deviation; BMI = body mass index; TST = tuberculin skin test; AFB = acid-fast bacilli
Our primary outcome was lung destruction defined by cavities on chest xray. At presentation 22/73 (30%) had lung cavities and in 51/73 (70%) no identifiable cavities were detected. There was no significant association between cavities and age, gender, BMI, race, tuberculin skin test (TST) size, or the presence of AFBs on sputum smear (Table 1).
We assessed the immune response using BAL cell differential and cytokine release after 24 hours of ex vivo culture. Patients with cavities had a higher proportion of PMN on BAL differential (41% vs 26%, p=0.043, Table 2). Consequently, in patients with cavitary TB the proportion of alveolar macrophages was lower (49%% vs 62%%, p=0.042, Table 2).
Table 2.
Bronchoalveolar lavage cell differential
| Cavitary Disease | |||
|---|---|---|---|
| Yes (n = 22) Median (IQR) | No (n = 51) Median (IQR) | P value* | |
| Cells/mL × 104 | 16.35 (14-25) | 17.65 (9-32) | 0.965 |
| Alveolar macrophages, % | 49 (10-69) | 62 (30-82) | 0.042 |
| Lymph,% | 5 (3-9) | 4 (1-10) | 0.443 |
| PMN, % | 41 (15-87) | 26 (4-58) | 0.043 |
| Eosinophil, % | - | 1 (1-1) | - |
Mann-Whitney U test
PMN = polymorphonuclear neutrophil
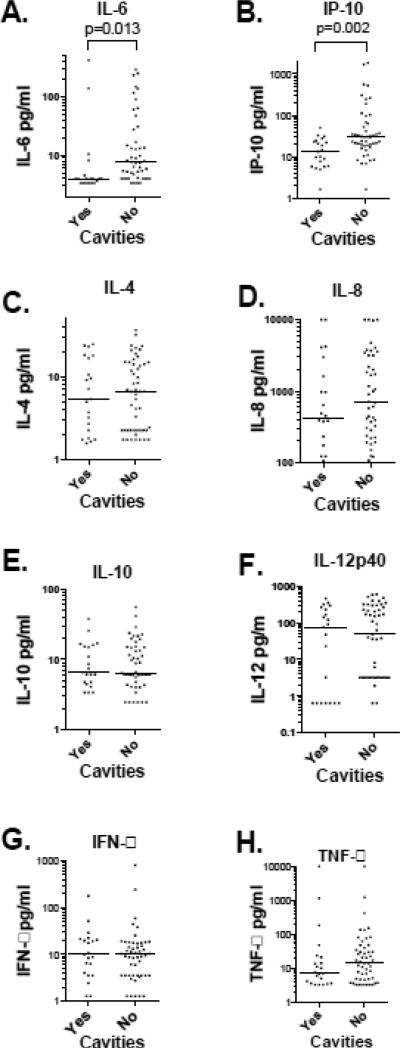
Cytokine release in 24-hour culture supernatants was measured by multiplex bead fluorescence assay. Patients with cavities had significantly lower IL-6, and IP-10 production (4.0 pg/ml vs. 7.67 pg/ml p=0.013 and 14.19 pg/ml vs. 30.68 pg/ml p= 0.002, respectively (Figure 1 panel A and B). There were no significant differences in IL-4, IL-8, IL-10, IL-12p40, IFN-γ, or TNF-α release between those with and without cavities. IL-1β, IL-2 and IL-17 were expressed a levels near or below the limits of detection of the assay (data not shown). There was a significant negative correlation between BAL PMN and IP-10 production (R2=0.07, p=0.026, Figure 2). There was no significant correlation between BAL cell differential and IL-6. There was no correlation between BAL cell cytokine production and serum cytokine levels (data not shown).
Figure 1. BAL cells cytokine elaboration in patients with an without cavities.
Cavity status is shown on the X axis. Each cytokine measured in this investigation is shown by log transformed concentration on the Y axis. The median value is represented by the horizontal line. Only IL-6 and IP-10 showed significant differences with Mann–Whitney U test between patients with and without cavities, p value shown above the bracket.
Figure 2. Correlation of BAL supernatant IP-10 with BAL Polymorphonuclear Neutrophils (%).
