Abstract
The liver is an essential metabolic organ, and its metabolic activity is tightly controlled by insulin and other metabolic hormones. Glucose is metabolized into pyruvate through glycolysis in the cytoplasm, and pyruvate is completely oxidized to generate ATP through the TCA cycle and oxidative phosphorylation in the mitochondria. In the fed state, glycolytic products are used to synthesize fatty acids through de novo lipogenesis. Long-chain fatty acids are incorporated into triacylglycerol, phospholipids, and cholesterol esters in hepatocytes, and these complex lipids are stored in lipid droplets and membrane structures, or secreted into the circulation as VLDL particles. In the fasted state, the liver secretes glucose through both breakdown of glycogen (glycogenolysis) and de novo glucose synthesis (gluconeogenesis). During pronged fasting, hepatic gluconeogenesis is the primary source of endogenous glucose production. Fasting also promotes lipolysis in adipose tissue to release nonesterified fatty acids which are converted into ketone bodies in the liver though mitochondrial β oxidation and ketogenesis. Ketone bodies provide a metabolic fuel for extrahepatic tissues. Liver metabolic processes are tightly regulated by neuronal and hormonal systems. The sympathetic system stimulates, whereas the parasympathetic system suppresses, hepatic gluconeogenesis. Insulin stimulates glycolysis and lipogenesis, but suppresses gluconeogenesis; glucagon counteracts insulin action. Numerous transcription factors and coactivators, including CREB, FOXO1, ChREBP, SREBP, PGC-1α, and CRTC2, control the expression of the enzymes which catalyze the rate-limiting steps of liver metabolic processes, thus controlling liver energy metabolism. Aberrant energy metabolism in the liver promotes insulin resistance, diabetes, and nonalcoholic fatty liver diseases (NAFLD).
Introduction
The liver is a key metabolic organ which governs body energy metabolism. It acts as a hub to metabolically connect to various tissues, including skeletal muscle and adipose tissue. Food is digested in the gastrointestinal (GI) tract, and glucose, fatty acids, and amino acids are absorbed into the bloodstream and transported to the liver through the portal vein circulation system. In the postprandial state, glucose is condensed into glycogen and/or converted into fatty acids or amino acids in the liver. In hepatocytes, free fatty acids are esterified with glycerol-3-phosphate to generate triacylglycerol (TAG). TAG is stored in lipid droplets in hepatocytes or secreted into the circulation as very low-density lipoprotein (VLDL) particles. Amino acids are metabolized to provide energy or used to synthesize proteins, glucose, and/or other bioactive molecules. In the fasted state or during exercise, fuel substrates (e.g. glucose and TAG) are released from the liver into the circulation and metabolized by muscle, adipose tissue, and other extrahepatic tissues. Adipose tissue produces and releases nonesterified fatty acids (NEFAs) and glycerol via lipolysis. Muscle breaks down glycogen and proteins and releases lactate and alanine. Alanine, lactate, and glycerol are delivered to the liver and used as precursors to synthesize glucose (gluconeogenesis). NEFAs are oxidized in hepatic mitochondria through fatty acid β oxidation and generate ketone bodies (ketogenesis). Liver-generated glucose and ketone bodies provide essential metabolic fuels for extrahepatic tissues during starvation and exercise.
Liver energy metabolism is tightly controlled. Multiple nutrient, hormonal, and neuronal signals have been identified to regulate glucose, lipid, and amino acid metabolism in the liver. Dysfunction of liver signaling and metabolism causes or predisposes to nonalcoholic fatty liver disease (NAFLD) and/or type 2 diabetes.
1. LIVER GLUCOSE METABOLISM
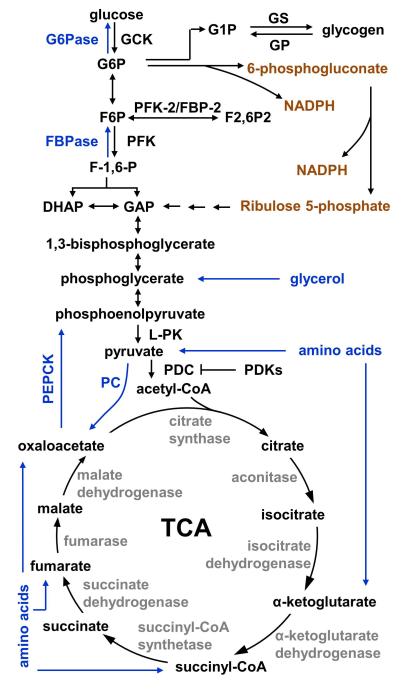
Hepatocytes are the main cell type in the liver (~80%). Blood glucose enters hepatocytes via GLUT2, a plasma membrane glucose transporter. Hepatocyte-specific deletion of GLUT2 blocks hepatocyte glucose uptake (231). GLUT2 also mediates glucose release from the liver; however, deletion of GLUT2 does not affect hepatic glucose production in the fasted state (231), suggesting that glucose is able be released from hepatocytes through additional transporters (e.g. GLUT1) or by other mechanisms. Glucose is phosphorylated by glucokinase in hepatocytes to generate glucose 6-phosphate (G6P), leading to a reduction in intracellular glucose concentrations which further increases glucose uptake (Fig. 1). Moreover, G6P is unable to be transported by glucose transporters, so it is retained within hepatocytes. In the fed state, G6P acts as a precursor for glycogen synthesis (Fig. 1). It is also metabolized to generate pyruvate through glycolysis. Pyruvate is channeled into the mitochondria and completely oxidized to generate ATP through the tricarboxylic acid (TCA) cycle (Fig. 1) and oxidative phosphorylation. Alternatively, pyruvate is used to synthesize fatty acids through lipogenesis (Fig. 3). G6P is also metabolized via the pentose phosphate pathway to generate NADPH (Fig. 1). NADPH is required for lipogenesis and biosynthesis of other bioactive molecules. In the fasted state, G6P is transported into the endoplasmic reticulum (ER) and dephosphorylated by glucose-6-phosphatase (G6Pase) to release glucose.
Fig. 1. Glucose metabolism pathways.
Gluconeogenic pathways are marked in blue, and the pentose phosphate pathway is marked in orange. GCK: glucokinase; G6Pase: glucose-6-phosphatase; G6P: glucose 1-phosphate; G1P: glucose 1-phosphate; GP: glycogen phosphorylase; GS: glycogen synthase; PFK: 6-phosphofructo-1 kinase; FBPase: fructose 1,6 bisphosphatase; F-1,6-P:; GAP: glyceraldehyde 3-phosphate; DHAP: dihydroxyacetone phosphate; L-PK: liver pyruvate kinase; PC: pyruvate carboxylase; PDC: pyruvate dehydrogenase complex; PDKs: pyruvate dehydrogenase kinases.
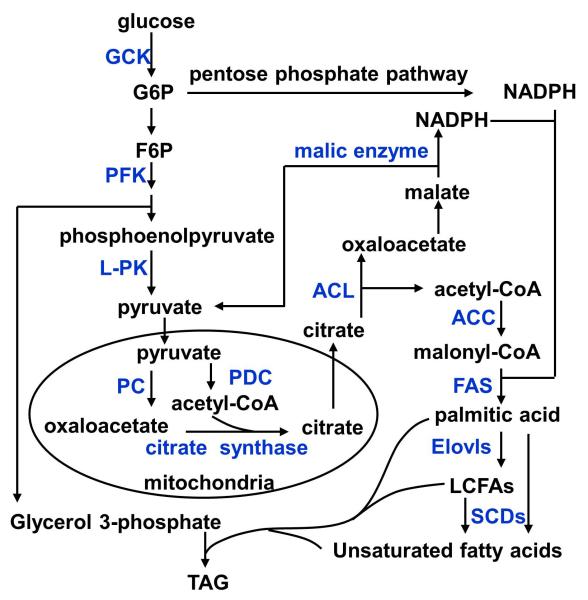
Fig. 3. Lipogenic pathways.
Lipogenic enzymes are marked in blue. ACL: ATP-citrate lyase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthase; Elovls: fatty acyl-CoA elongases; SCDs: stearoyl-CoA desaturases; TAG: triacylglycerol.
1.1. Glycogen metabolism
In the fed state, glucose enters hepatocytes via GLUT2 and is phosphorylated by glucokinase and used to synthesize glycogen by glycogen synthase (4). In the fasted state, glycogen is hydrolyzed by glycogen phosphorylase to generate glucose (glycogenolysis) (Fig. 1). G6P is a precursor for glycogen synthesis, and it also is an allosteric inhibitor of glycogen phosphorylase and an allosteric activator of glycogen synthase, thus further increasing liver glycogen levels (4). The activity of both glycogen synthase and glycogen phosphorylase is also regulated by posttranslational modifications. Phosphorylation of glycogen synthase, mainly by glycogen synthase kinase 3 (GSK-3), inhibits glycogen synthase activity; in contrast, phosphorylation of glycogen phosphorylase increases its activity. Both glycogen synthase and glycogen phosphorylase are able to be dephosphorylated by protein phosphatase 1. In the fed state, pancreatic β cells secret insulin in response to an increase in blood glucose, amino acids, and fatty acids. Insulin stimulates glycogen synthase by activating Akt which phosphorylates and inactivates GSK-3, thus increasing glycogen synthesis. Insulin stimulates acetylation of glycogen phosphorylase, which promotes dephosphorylation and inhibition of glycogen phosphorylase by protein phosphatase 1, thus suppressing glycogenolysis (288). Insulin stimulates the expression of glucokinase which increases hepatocyte glucose uptake indirectly by phosphorylating glucose and generating G6P (4). G6P in turn stimulates glycogen synthesis and inhibits glycogenolysis. Additionally, in the fasted state, the GI secretes fibroblast growth factor 15/19 (FGF15/19) which also stimulates glycogen synthesis (112). FGF15/19 stimulates the ERK/RSK pathway by activating its receptors FGFR4 and β-klotho, and activated RSK phosphorylates and inactivates GSK-3, a negative regulator of glycogen synthase (112).