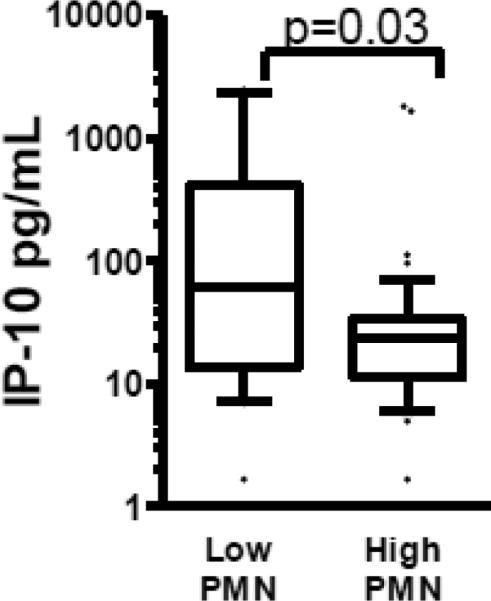
Linear regressions with 95% confidence intervals. R2 0.071, p=0.026.
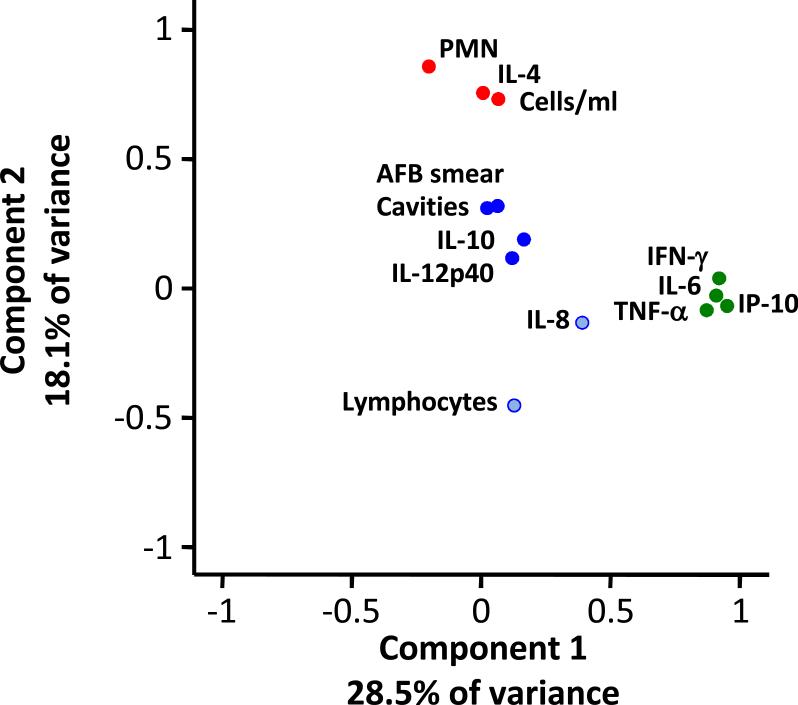
We then tested the ability of BAL cell cytokines to predict cavities on the chest radiograph. Individuals with BAL IP-10 expression above the median had reduced odds ratios of cavities on chest radiograph by 79% (p=0.01) in a logistic model adjusted for BMI, ethnicity and gender (Table 3). IL-6 expression above median also reduced the odds of cavities on chest radiograph by 78% (p=0.02). When added together in a multi-variable logistic model, IP-10 and IL-6 become insignificant. We further investigated the inter-relationship of cytokines, using principal component analysis. IL-6 and IP-10 grouped with grouped with IFN-γ and TNF-α. Cavities grouped with AFB smear positivity, IL-12p40, and IL-10. PMN grouped with IL-4 and BAL cells concentration (Figure 2). Component 1 accounted for 28.5 % of variance and Component 2 accounted for 18.1 %. A total of four component axes accounted for 70% of the variance (data not shown).
Table 3.