In the fasted state, insulin and FGF15/19 secretion is downregulated, leading to inhibition of glycogen synthase and activation of glycogen phosphorylase. Moreover, glucagon and catecholamines (e.g. epinephrine and norepinephrine), collectively called counterregulatory hormones, are secreted from pancreatic α cells and the adrenal medulla, respectively. These counterregulatory hormones bind to their cognate G protein-coupled receptors and activate protein kinase A (PKA) by increasing intracellular cAMP levels. PKA phosphorylates and activates glycogen phosphorylase directly or indirectly by phosphorylating and activating phosphorylase kinases. Glucagon inhibits acetylation of glycogen phosphorylase, which decreases the ability of protein phosphatase 1 to bind to, dephosphorylate, and inactivate glycogen phosphorylase (288). Glycogen is also able to be hydrolyzed to generate glucose through autophagy in the fasted state (116).
1.2. Gluconeogenesis
During short-term fasting periods, the liver produces and releases glucose mainly through glycogenolysis. During prolonged fasting, glycogen is depleted, and hepatocytes synthesize glucose through gluconeogenesis using lactate, pyruvate, glycerol, and amino acids (Fig. 1). These gluconeogenic substrates are either generated in the liver or delivered to the liver through the circulation from extrahepatic tissues. Lactate is oxidized by lactate dehydrogenase to generate pyruvate. Pyruvate is transported into the mitochondria and converted to oxaloacetate by pyruvate carboxylase (Fig. 1). Oxaloacetate is reduced to malate by mitochondrial malate dehydrogenase, exported into the cytoplasm, and oxidized by cytoplasmic malate dehydrogenase to regenerate oxaloacetate. Cytoplasmic oxaloacetate is converted to phosphoenolpyruvate by cytoplasmic phosphoenolpyruvate carboxylase (PEPCK-C), a key step of gluconeogenesis. Systemic deletion of PEPCK-C causes postnatal death within 3 days after birth (233). Mice with hepatocyte-specific deletion of PEPCK-C are viable but are unable to produce glucose from lactate and amino acids via gluconeogenesis, leading to accumulation of TCA cycle intermediates in hepatocytes and hepatic steatosis in the fasted state (21). However, liver-specific PEPCK-C knockout mice are able to generate glucose from glycerol and maintain relatively normal blood glucose levels after 24 h of fasting (21, 233). Phosphoenolpyruvate, after multiple biochemical reactions, is converted into fructose 1,6-biphosphate (F1,6P) which is then dephosphorylated by fructose 1,6 bisphosphatase (FBPase) to generate fructose-6-phosphate (F6P). F6P is converted to G6P, transported into the ER, and dephosphorylated by G6Pase to generate glucose. Dephosphorylation of G6P is a rate-limiting step common for both glycogenolysis and gluconeogenesis. Mice with hepatocyte-specific deletion of G6Pase (which encodes the catalytic subunit) develop hyperlipidemia, lactic acidosis, uricemia, and hepatomegaly with glycogen accumulation and hepatic steatosis (175). Glycerol enters into hepatocytes via aquaporin-9 and is phosphorylated by glycerol kinase to generate glycerate-3 phosphate, a precursor for gluconeogenesis (92). Amino acids are converted to α-ketoacids through deamination reactions catalyzed by glutaminase, glutamate dehydrogenase, and/or aminotransferase. The α-ketoacids are further converted to intermediates of the TCA cycle (e.g. pyruvate, oxaloacetate, fumarate, succinyl-CoA, or α-ketoglutarate) which serve as precursors for gluconeogenesis.
1.2.1. Gluconeogenesis is regulated by the availability of gluconeogenic substrates
The rate of gluconeogenesis is determined by both the availability of gluconeogenic substrates and the expression/activation of gluconeogenic enzymes (e.g. PEPCK-C and G6Pase) which control key steps of gluconeogenesis (Fig. 1). During exercise or fasting, skeletal muscles produce pyruvate through glycogenolysis and glycolysis. Pyruvate has two fates. It can be catabolized by mitochondrial pyruvate dehydrogenase complex (PDC) to produce acetyl-CoA, which is then completely oxidized in the TCA cycle (Fig. 1). Alternatively, pyruvate can be converted into lactate, released into the circulation, and utilized by hepatocytes to produce glucose through gluconeogenesis. PDC is phosphorylated and inactivated by pyruvate dehydrogenase kinases (PDKs, 4 isoforms) (Fig. 1), and it is dephosphorylated and activated by pyruvate dehydrogenase phosphatases (93). PDK2 and PDK4 levels are higher in the fasted state and in diabetes (93). Deletion of PDK4 increases PDC activity, which allows pyruvate to be channeled to the TCA cycle for complete oxidation (95). As a result, pyruvate is not available for gluconeogenesis, leading to hypoglycemia in fasted PDK4 knockout mice (95). Glycerol, which is released from adipose tissue through lipolysis, is also a gluconeogenic substrate. Fatty acid β oxidation is unable to produce gluconeogenic substrates, but it does generate ATP which is required for gluconeogenesis. Prolonged starvation leads to protein degradation and release of amino acids, which are important gluconeogenic substrates.
1.2.2. Gluconeogenesis is regulated by gluconeogenic enzymes
Gluconeogenic enzymes are regulated by posttranslational modifications and/or allosteric regulation. Most liver enzymes, which regulate glycolysis, gluconeogenesis, the TCA cycle, the urea cycle, and fatty acid and glycogen metabolism, are acetylated (292). Acetylation states of these enzymes are regulated by nutrient availability (292). Glucose stimulates acetylation of PEPCK-C by p300, which promotes PEPCK-C ubiquitination and degradation (97). In contrast, cytosolic SIRT2 deacetylates and stabilizes PEPCK-C (97), which may contribute to increased gluconeogenesis in the fasted state. Fructose-2,6-bisphosphate (F-2,6-P2), which is derived from G6P (Fig. 1), binds to FBPase and inhibits the catalytic activity of FBPase, thus inhibiting gluconeogenesis in the fed state (215).
1.2.3. Gluconeogenesis is controlled by multiple transcription factors and coregulators
Hepatic gluconeogenesis is largely controlled in by transcriptional regulation of the enzymes which catalyze the key reactions of gluconeogenesis. Numerous transcription factors, including CREB, FOXO1, and C/EBPα/β, have been identified to stimulate the expression of PEPCK-C and G6Pase. CREB is a well-documented gluconeogenic transcription factor which is activated by PKA-mediated phosphorylation, and it stimulates the expression of PEPCK-C, G6Pase, and peroxisome proliferator γ-activated receptor coactivator 1-α (PGC-1α) (75). Inhibition of liver CREB, by liver-specific transgenic overexpression of a dominant negative form of CREB, decreases the expression of PEPCK-C, G6Pase, and PGC-1α, leading to reduced hepatic glucose production (HGP) and hypoglycemia (75). Knockdown of CREB in the liver reduces HGP in rodents with type 2 diabetes (52). Hepatocyte-specific deletion of FOXO1 decreases both glycogenolysis and gluconeogenesis in fasted mice, leading to hypoglycemia (158). Deletion of C/EBPα also decreases gluconeogenesis, and the mutant mice die from hypoglycemia within 8 h after birth (261). C/EBPα stimulates the expression of carbamoyl phosphate synthetase-1 (CPS-1) which controls the rate-limiting reaction of the urea cycle; therefore, C/EBPα is able to increase production of gluconeogenic substrates by promoting amino acid catabolism (89, 111). However, hepatocyte-specific deletion of C/EBPα does not affect the expression of PEPCK-C and G6Pase, and the mutant mice have normal blood glucose levels (89). These observations suggest that other C/EBP family members may have a compensatory function in the mutant mice, and indeed, deletion of C/EBPβ also decreases HGP and blood glucose in mice (144).
Several coactivators have been described to stimulate the expression of PEPCK-C and G6Pase in the liver. Both p300/CBP and cAMP-regulated transcriptional coactivator 2 (CRTC2) binds to CREB and stimulate the expression of PEPCK-C and G6Pase, thus increasing hepatic gluconenogenesis (115, 295). Systemic deletion of CRTC2 impairs both the expression of liver gluconeogenic genes and the ability of glucagon to stimulate glucose production in hepatocytes (123, 264). PGC-1α is higher in the fasted state and in diabetes (75, 284), and it promotes gluconeogenesis by coactivating HNF-4α (284). Steroid receptor coactivator-1 (SRC-1) coactivates C/EBPα and promotes expression of pyruvate carboxylase and other gluconeogenic genes, and deletion of SRC-1 results in hypoglycemia (149). SRC-2 stimulates G6Pase promoter activity by coactivating retinoid-related orphan receptor α (RORα), and genetic deletion of SRC-2 results in decreased G6Pase expression and hypoglycemia in fasted mice (38).