Bronchoalveolar lavage biomarkers of pulmonary cavities
| Model* | Analyte§ | OR (95% CI) | P value |
|---|---|---|---|
| Single Analyte | IP-10 | 0.21 (0.56-0.68) | 0.01 |
| IL-6 | 0.22 (0.066-0.68) | 0.02 | |
| Multi-Analyte | IP-10 | 0.30 (0.086-1.07) | 0.06 |
| IL-6 | 0.36 (0.095-1.35) | 0.13 | |
Adjusted for BMI, ethnicity and sex
Cut-off points ≥ median IP-10 23.31 pg/ml and IL-6 5.89 pg/ml
OR = odds ratio; CI = confidence interval; IP = inducible protein; IL = interleukin; BMI = body mass index
DISCUSSION
We performed a cross sectional case control study of cytokine release into 24-hour BAL supernatants on 73 HIV-negative urban South African patients who presented with pulmonary tuberculosis. All had bilateral infiltrates on chest x-ray and a minority had cavities. They were severely underweight with median BMI of 18.9, but maintained active antigen-specific cellular immunity to Mtb with strongly reactive tuberculin skin tests. An improved understanding of the risk factors for lung destruction is therefore important.
In addition to routine clinical evaluation, we performed research bronchoscopy to retrieve lung immune cells from the site of infection. This allowed us to assess cytokine production and BAL cell differential in association with radiographic evidence of cavities. We observed that elevated IP-10 expression reduced the odds of being cavity positive by 79%. Similarly, increased IL-6 was associated with 78% protection from cavities. These protective factors are correlated with one another since they became less significant in a multi-analyte logistic model that included both IP-10 and IL-6. They cluster closely with IFN-γ in principal component analysis suggesting they are parts of a single Th-1 pathway. We have previously shown that IFN-γ induces a signaling pathway leading to IP-10 induction (17), and promotes autophagy and other anti-mycobacterial defenses including phagosome acidification and phagolysosomal fusion (18). BAL and flow cytometry of lung lymphocytes has shown that there are more CD4+ cells in non-cavitary TB compared to cavitary TB (19). PPD-specific clones derived from peripheral blood or pleural fluid showed a Th0 profile with IFN-γ and IL-4 release at baseline but polarized to a Th-1 profile with only IFN-γ production after 6 months of anti-mycobacterial therapy (20). Similarly, in The Gambia, TB patients had evidence of blood Th-2 profile compared to household contacts without TB, and the blood Th-2 profile converted to Th-1 activity after successful treatment (21). IP-10 and IL-6 also cluster tightly with TNF-α, another cytokine essential to tuberculosis control in humans (22).
IP-10 has been implicated in protection from pulmonary cavities in pulmonary TB (14). There was no association between BAL-cell IP-10 and serum IP-10 levels suggesting the pulmonary inflammation during TB is not accurately reflected in the systemic compartment or venous blood. There was a negative association between BAL IP-10 and BAL PMN. This could underlie the impact of IP-10 on cavitary disease since these patients have higher BAL PMN than patients without cavities.
Interestingly, BAL PMN tightly clustered with the Th-2 cytokine IL-4, a marker of disease progression in tuberculosis (4, 6, 23, 24). Our observations on IL-4 are consistent with the conclusion that a Th-2 predominant response to Mtb could lead to tissue destruction. This is biologically plausible since a Th-2 response in leprosy, another mycobacterial disease, produces much more skin destruction than the Th-1 response or tuberculoid response (25, 26). Circulating T cells produce more IL-4 from TB patients with cavities than without cavities (27). Also mRNA for IL-4 was higher in unstimulated PBMC from TB patients with cavities versus TB patients without cavities (23), but following stimulation with phytohemagglutinin, the IL-4 mRNA in PBMC from TB patients with cavities was less than controls, and the ratio of IL-4δ2 to IL-4 was significantly lower in TB patients with a cavity (28, 29). IL-10 is the cytokine most tightly clustered with cavities and AFB smears in the principal component analysis. This is also consistent with the known inhibitory effect of IL-10 on the immune response to tuberculosis in humans (30-32). Further investigation of the relationships observed in the principal component analysis of tuberculosis is needed to better understand these observations.
Cases and controls were drawn from the same parent cohort minimizing the potential for selection bias. Nevertheless, this study has several limitations. It is a cross sectional natural history investigation; therefore, the observed associations do not imply causality. In addition, the findings on BAL-cell cytokine production are unlikely to be clinically useful since they require invasive and expensive testing. Finally, there are likely to be cohort specific effects on our results; these findings need to be replicated in other cohorts to assess their generalizability. These human observations are unique since they come from the site of disease, and importantly, should drive investigation as to why the Th-1 response in tuberculosis is less destructive than the Th-2 response.