1.3. Gluconeogenesis is regulated by metabolic states and the circadian clock
Low energy states under fasting conditions are associated with activation of both SIRT and AMPK family members, whereas high energy states are associated with mTORC1 activation. SIRT, AMPK, and mTORC1 are considered molecular energy sensors. Many gluconeogenic transcriptional regulators are substrates of SIRT1, AMPK and/or TORC1. PGC-1α is acetylated by GCN5, and acetylation decreases the ability of PGC-1α to activate gluconenogenic genes (133). SIRT1 deacetylates PGC-1α, thus increasing its ability to coactivate HNF-4α for gluconeogenesis (216). Knockdown of SIRT1 in the liver decreases hepatic gluconeogenesis in mice with obesity (53, 217). Surprisingly, mice with hepatocyte-specific deletion of SIRT1 appear to be able to maintain relatively normal blood glucose levels (32, 270). Hepatic gluconeogenesis is even higher in these mice (263). In addition to deacetylating PGC-1α, SIRT1 also deacetylates CRTC2 during prolonged fasting, leading to degradation of CRTC2 and decreased gluconeogenesis (146). Both SIRT3 and SIRT5 are located in mitochondria, and their activity is higher in the fasted state (69, 179). SIRT3 deacetylates and activates ornithine transcarbmoylase (OTC), a key enzyme of the urea cycle (69). SIRT5 deacetylates and activates CPS-1 (179). Mitochondrial SIRT3 and SIRT5 are able to increase gluconeogenic substrate availability and hepatic gluconeogenesis during starvation by stimulating amino acid catabolism. The LKB1/AMP pathway suppresses hepatic glucose production. AMPK phosphorylates CRTC2 and blocks nuclear translocation of CRTC2, thus inhibiting the ability of CRTC2 to promote hepatic gluconeogenesis (115). Genetic deletion of AMPKα2 in the liver increases hepatic gluconeogenesis and glucose intolerance (5). Liver-specific deletion of LKB1 also increases hepatic gluconeogenesis and blood glucose levels (232). S6 kinase, a downstream effector of mTORC1, phosphorylates PGC-1α and inhibits its ability to bind to HNF-4α, thus inhibiting gluconeogenesis (152).
Circadian clock genes have been reported to regulate hepatic gluconeogenesis. Cryptochrome 1 (Cry1) and Cry2 bind to and inhibit glucocorticoid receptors (GR) (121). Glucocorticoids are important counterregulatory hormones and stimulate hepatic gluconeogenesis. Cry1 also inhibits the ability of glucagon, another important counterregulatory hormone, to stimulate HGP by uncoupling glucagon receptors from G α (287). Ubiquitin-specific protease 2 (UPS2) is a clock-regulated gene in the liver, and it increases hepatic gluconeogenesis by stimulating the expression of 11-hydroxysteroid dehydrogenase 1 (HSD1) (168). HSD1 converts inactive glucocorticoids into their active forms.
1.4. Regulation of gluconeogenesis by the ER
The ER is able to both positively and negatively regulate hepatic gluconeogenesis depending on the cellular context and the nature of downstream signaling pathways. CREBH is an ER-membrane protein, and its levels are higher in the fasted state (128). CREBH binds to CRTC2 and promotes the expression of gluconeogenic genes, including PEPCK-C and G6Pase (128). ER stress activates the unfolded protein response (UPR). Three UPR pathways, the protein kinase-like ER kinase (PERK)/elF2α, the inositol-requiring enzyme 1 (IRE1)/XBP1, and the ATF6 pathways, have been extensively characterized (101). The PERK/elF2α pathway stimulates HGP by increasing translation of C/EBPα and C/EBPβ (191). In contrast, XBP1 is able to bind to FOXO1 and target FoxO1 for degradation, thus inhibiting the hepatic gluconeogenesis (296). ATF6 binds to CRTC2 and inhibits the expression of gluconeogenic genes by sequestering CRTC2 from CREB (266). Moreover, chronic activation of the UPR pathways promotes insulin resistance, thus indirectly increasing HGP (101, 193).
1.5. Insulin suppresses hepatic gluconeogenesis
Insulin potently suppresses gluconeogenesis, and hepatocyte-specific deletion of insulin receptors markedly increases hepatic gluconeogenesis in mice, resulting in hyperglycemia and glucose intolerance (165). Insulin resistance is a determinant for the development of type 2 diabetes, and it also contributes to the pathogenesis of NAFLD. Insulin receptors bind to IRS1 and IRS2 and phosphorylate them on tyrosine residues (223, 272). Hepatocyte growth factor receptor Met is able to form a hybrid complex with insulin receptors in the liver to promote insulin signaling (55). Tyrosine phosphorylated IRS proteins activate the PI 3-kinase/Akt pathway (223, 272). Liver-specific inhibition of either IRS1 or IRS2 partially impairs insulin action; deletion of both IRS1 and IRS2 in the liver completely blocks hepatic insulin action, resulting in increased hepatic gluconeogenesis, hyperglycemia, and type 2 diabetes (49). Insulin stimulates mTORC2, which phosphorylates Akt at Ser473 and enhances Akt activity (90). Mice with hepatocyte-specific deletion of rictor, an essential component of the mTORC2 complex, have higher hepatic gluconeogenesis and develop hyperglycemia and insulin resistance (68). Akt phosphorylates and inactivates FOXO1 in the liver, thus suppressing gluconeogenesis (Fig. 3A) (67, 158, 178, 204). In contrast, MAPK phosphatase-3 (MKP-3) dephosphorylates FOXO1 at pSer256 and promotes nuclear translocation of FOXO1, which activates gluconeogenic genes and increases hyperglycemia (276). FOXO1 activity is also regulated by additional mechanisms. FOXO1 is acetylated on multiple sites by p300/CBP, and acetylation decreases the ability of FOXO1 to bind to the promoters of its target genes (162). FOXO1 interacts with C/EBPα, and these two proteins act cooperatively to promote gluconeogenesis (229). Wnt ligands in the liver are higher in the fasted state, and they increase the expression of PEPCK-C and G6Pase by stimulating the binding of β-catenin to FOXO1; deletion of β-catenin impairs HGP (143).
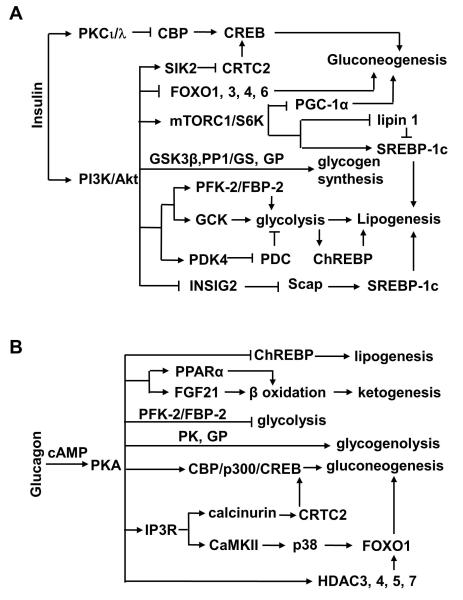
In addition to FOXO1, insulin also stimulates phosphorylation of FOXO3, FOXO4, and FOXO6 by Akt and inhibits their ability to stimulate hepatic gluconeogenesis (67, 105). Insulin stimulates phosphorylation of PGC-1α by Akt and decreases the ability of PGC-1α to activate gluconeogenic genes (Fig. 2A) (138). Insulin still suppresses HGP in mice with liver-specific triple knockout of Akt1, Akt2, and FoxO1 (150), suggesting that insulin is able to suppress HGP by Akt1/2/FOXO1-independent mechanisms. Insulin stimulates activation of SIK2 which phosphorylates CRTC2 and promotes cytoplasmic translocation and degradation of CRTC2, thus suppressing gluconeogenesis in hepatocytes (Fig. 2A) (46). Insulin also stimulates phosphorylation of CBP on Ser436 by atypical PKCι/λ, which disrupts the CREB/CBP/CRTC2 complex and inhibits gluconeogenesis (Fig. 2A) (72, 295); however, mice with liver-specific deletion of CBP have relatively normal insulin sensitivity, hepatic glucose production, and blood glucose (11).
Fig. 2.
Regulation of glucose and fatty acid metabolism by insulin (A) and glucagon (B) in the liver.
1.6. Glucagon stimulates hepatic gluconeogenesis
Glucagon is secreted from pancreatic α cells, and glucagon secretion is higher in the fasted state and during exercise (268). Genetic depletion of pancreatic α cells causes glucagon deficiency, resulting in improved glucose tolerance and decreased gluconeogenic gene expression, HGP, and blood glucose in the fasted state (70). Systemic deletion of glucagon receptors decreases blood glucose levels and improves glucose tolerance (62, 196). Glucagon receptor knockout mice resist diet-induced obesity, glucose intolerance, and hepatic steatosis (40). Streptozotocin (STZ)-induced insulin deficiency is associated with increased α cell number and hyperglucagonemia, and deletion of glucagon receptors decreases hepatic gluconeogenesis and fully rescues STZ-induced hyperglycemia and glucose intolerance (129). Silencing of liver glucagon receptors also reduces blood glucose and improves glucose tolerance in db/db mice and Zucker diabetic fatty rats (140, 238). Glucagon receptors, members of the G protein-coupled receptor family, activate the Gα-cAMP-PKA pathway (96). Liver-specific deletion of Gα results in glucagon resistance, hypoglycemia, and reduced expression of gluconeogenic genes (34). PKA phosphorylates and activates CREB which stimulates hepatic gluconeogenesis (Fig. 2B). CRTC2, a critical CREB coactivator, is phosphorylated by SIK2, and phosphorylated CRTC2 is then translocated from the nucleus to the cytoplasm, ubiquitinated, and degraded (46). PKA promotes dephosphorylation of CRTC2 and inhibits CRTC2 degradation (Fig. 2B) (146). PKA also phosphorylates and activates inositol-1,4,5-triphosphate receptors (IP3Rs), thus increasing the release of Ca2+ from the ER into the cytoplasm (Fig. 2B) (265). Ca2+ activates calcineurin which in turn dephosphorylates and stabilizes CRTC2, thus promoting gluconeogenesis (265). Glucagon also stimulates acetylation of CRTC2 by p300/CBP, which increases both the stability and gluconeogenic activity of CRTC2 (146).