Conclusion
Increased production of the Th-1 cytokine IP-10 by BAL cells is a strong protective factor for preserved lung architecture in this cohort of pulmonary TB patients. IL-6 is in the same immune pathway, and protects from tissue destruction. Further investigation of the Th-1 immune pathway in protecting lung integrity in TB is needed to improve our understanding of immune states that predispose to tissue damage.
Figure 3. Principal Component Analysis.
Component 1 explains 28.5% of variance and Component 2 18.1% variance. IFN-γ, IL-6, TNF-α, and IP-10 cluster together as a Th-1 response, and PMN, IL-4 and Total Cells/ml clustered as a Th-2 response. Cavities and IL-10 and IL-12p40 clustered together.
Acknowledgements
This work was supported by National Institutes of Health HL090316, K23HL084191 and K24A1080298
REFERENCES
- 1.Global Tuberculosis Control World Health Organization [Internet] 20112011 Available from: http://www.who.int/tb/publications/global_report/2011.
- 2.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. The New England journal of medicine. 1989;320(9):545–50. doi: 10.1056/NEJM198903023200901. Epub 1989/03/02. [DOI] [PubMed] [Google Scholar]
- 3.Dheda K, Migliori GB. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue? Lancet. 2012;379(9817):773–5. doi: 10.1016/S0140-6736(11)61062-3. Epub 2011/10/29. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Chang JS, Breen RA, Haddock JA, Lipman MC, Kim LU, et al. Expression of a novel cytokine, IL-4delta2, in HIV and HIV-tuberculosis co-infection. AIDS. 2005;19(15):1601–6. doi: 10.1097/01.aids.0000183520.52760.ef. Epub 2005/09/27. [DOI] [PubMed] [Google Scholar]
- 5.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. The Journal of infectious diseases. 2005;192(7):1201–9. doi: 10.1086/444545. Epub 2005/09/02. [DOI] [PubMed] [Google Scholar]
- 6.Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, et al. In vivo and in vitro studies of a novel cytokine, interleukin 4delta2, in pulmonary tuberculosis. American journal of respiratory and critical care medicine. 2005;172(4):501–8. doi: 10.1164/rccm.200502-278OC. Epub 2005/05/20. [DOI] [PubMed] [Google Scholar]
- 7.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375(9728):1798–807. doi: 10.1016/S0140-6736(10)60492-8. Epub 2010/05/22. [DOI] [PubMed] [Google Scholar]
- 8.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. The Journal of experimental medicine. 2006;203(7):1805–15. doi: 10.1084/jem.20052545. Epub 2006/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson R, Condos R, Tse D, Huie ML, Ress S, Tseng CH, et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PloS one. 2009;4(9):e6984. doi: 10.1371/journal.pone.0006984. Epub 2009/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raju B, Hoshino Y, Kuwabara K, Belitskaya I, Prabhakar S, Canova A, et al. Aerosolized gamma interferon (IFN-gamma) induces expression of the genes encoding the IFN-gamma-inducible 10-kilodalton protein but not inducible nitric oxide synthase in the lung during tuberculosis. Infection and immunity. 2004;72(3):1275–83. doi: 10.1128/IAI.72.3.1275-1283.2004. Epub 2004/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raju B, Hoshino Y, Belitskaya-Levy I, Dawson R, Ress S, Gold JA, et al. Gene expression profiles of bronchoalveolar cells in pulmonary TB. Tuberculosis (Edinb) 2008;88(1):39–51. doi: 10.1016/j.tube.2007.07.003. Epub 2007/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alsleben N, Ruhwald M, Russmann H, Marx FM, Wahn U, Magdorf K. Interferon-gamma inducible protein 10 as a biomarker for active tuberculosis and latent tuberculosis infection in children: a case-control study. Scandinavian journal of infectious diseases. 2012;44(4):256–62. doi: 10.3109/00365548.2011.632644. Epub 2011/11/23. [DOI] [PubMed] [Google Scholar]
- 13.Petrucci R, Abu Amer N, Gurgel RQ, Sherchand JB, Doria L, Lama C, et al. Interferon gamma, interferon-gamma-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. The Pediatric infectious disease journal. 2008;27(12):1073–7. doi: 10.1097/INF.0b013e31817d05a3. Epub 2008/10/24. [DOI] [PubMed] [Google Scholar]