Aside from stimulating the CREB/CRTC2 pathway, glucagon is able to stimulate gluconeogenesis through additional mechanisms. Glucagon stimulates Ca2+ release from the ER in hepatocytes via PKA-mediated phosphorylation of IP3R as described above. Ca2+ activates CaMKII which in turn promotes nuclear translocation of FOXO1 (Fig. 2B) (192). Hepatic gluconeogenesis is lower in CaMKIIγ null mice, and liver-specific overexpression of CaMKII increases gluconeogenesis (192). CaMKII activates p38 MAPK which in turn increases nuclear translocation and activity of FOXO1 (192). Activation of the p38 MAPK pathway stimulates HGP (26). FOXO1 is acetylated at multiple sites by p300/CBP, which reduces its ability to bind to the promoters of its target genes (162). Glucagon promotes deacetylation of FOXO1 (166). Glucagon stimulates dephosphorylation and nuclear translocation of HDAC4/5/7 which interact with both HDAC3 and FOXO1 at the promoters of FOXO1 target genes (Fig. 2B), thus allowing HDAC3 to deacetylate and activate FOXO1 (166). Glucagon also stimulates phosphorylation of IRE1α by PKA, and silencing of hepatic IRE1α impairs HGP (155).
1.7. Regulation of gluconeogenesis by growth hormone (GH) and nuclear receptors
GH and glucocorticoids are important counterregulatory hormones. GH stimulates the JAK2/STAT5 pathway (174). STAT5 directly binds to and stimulates the PEPCK-C promoter (110). GH also stimulates the expression of PDK4 through STAT5 (109). PDK4 phosphorylates PDC and inhibits PDC activity (94, 95), which blocks TCA cycle-mediated oxidation of pyruvate, thus channeling pyruvate to gluconeogenesis. The gluconeogenic action of GH is negatively regulated by multiple factors, including bile acids and fibroblast growth factor (FGF) 21. Bile acids activate nuclear receptor farnesoid X receptor (FXR) which stimulates the expression of SHP, a transcription repressor (249). SHP in turn inhibits the ability of STAT5 to bind to PEPCK-C and PDK4 promoters (109, 110), thus inhibiting hepatic gluconeogenesis. Liver-specific overexpression of constitutively active FXR decreases blood glucose (290). FGF21 is largely produced and secreted by hepatocytes (9, 85). It decreases STAT5 levels and causes GH resistance in the liver in an autocrine fashion, thus inhibiting GH-stimulated HGP (86).
The glucocorticoid receptor (GR), a member of the nuclear receptor family, resides primarily in the cytoplasm in quiescent cells in a complex with chaperones heat shock protein (HSP) 90 and HSP70 and cochaperones HSP40 and p23 (257). Ligand binding stimulates nuclear translocation of GR, which activates gluconeogenic genes (273). Hepatocyte-specific deletion of GR decreases both the expression of gluconeogenic genes and blood glucose levels in the fasted state and protects against STZ-induced hyperglycemia (190). Knockdown of GR in the liver also inhibits the expression of gluconeogenic genes and reduces hyperglycemia in db/db mice (131). HDAC6 dephosphorylates HSP90 and promotes GR-HSP90 complex assembly, and deletion of HDAC60 blocks ligand-induced nuclear translocation of GR and GR-stimulated expression of gluconeogenic genes PEPCK-C, G6Pase, FBPase, and pyruvate carboxylase in the liver (273). Additional, GR binds to STAT5 as a cofactor to promote GH-stimulated gluconeogenesis (174). GR expression is upregulated in hepatocytes by transcription factor Yin Yang 1 (YY1) which is elevated in the fasted state (151). Knockdown of YY1 in the liver ameliorates hyperglycemia in db/db mice (151). The liver X receptor (LXR), another member of the nuclear receptor family which is activated by oxysterols and controls cholesterol homeostasis (23), inhibits the gluconeogenic action of GR by competing for GR binding sites in the promoter of gluconeogenic genes (176). LXR activation also suppresses GR expression in hepatocytes (147). Surprisingly, genetic deletion of LXRb has been reported to impair the ability of GR to stimulate the expression of gluconeogenic genes and HGP (197).
1.8. Cytokines regulate hepatic gluconeogenesis
The liver houses many types of immune cells, including Kupffer, NK, NKT, and CD4+ T cells (10, 134, 211). These immune cells as well as hepatocytes secrete numerous cytokines which regulate hepatocyte metabolism in an autocrine/paracrine fashion. Insulin signaling in the hypothalamus stimulates IL-6 production in the liver, and IL6 in turn suppresses gluconeogenesis by activating STAT3 (87). STAT3 directly binds to the promoters of PEPCK-C and G6Pase and inhibits promoter activity (209). Hepatocyte specific deletion of STAT3 increases the expression of PEPCK-C, G6Pase, and PGC-1α; conversely, liver-specific overexpression of a constitutively active form of STAT3 decreases HGP and blood glucose levels in diabetic mice (88). IL-13 also stimulates tyrosine phosphorylation of STAT3 in hepatocytes, and genetic deletion of IL-13 increases hepatic gluconeogenesis (243). IL-13 null mice develop hyperglycemia and glucose intolerance (243). SIRT1 deacetylates STAT3 and inhibits tyrosine phosphorylation of STAT3, thus decreasing the ability of STAT3 to suppress HGP (182). However, chronic inflammation in the liver causes insulin resistance, leading to increased HGP (79). Liver inflammation also increases the ability of glucagon to stimulate HGP (36, 234).
1.9. GI hormones regulate hepatic gluconeogenesis
Several GI hormones, including glucagon-like peptide 1 (GLP-1), have been well established to regulate HGP indirectly by stimulating insulin secretion. GI-derived factors are also able to act directly on hepatocytes. Bile acids stimulate the expression and secretion of FGF15/19 from small intestines by activating FXR (84). Circulating FGF15/19 levels increase after food ingestion (202). FGF15/19 promotes dephosphorylation of CREB and inhibits the ability of CREB to activate PGC-1α and G6Pase genes, thus suppressing gluconeogenesis (202). Deletion of FGF19 or its receptor FGFR4 increases gluconeogenesis and blood glucose levels (202). Circulating serotonin levels are lower in the fed state and markedly increase during chronic fasting due to increased secretion from the gut, (247). Serotonin directly increases gluconeogenesis in hepatocytes by activating Htr2b receptors (247). Gut-specific deletion of tryptophan hydroxylate 1, which controls a rate-limiting reaction of the serotonin biosynthesis in peripheral tissues, impairs gluconeogenesis and protects against dietary glucose intolerance and insulin resistance (247). Hepatocyte-specific deletion of Htr2b also decreases hepatic gluconeogenesis (247).
1.10. Regulation of glycolysis
Hepatocytes have great flexibility in selecting metabolic fuels (glucose and/or fatty acids). Fuel selection is regulated by both nutrient and hormonal signals. Glycolysis is dominant in the fed state in which glucose is abundant. Glycolytic intermediates and products are used to synthesize lipids, amino acids, and other important molecules in addition to be completely oxidized to generate ATP. In the fasted state in which glucose levels are low, hepatocytes switch to fatty acid β oxidation for energy supply.
Glycolytic flux is controlled largely by four kinases: glucokinase (GCK), 6-phosphofructo-1 kinase (PFK), liver pyruvate kinase (L-PK), and PDKs (Fig. 1). The levels and activity of these glycolytic enzymes are lower in the fasted state and increase in the postprandial period (106). LRH-1, a nuclear receptor family member which is activated by several phosphatydylcholine species (127), stimulates GCK expression, and hepatocyte-specific deletion of LRH-1 decreases GCK levels and glycolysis (189). GCK binds to glucokinase regulatory protein (GKRP) at low glucose concentrations (4). GKRP, which is exclusively expressed in the liver, inhibits GCK activity by sequestering GCK in the nucleus (4). Glucose induces dissociation of GCK from GKRP, allowing GCK to be translocated into the cytoplasm and phosphorylate glucose (4). F-2,6-P2 is a potent allosteric activator of PFK and stimulates glycolysis in hepatocytes (215). F-2,6-P2 also suppresses gluconeogenesis by inhibiting FBPase (215). Both the generation and clearance of F-2,6-P2 is controlled by a single enzyme called bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBP-2) (215). In the fed state, both insulin and carbohydrates stimulate the kinase activity of PFK-2/FBP-2 which phosphorylates fructose-6-phosphate, a glycolytic intermediate, to generate F2,6P2 (188). In the fasted state, glucagon stimulates the phosphatase activity of PFK-2/FBP-2 by PKA-mediated phosphorylation, thereby decreasing F2,6P2 levels and glycolysis (188, 215). Glucose activates carbohydrate response element binding protein (ChREBP), also called Williams-Beuren syndrome critical region 14 (WBSCR14) (255). ChREBP binds to the E-box motifs in the L-PK promoter and activates L-PK expression in hepatocytes (280). Insulin suppresses the expression of PDK4 (Fig. 2A), a negative regulator of PDC as described above, by inhibiting FOXO1, thus increasing pyruvate consumption and glycolysis (93).
2. LIVER FATTY ACID METABOLISM
When carbohydrates are abundant, the liver not only utilizes glucose as the main metabolic fuel but also converts glucose into fatty acids. Hepatocytes also obtain fatty acids from the bloodstream, which are released from adipose tissue or absorbed from food digestion in the GI. Fatty acids are esterified with glycerol 3-phosphate to generate TAG (Fig. 3) or with cholesterol to produce cholesterol esters. TAG and cholesterol esters are either stored in lipid droplets within hepatocytes or secreted into the circulation as VLDL particles. Fatty acids are also incorporated into phospholipids, which are an essential component of cell membranes, and the surface layer of lipid droplets, VLDL, and bile particles. In the fasted state, fatty acids are oxidized mainly in the mitochondria to generate energy supply as well as ketone bodies.
2.1. Hepatocyte fatty acid uptake and trafficking
After a meal, dietary fat is digested mainly in the small intestine and absorbed into enterocytes in which fatty acids are resynthesized into TAG and secreted into the gut lymphatic system as chylomicrons. Chylomicrons arrive at the liver through the circulation and release NEFAs through lipolysis which is mainly mediated by lipoprotein lipase (LPL). Transgenic mice with liver-specific overexpression of LPL develop hepatic steatosis and insulin resistance (108). Hepatic CREBH stimulates the expressing of LPL coactivators (e.g Apoa4, Apoa5, and Apoc2) and suppresses the expression of LPL inhibitor Apoc3, thus promoting plasma TAG clearance from the circulation (125). NEFAs enter into hepatocytes mainly through CD36, fatty acid transport protein 2 (FATP4), FATP4, and FATP5. Pregnane X receptor (PXR) activates the expression of CD36 in hepatocytes, increasing hepatocyte fatty acid uptake and TAG levels (294). Aryl hydrocarbon receptor (AhR) activation also increases hepatocyte CD36 expression, fatty acid uptake, and steatosis (126). FATP5 is exclusively expressed in the liver, and deletion of FATP5 decreases hepatocyte fatty acid uptake and lipid levels in FATP5 null mice (48). FATP2 also mediates liver fatty acid uptake, and knockdown of FATP2 in the liver decreases NEFA uptake and reduces high fat diet (HFD)-induced hepatic steatosis (56). FATP2 and FATP4 reside mainly in peroxisomes and mediate transport of long-chain fatty acids (LCFAs) into peroxisomes (56, 245). FATP2 also has very long-chain acyl-CoA synthetase activity (56, 245). LCFAs are activated and converted to LCFA-CoA by long chain acyl-CoA synthetase (ACSL) 128,129. Mammals express five ACSL family members (ACSL1 and 3-6) 128,129. ACSL1 and 5 are highly expressed in the liver (18, 135), and knockdown of ACSL5 decreases lipid levels in cultured hepatocytes (18); however, liver-specific deletion of ACSL1 does not alter lipid levels in the liver (135). Fatty acid binding proteins (FABPs) bind to both LCFAs and LCFA-CoA and act as intracellular fatty acid chaperones and carriers. Mammals express a single FABP in the liver (L-FABP). L-FABP delivers its bound LCFAs to the nucleus to activate PPARα, a nuclear receptor family member which promotes fatty acid β oxidation (156, 245). Deletion of L-FABP decreases hepatocyte fatty acid uptake, suppresses β oxidation, and protects against dietary steatosis (180, 181). A separated study reported that the liver pool of NEFAs and TAG are relatively normal or higher in L-FABP null mice (156). The null mice have a compensatory increase in the expression of sterol carrier protein-2 (SCP-2) which also binds LCFAs (156).
2.2. De novo Fatty acid synthesis
The liver is the main organ which converts carbohydrates into fatty acids. Fatty acids are packed into VLDL particles and delivered to adipose tissue and other extrahepatic tissues through the bloodstream.
2.2.1. The hepatic lipogenic programs
Glucose is hydrolyzed into pyruvate through glycolysis. Pyruvate is imported into the mitochondria and metabolized by PDC to generate acetyl-CoA (Fig. 3). Acetyl-CoA is combined with oxaloacetate by citrate synthase to form citrate (Fig. 3). Citrate is exported into the cytoplasm and split into acetyl-CoA and oxaloacetate by ATP-citrate lyase (ACL). Oxaloacetate is reduced to malate which is converted into pyruvate by malic enzyme, releasing NADPH (Fig. 3). Pyruvate is recycled back into the mitochondria and carboxylated by pyruvate carboxylase (PC) to form oxaloacetate which drives continuous citrate synthesis (Fig. 3).
In the cytoplasm, acetyl-CoA is carboxylated by acetyl-CoA carboxylase (ACC) to form malonyl-CoA (Fig. 3). Both malonyl-CoA and NADPH are used as precursors to synthesize palmitic acid (a 16-carbon fatty acid) by fatty acid synthase (FAS). Mammals have two ACC genes, ACC1 and ACC2 whose products are located in the cytoplasm and mitochondrial outer membrane, respectively. Systemic deletion of ACC1 causes embryonic death (3). Hepatocyte-specific deletion of ACC1 decreases the levels of malonyl-CoA, TAG, and de novo lipid synthesis (154). However, a separate study has reported that hepatocyte-specific deletion of ACC1 does not alter malonyl-CoA levels and lipogenesis in the liver, presumably due to a compensatory increase in ACC2 expression (71). Transient inhibition of both ACC1 and ACC2 in the liver decreases levels of hepatic malonyl-CoA and lipogenesis, increases β oxidation, and protects against hepatic steatosis (226). Mice with liver-specific deletion of FAS are relatively normal (31), suggesting that fatty acid uptake is sufficient to maintain normal hepatic lipid content in the absence of liver FAS. Surprisingly, after being fed a zero-fat/high carbohydrate diet, mutant mice develop fatty livers and hypoglycemia which are reversed by treatments with PPARα agonists (31). FAS products are believed to serve as endogenous ligands for PPARα and stimulate fatty acid β oxidation in the liver (30, 31).
Palmitic acid is elongated by fatty acyl-CoA elongase (Elovl) family members in the ER to generate LCFAs (>16 carbon-chain) (Fig. 3). Deletion of Elovl6 protects against hepatic steatosis and liver inflammation in mice fed an atherogenic high fat diet (AHF); conversely, liver-specific overexpression of Elovl6 increases AHF-induced fatty liver and liver fibrosis (161). LCFAs are desaturated by stearoyl-CoA desaturases (SCDs), ER membrane enzymes, to form mono- and poly-unsaturated LCFAs (Fig. 3). Global knockout of SCD1, which catalyzes the synthesis of monounsaturated LCFAs, protects against obesity (39, 184). Hepatocyte-specific deletion of SCD1 also protects against high carbohydrate diet-induced, but not HFD-induced, obesity and hepatic steatosis (167). SCD1 products, particularly oleate, appear to be important regulators of glucose and lipid metabolism in the liver (167).
2.2.2. Regulation of de novo lipogenesis by the availability of lipogenic substrates
Dietary carbohydrates drive lipogenesis. Pyruvate, the main glycolytic product, provides a carbon source for lipogenesis and links glycolysis to lipogenesis. GCK catalyzes the first chemical reaction of glycolysis, and GCK activity is negatively regulated by GCKR. A variant in the GCKR gene is associated with hepatic steatosis and hyperglycemia in patients with obesity (225). LRH stimulates GCK expression, and hepatocyte-specific deletion of LRH-1 decreases GCK levels, glycolysis, and de novo lipogenesis in the liver (189). NADPH provides the reducing power for lipogenesis. Malate is metabolized by malic enzyme to generate NADPH. Moreover, glucose catabolism through the pentose phosphate pathway provides an additional NADPH source for lipogenesis (Fig. 3). Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, which catalyze the reactions to generate NADPH, are likely to be involved in the regulation of lipogenesis.
2.3. Lipogenesis is controlled by multiple transcription factors and coregulators
Lipogenesis is controlled in a large part through transcriptional regulation of glycolytic genes and lipogenic genes. Numerous transcription regulators have been identified to activate these genes. Many regulators also regulate the expression of additional genes which are involved in the regulation of lipid uptake, trafficking, and/or storage.
2.3.1. ChREBP
ChREBP binds to and activates the L-PK promoter in hepatocytes (280). L-PK is a key glycolytic enzyme. ChREBP also stimulates the expression of lipogenic genes, including malic enzyme, ACL, ACC, FAS, SCD1, and Elovls (82). Systemic deletion of ChREBP decreases the expression of these genes, thus inhibiting glycolysis and hepatic lipogenesis, and glucose is then used to synthesize glycogen in the liver in ChREBP null mice (82). Conversely, overexpression of ChREBP in the liver causes hepatic steatosis without concomitant insulin resistance (13). ChREBP levels are elevated in obese mice, and genetic deletion of ChREBP, or liver-specific inhibition of ChREBP, decreases hepatic lipogenesis and steatosis in ob/ob mice (45, 83).
ChREBP binds to Max-like protein X (Mlx), and the heterodimer acts as a functional transcription factor (244). ChREBP is phosphorylated and inhibited by PKA, and dephosphoralated and activated by PP2A (102). Glucagon stimulates phosphorylation of ChREBP at Ser196 by activating the cAMP/PKA pathway (Fig. 2B), resulting in nuclear export and inactivation of ChREBP in the liver (44, 47). Phosphorylated ChREBP binds to 14-3-3 and is retained in the cytoplasm (163, 222). Glucose is a potent activator of ChREBP. Glucose is oxidized to generate xylulose 5-phosphate through the pentose phosphate pathway. Xylulose 5-phosphate activates PP2A, which dephosphorylates ChREBP, promoting nuclear translocation and activation of ChREBP (99). G6P, a glycolytic intermediate, binds to and activates ChREBP in hepatocytes (47). Additionally, F-2,6-P2, a G6P-drived product, also stimulates nuclear translocation of ChREBP (7). Glucose promotes acetylation of ChREBP on Lys672 by p300, which increases ChREBP activity (17). Additionally, ChREBP binds to and is glycosylated by O-linked β-N-acetylglucosamine transferase (OGT), and O-GlcNacylation of ChREBP increases ChREBP stability (65).