- 14.Kibiki GS, Myers LC, Kalambo CF, Hoang SB, Stoler MH, Stroup SE, et al. Bronchoalveolar neutrophils, interferon gamma-inducible protein 10 and interleukin-7 in AIDS-associated tuberculosis. Clinical and experimental immunology. 2007;148(2):254–9. doi: 10.1111/j.1365-2249.2007.03330.x. Epub 2007/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–204. doi: 10.4049/jimmunol.168.7.3195. Epub 2002/03/22. [DOI] [PubMed] [Google Scholar]
- 16.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. The Journal of experimental medicine. 2011;208(11):2251–62. doi: 10.1084/jem.20110919. Epub 2011/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condos R, Raju B, Canova A, Zhao BY, Weiden M, Rom WN, et al. Recombinant gamma interferon stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infection and immunity. 2003;71(4):2058–64. doi: 10.1128/IAI.71.4.2058-2064.2003. Epub 2003/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Current opinion in immunology. 2005;17(4):374–80. doi: 10.1016/j.coi.2005.06.006. Epub 2005/06/21. [DOI] [PubMed] [Google Scholar]
- 19.Mazzarella G, Bianco A, Perna F, D'Auria D, Grella E, Moscariello E, et al. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clinical and experimental immunology. 2003;132(2):283–8. doi: 10.1046/j.1365-2249.2003.02121.x. Epub 2003/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchant A, Amedei A, Azzurri A, Vekemans J, Benagiano M, Tamburini C, et al. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-alpha or interleukin-12. American journal of respiratory cell and molecular biology. 2001;24(2):187–94. doi: 10.1165/ajrcmb.24.2.4274. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 21.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. European journal of immunology. 2002;32(6):1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. Epub 2002/07/13. [DOI] [PubMed] [Google Scholar]
- 22.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. The New England journal of medicine. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. Epub 2001/10/13. [DOI] [PubMed] [Google Scholar]
- 23.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. The Journal of infectious diseases. 2000;181(1):385–9. doi: 10.1086/315200. Epub 1999/12/23. [DOI] [PubMed] [Google Scholar]
- 24.Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, et al. Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. The Journal of infectious diseases. 2004;190(4):756–66. doi: 10.1086/422532. Epub 2004/07/24. [DOI] [PubMed] [Google Scholar]
- 25.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254(5029):277–9. doi: 10.1126/science.254.5029.277. Epub 1991/10/11. [DOI] [PubMed] [Google Scholar]
- 26.Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301(5639):1527–30. doi: 10.1126/science.1087785. Epub 2003/09/13. [DOI] [PubMed] [Google Scholar]
- 27.van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. The Journal of infectious diseases. 2000;181(3):1194–7. doi: 10.1086/315325. Epub 2000/03/18. [DOI] [PubMed] [Google Scholar]
- 28.Wu HP, Wu CL, Yu CC, Liu YC, Chuang DY. Efficiency of interleukin-4 expression in patients with tuberculosis and nontubercular pneumonia. Human immunology. 2007;68(10):832–8. doi: 10.1016/j.humimm.2007.07.003. Epub 2007/10/27. [DOI] [PubMed] [Google Scholar]
- 29.Dheda K, Chang JS, Huggett JF, Kim LU, Johnson MA, Zumla A, et al. The stability of mRNA encoding IL-4 is increased in pulmonary tuberculosis, while stability of mRNA encoding the antagonistic splice variant, IL-4delta2, is not. Tuberculosis (Edinb) 2007;87(3):237–41. doi: 10.1016/j.tube.2006.11.001. Epub 2007/01/02. [DOI] [PubMed] [Google Scholar]
- 30.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infection and immunity. 1996;64(3):913–8. doi: 10.1128/iai.64.3.913-918.1996. Epub 1996/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. The Journal of clinical investigation. 2000;105(9):1317–25. doi: 10.1172/JCI9918. Epub 2000/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Leary S, O'Sullivan MP, Keane J. IL-10 blocks phagosome maturation in mycobacterium tuberculosis-infected human macrophages. American journal of respiratory cell and molecular biology. 2011;45(1):172–80. doi: 10.1165/rcmb.2010-0319OC. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]