2.3.2. SREBP
The SREBP family members (SREBP-1a, -1c and -2) are master regulators of lipid metabolism (78). Both SREBP-1a and SREBP-1c are encoded by a single gene and have different N-termini; SREBP-2 is encoded by a separate gene (78). Both SREBP-1c and SREBP-2 are abundantly expressed in the liver (78). SREBP1-c activates the genes that control fatty acid and TAG synthesis, and SREBP-2 activates the genes that control cholesterol biosynthesis (78). SREBP-1b promotes both fatty acid and cholesterol synthesis (78).
SREBPs are integral ER membrane proteins. They are translocated to the Golgi and cleaved sequentially by SIP1 and SIP2 proteases to release transcriptionally-active SREBPs (78). ER stress promotes proteolytic cleavage and activation of SREBP-1c in the liver, increasing lipogenesis (100). Low levels of cholesterol potently stimulate SREBP processes in hepatocytes (78). SREBP precursors bind to Scap which is a cholesterol sensor and required for ER-Golgi transport of SREBPs (78). Hepatocyte-specific deletion of Scap markedly decreases hepatic NEFAs, TAG synthesis, and hepatic steatosis in both ob/ob mice and mice with diet-induced obesity (169). Inhibition of phosphatidylcholine biosynthesis reduces phosphatidylcholine pools in hepatocytes, which promotes SREBP-1 cleavage and activation (258). Reduction in phosphatidylcholine/phosphatidylethanolamine ratios may cause relocation of S1P and S2P to the ER, increasing proteolytic activation SREBP-1 (258). SREBP activation is also subjected to posttranslational modifications. AMPK phosphorylates SREBP-1c at Ser372 and inhibits proteolytic cleavages and nuclear translocation of SREBP-1c, thus suppressing hepatic lipogenesis (139). SIRT1 deacetylates and inhibits SREBP-1c,suppressing lipogenesis in the liver (200, 259). SREBP activation is inhibited by nuclear translocation of lepin 1 (198). The mTORC1 complex phosphosphorylates lipin 1 and promotes cytoplasmic translocation of lipin 1, thus stimulating SREBP-1 activity and lipogenesis (198). PGC-1β is a coactivator for SREBP family members and simulates liver lipogenesis (141). Knockdown of PGC-1β in the liver decreases the expression of lipogenic genes and ameliorates fructose-induced hepatic steatosis (177).
2.3.3. LXR and FXR
LXR has two isoforms (α and β) in rodents, and each isoform forms heterodimers with the retinoid X receptor (RXR) to activate its target genes (23). LXR is activated by cholesterol metabolites called oxysterols (35, 91). LXR is well known to control reverse cholesterol transport by stimulating the expression of ATP-binding cassette transporter A1 (ABCA1) and ABCG1 (23). It also stimulates de novo fatty acid biosynthesis (23). LXR directly binds to the SREBP1 promoter and increases SREBP-1c expression (214, 228). It also stimulates ChREBP expression (29). Additionally, LXRα directly stimulates expression of PFK-2/FBP-2, which produces F-2,6-P2 to stimulate glycolysis (291). LXR agonists stimulate the expression of lipogenic genes and increase both liver and plasma TAG levels in wild-type, but not LXRα/β double knockout, mice (228).
Farnesoid X receptor (FXR), a nuclear receptor family member which is activated by bile acids, suppresses bile acid synthesis in a negative feedback fashion (23, 237). It stimulates the expression of SHP, a transcription repressor which inhibits the expression of Cyp7a (23, 237). Cyp7a catalyzes hydroxylation of cholesterol, a rate-limiting step of bile acid biosynthesis. FXR also regulates many genes which regulate NEFA and TAG metabolism (157, 199, 237, 249). SHP, the main transcriptional target of FXR, suppresses the ability of LXR to stimulate the expression of lipogenic SREBP-1 (269). FXR binds to and inhibits ChREBP, suppressing both glycolysis and lipogenesis (27). Bile acids stimulate the expression and secretion of FGF15/19 from the GI by activating FXR in enterocytes. FGF15/19 suppresses lipogenesis in the liver (16). FXR knockout mice have higher levels of TAG in both the circulation and the liver (237). FXR is acetylated by p300 and deacetylated by SIRT1, and SIRT1-mediated deacetylation increases FXR activity (103). Obesity is associated with higher levels of FXR acetylation in the liver (103). PGC-1α serves as a coactivator for some FXR target genes (289).
2.3.4. PPARγ and PPARδ
The levels PPARγ in the liver are low in normal mice and increase in mice with obesity (170)184,185. Hepatic PPARγ stimulates the expression of many genes which control fatty acid uptake, fatty acid trafficking, and TAG biosynthesis in the liver (130). PPARγ also stimulates the expression of Cidec, a lipid droplet protein (160). Hepatocyte-specific deletion of PPARγ suppresses the expression of many lipogenic genes and protects against hepatic steatosis in mice fed a HFD (170). Ablation of liver PPARγ ameliorates hepatic steatosis in ob/ob as well as in lipoatrophic A-ZIP/F-1 mice (61, 159). Expression of hepatic PPARγ is repressed by the hairy enhancer of split 1 (HES-1) in the liver (74). CREB stimulates the expression of HES-1 which in turn suppresses PPARγ expression and lipogenesis in the fasted state (74); however, a separate study has reported that knockdown of CREB in the liver decreases hepatic lipogenesis in rodents with type 2 diabetes (52). Like PPARγ, PPARδ also activates lipogenic genes, and liver-specific expression of PPARδ increases liver lipid levels in mice (145).
2.4. Insulin stimulates lipogenesis in the liver
Insulin is the primary hormone which stimulates hepatic lipogenesis in the fed state. The PI 3-kinase/Akt pathway is required for both insulin suppression of gluconeogenesis and insulin stimulation of lipogenesis; however, lipogenesis and gluconeogenesis are mediated by two distinct pathways downstream of Akt (136). Insulin stimulates activation of mTORC1 through the PI 3-kinase/Akt pathway, and mTORC1 is required for insulin to stimulate SREBP-1 expression and lipogenesis (Fig. 2A) (136). Akt, particularly Akt 2, stimulates SREBP-1 activation and lipogenesis (260, 283). Inhibition of hepatic Akt by hepatocyte-specific deletion of rictor inhibits both glycolysis and lipogenesis (68). Disruption of mTORC1 signaling in the liver, by deleting Raptor, prevents dietary hepatic steatosis (198). mTORC1 phosphosphorylates lipin 1 and blocks its ability to suppress SREBP-1 (Fig. 2A) (198). Activation of mTORC1 alone is not sufficient to stimulate lipogensis in the liver (260, 283). Akt suppresses the activity of INSIG2 (Fig. 2A), an ER membrane protein which binds to Scap, blocks the ER-Golgi translocation of SREBPs, and inhibits proteolytic activation of SREBPs (283). Insulin stimulates the expression of SREBP-1, and LXR is involved in mediating insulin action (33, 250). Insulin stimulates phosphorylation of upstream stimulatory factor-1 (USF-1) through DNA-PK (274). USF-1 stimulates expression of FAS and mitochondrial glycerol-3-phosphate acyltransferase (mGPAT); phosphorylation increases acetylation and activation of USF-1 (274). Deletion of USF-1 or USF-2 markedly suppresses carbohydrate-stimulated expression of FAS in the liver during a fasting/feeding transition (28). Additionally, insulin stimulates glycolysis as described before, thus increasing the availability of lipogenic precursors.
2.5. Regulation of lipogenesis by metabolic states, the circadian clock, and ER stress
Hepatic lipogenesis is low in the fasted state and high in the fed state. SIRT1 is activated in the fasted state, and it deacetylates and inhibits lipogenic SREBP-1c (200, 259). Hepatocyte-specific deletion of SIRT1 exacerbates dietary hepatic steatosis (206). SIRT1 binds to and is inhibited by deleted in breast cancer-1 (DBC1) (107, 293). Fasting decreases DBC1-SIRT1 interaction in the liver, increasing SIRT1 activity (54). Deletion of DBC1 increases SIRT1 activity in the liver and protects against dietary hepatic steatosis in mice (54). AMPK phosphorylates SREBP-1c at Ser372 and inhibits proteolytic cleavage and nuclear translocation of SREBP-1c, thus suppressing hepatic lipogenesis (139). In the liver, mTORC1 is inhibited in the fasted state and activated in the fed state (230), and mTORC1 stimulates lipogenesis as described above.
Chronic ER stress promotes hepatic steatosis (219). The PERK/elF2α pathway stimulates both hepatic glucose production and lipogenesis by increasing the translation of C/EBPα and C/EBPβ as well as the expression of PPARγ (191). Liver-specific overexpression of C/EBPβ induces hepatic steatosis (207); conversely, deletion of C/EBPβ ameliorates hepatic steatosis in db/db mice (227). XBP1 activates the expression of key lipogenic genes in hepatocytes, including SREBP1, DGAT2 and ACC2 (124, 183); however, IRE1α is able to degrade the mRNAs of some lipogenic enzymes, suppressing hepatic lipogenesis (239).
Core circadian genes are involved in hepatic lipid metabolism. Mice with liver-specific deficiency of molecular clock Rev-erbα/β develop hepatic steatosis (20, 59). Genomic recruitment of HDAC3 displays a circadian rhythm and is controlled by circadian clocks, and the rhythmic recruitment of HDAC3 regulates circadian rhythm of hepatic lipogenesis (59). Hepatocyte-specific deletion of HDAC3, increases lipogenic gene expression, resulting in hepatic steatosis in mice (113).
2.6. Fatty acid β oxidation and ketogenesis
Liver fatty acid β oxidation is high in the fasted state and low in the fed state. Mitochondrial β oxidation not only provides energy for hepatocytes but also generates ketone bodies (β-hydroxybutyrate, acetoacetate, and acetone) which are exported into the circulation and provide metabolic fuels for extrahepatic tissues during fasting. LCFA-CoA translocation into mitochondria, which is mediated by carnitine palmitoyltransferase 1 (CPT-1), is a rate-limiting step for fatty acid β oxidation. CPT-1 activity is inhibited by malonyl-CoA. Mitochondrial ACC2 generates malonyl-CoA and increases local malonyl-CoA concentrations, thus inhibiting CPT-1 activity and β oxidation (1). Systemic deletion of ACC2 increases mitochondrial fatty acid β oxidation, leading to lean phenotypes (2). Long-chain acyl-CoA dehydrogenase (LCAD) activity is also regulated through posttranslational modifications. Deletion of LCAD leads to hepatic steatosis and insulin resistance (286).
PPARα is the master regulator of fatty acid β oxidation and promotes fatty acid β oxidation in both the mitochondria and peroxisomes (104). PPARα expression in the liver is higher in the fasted state, and deletion of PPARα decreases hepatic fatty acid β oxidation in the fasted state and exacerbates fasting-induced hepatic steatosis, hypoglycemia, hypoketonemia, and hypothermia (104, 132). PPARα is a nuclear receptor family member activated by a subtype of LCFAs and phosphatidylcholines (30). FAS products appear to generate endogenous PPARα ligands in the liver (30, 31). PPARα agonist treatments correct hepatic steatosis and hypoglycemia in mice with liver-specific deletion of FAS that are fed a zero-fat, high carbohydrate diet (31). PPARα ligands are able to be inactivated through peroxisomal β oxidation, and deletion of peroxisomal fatty acyl-CoA oxidase increases PPARα activity in the liver (57), and decreases hepatic steatosis and obesity in ob/ob mice (80).
Multiple PPARα coactivators have been identified to promote β oxidation in the liver. PGC-1α is a well-characterized PPARα coactivator which promotes β oxidation (256). In the fasted state, SIRT1 deacetylates PGC-1α and increases its activity (216). SIRT1 also physically interacts with PPARα and promotes PPARα transcriptional activity in the liver (206). Hepatocyte-specific deletion of SIRT1 decreases the expression of β oxidative genes and β oxidation, increases fasting-induced lipid accumulation in the liver, and exacerbates diet-induced steatosis (206). Hepatic lipin 1, which is higher in the fasted state, binds to both PPARα and PGC-1α in the nucleus and promotes β oxidation (60). In the fed state, insulin stimulates phosphorylation of PGC-1α by Akt, which impairs the ability of PGC-1α to stimulate fatty acid β oxidation (138). Activation of mTORC1 also inhibits PPARα activity, β oxidation, and ketogenesis in the fed state (230). PGC-1α binds to BAF60α, a subunit of the SWI/SNF chromatin-remodeling complex; the PGC-1α/BAF60α complex interacts with PPARα and mediates PPARαactivated expression of β oxidation genes in the liver (137). BAF60α overexpression in the liver increases β oxidation and ameliorates hepatic steatosis in mice with obesity (137). PGC-1β and transducin beta-like 1 (TBL) also serve as PPARα coactivators. Hepatocyte-specific overexpression of PGC-1β increases the expression of β oxidative genes and protects PGC-1β transgenic mice from diet-induced steatosis (12). Knockdown of TBL1 or its partner TBLR1 in the liver inhibits PPARα activity, decreases β oxidation and ketogenesis, and promotes hepatic steatosis (117).
Multiple factors regulate β oxidation through PPARα. Fasting stimulates expression and secretion of FGF21 from the liver (9, 85). Glucagon stimulates FGF21 secretion in both rodents and humans (6, 66). FGF21 stimulates the expression of PGC-1α in the liver in an autocrine/paracrine fashion, thus increasing fatty acid β oxidation (Fig. 2B), TCA flux, and ketogenesis (203). Glucagon also stimulates β oxidation in the liver by a PPARα-dependent mechanism (Fig. 2B) (148). Glucagon deficiency is associated with higher TAG levels in the liver (70). Deletion of glucagon receptors abolishes fasting-stimulated β oxidation in hepatocytes (148). Glucagon secretion increases during exercise, and exercise attenuates hepatic steatosis in mice with dietary obesity (14). Deletion of glucagon receptors abrogates protection against hepatic steatosis by exercise (14). FGF15/19, a GI-derived hormone, inhibits PGC-1α expression and β oxidation in the liver (202).
In addition to PPARα, hepatic PPARβ/δ is believed to act as a plasma free fatty acid sensor and promote hepatic β oxidation in the liver (224). A subset of PPARα target genes, which stimulate fatty acid β oxidation, may also be stimulated by PPARβ/δ in the liver (224). Mitochondrial SIRT3, which is upregulated in the fasted state, deacetylates and activates LCAD in the liver, thus promoting fatty acid β oxidation (76). SIRT3 also deacetylates and activates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 (HMGCS2), promoting ketogenesis in the fasted state (236). SIRT6 is a nuclear, chromatin-associated protein which promotes resistance to DNA damage and suppresses genomic instability (173). Hepatic SIRT6 is higher in the fasted state, promotes β oxidation, and suppresses glycolysis; liver-specific deletion of SIRT6 promotes hepatic steatosis (106).
2.7. Liver-extrahepatic tissue crosstalk
The liver has close communications with extrahepatic tissues, including adipose tissue and skeletal muscle. Liver-produced glucose and ketone bodies are delivered to muscle and other extrahepatic tissues and are used as metabolic fuels during fasting and exercise; in return, skeletal muscle provides the liver with lactate and amino acids which serve as gluconeogenic substrates for hepatocytes to synthesize glucose. Adipose tissue produces NEFAs and glycerol through lipolysis during fasting and exercise. Hepatocytes oxidize fatty acids to generate ketone bodies or pack NEFAs into VLDL particles. Ketone bodies and VLDL are secreted from the liver and utilized by extrahepatic tissues. Glycerol is used by hepatocytes to synthesize glucose or TAG.
2.7.1. Liver-adipose tissue crosstalk
NEFAs released from adipose tissue through lipolysis are the main source for liver TAG pools, and hepatocyte fatty acid uptake provides ~59% of the supply of liver fat in humans with NAFLD (50). In adipocytes, TAG is stored in lipid droplets (LDs) which consist of a neutral lipid core (triacylglycerol and cholesterol esters) covered by a phospholipid monolayer (64). LDs are believed to be generated from the ER and coated with perilipin family proteins, enzymes, and vesicle trafficking proteins. LD proteins, including perilipins, tail interacting protein 47, (TIP47), and adipose differentiation related protein (ADRP), and cell death-inducing DNA fragmentation factor-like effector (CIDE) family members, are involved in the regulation of lipolysis (64, 205). TAG is hydrolyzed mainly by ATGL to release NEFAs and diacylglycerol (DAG) (285). DAG is further hydrolyzed by hormone-sensitive lipase (HSL) to release NEFAs and monoacylglycerol (MAG), and MAG is completely hydrolyzed by MAG lipase (MGL) and generate glycerol and NEFAs (285). HSL is also able to hydrolyze retinyl esters and cholesterol esters (285). CGI-58, an endogenous activator of ATGL, binds to perilipins under basal conditions; catecholamine hormones stimulate phosphorylation of perilipins which releases CGI-58, allowing it to activate ATGL and stimulate lipolysis (63, 122). In contrast to CGI-58, G0S2 binds to and inhibits ATGL (282). Adipocyte-specific deletion of ATGL blocks lipolysis and the release of NEFAs, thus reducing β oxidation and ketogenesis in the liver during starvation (275).
Adipose tissue also regulates liver energy metabolism by secreting a variety of adipokines, including adiponectin and cytokines (208). Adiponectin stimulates β oxidation in the liver and improves liver insulin sensitivity (278, 281). IL-6 is able to suppress insulin signaling by stimulating expression of SOCS3 in the liver (221). SOCS3 inhibits insulin signaling by both promoting IRS protein degradation and uncoupling IRS proteins from insulin receptors (218, 253). Adipocyte-specific deletion of JNK1 decreases secretion of IL-6 by adipose tissue and improves liver insulin sensitivity and hepatic steatosis in mice with dietary obesity (221). C1q/TNF-related protein-12 (CTRP12), an adiponectin-related adipokine secreted mainly from adipocytes, activates the PI 3-kinase/Akt pathway and suppresses hepatic gluconeogenesis (271). FABP4 (also called aP2) is secreted by white adipose tissue, and secretion is higher in the fasted state (25). FABP4 directly stimulates gluconeogenesis in hepatocytes (25). C16:1n7-palmitoleate is secreted by adipose tissue and acts as a lipid hormone to suppress hepatic steatosis (24). Additionally, adipose tissue is able to regulate liver metabolism indirectly by secreting hormones (e.g. leptin) which act on the brain to regulate liver metabolism (172). The liver also regulates the metabolic activity of adipose tissue. FGF21 is an important metabolic hormone secreted mainly from the liver in the fasted state (9, 85). Glucagon stimulates FGF21 secretion in both rodents and humans (6, 66). FGF21 stimulates both lipolysis and the expression and secretion of adiponectin by adipose tissue (6, 66, 77, 142). GH is secreted from the pituitary gland. It stimulates not only hepatic gluconeogenesis but also adipocyte lipolysis. Liver-specific deletion of GH receptors causes liver GH resistance, resulting in a compensatory increase in the levels of circulating GH which promotes adipocyte lipolysis and hepatic steatosis (58). Liver-specific deletion of JAK2 or STAT5 also causes GH resistance in the liver and increases compensatory GH secretion, thus increasing adipocyte lipolysis and hepatic steatosis (42, 240).
2.7.2. Liver-gut crosstalk
The gut is anatomically connected to the liver by the portal vein circulation. Most absorbed nutrients, GI hormones, and GI metabolites are directly delivered to the liver. Some metabolites from gut microbiota are also delivered to the liver via the portal vein circulation (73). These biologically active molecules directly regulate liver glucose and lipid metabolism. The GI also regulates liver metabolism indirectly through the central nervous system (CNS). In response to food ingestion, nutrient signals, encoded by duodenum lipid sensors, are transmitted via intestinal vagal afferent fibers to the nucleus of the solitary tract (NTS) in the hindbrain (262). The NTS in turn suppresses HGP via the hepatic branch of vagus nerve fibers (262). Intestinal cholecystokinin (CCK) activates CCK-A receptors in the intestinal afferent fibers and decreases HGP via the gut-brain-liver axis (37).
2.7.3. Liver-brain crosstalk
The CNS regulates liver energy metabolism directly via both the sympathetic nervous system (SNS) and the parasympathetic nervous system which directly innervate the liver. The neural circuitry in the hypothalamus and the hindbrain regulate the activity of most internal organs, including the liver, and maintains internal homeostasis (242). The SNS promotes HGP and mobilization of metabolic fuels for extrahepatic tissues, whereas the parasympathetic system antagonizes SNS action and inhibits HGP and promotes fuel storage in the liver.
Insulin directly regulates glucose and lipid metabolism in the liver as described above. It also regulates hepatic energy metabolism indirectly by activating insulin receptor signaling in the hypothalamus. Insulin stimulates the PI 3-kinase/Akt pathway in the brain, which in turn causes downregulation of GSK-3β in the liver and increases glycogen synthesis (210). Insulin activates its receptors in hypothalamic neurons and suppresses HGP in a vagus nerve output-dependent manner (185, 187). Hypothalamic insulin signaling promotes production of hepatic IL-6 which in turn activates STAT3 and suppresses gluconeogenesis in the liver (87). AgRP neuron-specific deletion of insulin receptors blocks the ability of central insulin to suppress HGP (114). Leptin, an adipose hormone, also regulates liver energy metabolism in addition to controlling food intake and body weight (172). Central administration of leptin suppresses glycogenolysis, gluconeogenesis, and the expression of G6Pase and PEPCK-C in the liver (19). Leptin, by activating the PI 3-kinase pathway in hypothalamic neurons, suppresses hepatic lipogenesis indirectly by increasing SNS outflow to the liver (267). Hypothalamic neurons are also able to directly sense glucose, amino acids, and lipids, and they suppress HGP by increasing vagal nervous outflow to the liver (119, 120, 186). Injection of leucine into the mediobasal hypothalamus suppresses hepatic glycogenolysis and gluconeogenesis in rats (246). Activation of glucose sensing pathways in the brain also suppresses SCD1 expression, lipogenesis, and VLDL secretion in the liver (118).
The CNS also regulates liver activity indirectly by controlling secretion of various metabolic hormones. Disruption of glutamatergic transmission in the ventromedial hypothalamus by deleting VGLUT2 in SF1-expressing neurons decreases secretion of glucagon from pancreatic α cells in the fasted state, resulting in a decrease in hepatic gluconeogenesis and blood glucose levels (251). Mice with leptin receptor LepRb deficiency develop obesity, type 2 diabetes, and high levels of circulating insulin and glucagon. Restoration of LepRb specifically in POMC neurons, an important subpopulation of hypothalamic neurons, markedly decreases hyperglucagonemia, leading to reduction in HGP and blood glucose levels (15).
3. OBESITY, NAFLD, AND TYPE 2 DIABETES
The prevalence of obesity has been increasing rapidly, and it is associated with NAFLD and type 2 diabetes. Glucose produced from the liver contributes significantly to hyperglycemia in humans with type 2 diabetes (41, 153, 164). GCK promotes glucose utilization in the liver and inhibits HGP, and GCK activity is lower in Zucker diabetic, obese rats (252). Liver-specific overexpression of GCK decreases HGP and hyperglycemia in these rats (252). Conversely, hepatocyte-specific deletion of GCK results in mild hyperglycemia and hyperinsulinemia (201).
Obesity, NAFLD, and type 2 diabetes are associated with insulin resistance. Insulin resistance is a primary causal factor for the pathogenesis of NAFLD and type 2 diabetes. NAFLD is associated with increased gluconeogenesis in humans (248). Multiple factors have been described to induce insulin resistance in the liver. Insulin signaling is negatively regulated by protein phosphatases, including PTP1B and Shp-1. Liver-specific deletion of PTP1B enhances insulin signaling in the liver and the ability of insulin to suppress gluconeogenesis, protecting against diet-induced NAFLD (43). Hepatocyte-specific deletion of Shp1 protects against liver insulin resistance in mice fed a HFD (279). Insulin signaling is negatively regulated by SOCS1 and SOCS3 in the liver (218, 253). Insulin signaling is positively regulated by SH2B1, a SH2 domain-containing adaptor protein which recruits IRS proteins to the insulin receptors (51, 171). Deletion of SH2B1 results in leptin resistance, insulin resistance, obesity, NAFLD, and type 2 diabetes (171, 212, 213). Systemic insulin resistance is a causal factor for the development of NAFLD, and lipid accumulation in the liver further promotes hepatic insulin resistance, thus forming a vicious cycle. Saturated NEFAs are able to cause insulin resistance by activating TLR4 (235, 241). Fetuin-A, a glycoprotein secreted by the liver, acts as a NEFA carrier in the circulation, and the NEFA-fetuin-A complex binds to TLR4 and promotes inflammation and insulin resistance (195). DAG induces hepatic insulin resistance by activating PKCε which phosphorylates IRS proteins at inhibitory Ser/Thr residues (98). Ceramides promote insulin resistance by inhibiting Akt activation through PP2A and JNK (194). Obesity and NAFLD are associated with ER stress, which promotes insulin resistance, in the liver (193). Proinflammatory cytokines activate the IKKβ and the JNK pathways which inhibit insulin signaling (22, 79). Liver-specific deletion of IKKβ improves hepatic insulin sensitivity and reduces hepatic gluconeogenesis in HFD-fed mice and ob/ob mice (8). Hepatocyte-specific deletion of JNK1 results in liver inflammation and steatosis in mice fed a normal chow diet (220). C-reactive protein (CRP), an acute phase protein secreted by the liver, inhibits insulin signaling through the ERK pathway in primary hepatocytes and inhibits the ability of insulin to suppress HGP in rats (277). Kupffer cells are a major source of cytokines, and depletion of Kupffer cells improves NAFLD and insulin resistance in the liver (81).
In addition to insulin, aberrant counterregulatory hormone signaling and action in the liver also contribute to the progression of type 2 diabetes (254). Silencing of glucagon receptors in the liver reduces blood TAG levels and improves glucose intolerance in both db/db mice and Zucker diabetic fatty rats (140, 238). We recently reported that inflammatory pathways enhance the ability of glucagon to stimulate gluconeogenesis, contributing to hyperglycemia and glucose intolerance in mice with obesity (36, 234).
Conclusion
The liver has long been recognized to be an essential metabolic organ. When carbohydrates are abundant during the postprandial phase, the liver converts glucose into glycogen and lipids, which provide metabolic fuels during fasting. In the fasted state, the liver produces and secretes glucose through both glycogenolysis and gluconeogenesis. The liver also converts fatty acids into ketone bodies which provide additional metabolic fuels for extrahepatic tissues during fasting. The metabolic switch between the fasted and fed states in the liver is tightly controlled by neuronal and hormonal systems. Insulin suppresses glucose production and ketogenesis and stimulates glycolysis and lipogenesis in the liver. Insulin resistance is not only a hallmark of type 2 diabetes but also promotes type 2 diabetes progression in obesity. Glucagon counteracts insulin action, and defects in glucagon signaling lead to hypoglycemia. Hepatic energy metabolism is largely controlled at the genomic levels by numerous transcription factors and coregulators. The activity of these nuclear proteins is regulated by insulin, glucagon and other metabolic hormones, which dynamically regulates gluconeogenesis, β oxidation, and lipogenesis in the liver in order to meet a systemic metabolic demand. Dysregulation of these transcription factors and coregulators contributes to NAFLD in obesity. Moreover, systemic insulin resistance promotes NAFLD, and hepatocyte lipid accumulation further impairs insulin action, thus activating the insulin resistance-lipotoxicity vicious cycle which drives NAFLD and/or type 2 diabetes progression.
Acknowledgements
Click here to insert Acknowledgements text
I thank Crystal Rui for editing of the manuscript. This study has been supported by the NIH grants RO1 DK 065122, RO1 DK091591 and RO1 DK094014.
References
- 1.Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci U S A. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 5.Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- 6.Arafat AM, Kaczmarek P, Skrzypski M, Pruszynska-Oszmalek E, Kolodziejski P, Szczepankiewicz D, Sassek M, Wojciechowicz T, Wiedenmann B, Pfeiffer AF, Nowak KW, Strowski MZ. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia. 2013;56:588–597. doi: 10.1007/s00125-012-2803-y. [DOI] [PubMed] [Google Scholar]
- 7.Arden C, Tudhope SJ, Petrie JL, Al-Oanzi ZH, Cullen KS, Lange AJ, Towle HC, Agius L. Fructose 2,6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem J. 2012;443:111–123. doi: 10.1042/BJ20111280. [DOI] [PubMed] [Google Scholar]
- 8.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 9.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. Journal of hepatology. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedford DC, Kasper LH, Wang R, Chang Y, Green DR, Brindle PK. Disrupting the CH1 domain structure in the acetyltransferases CBP and p300 results in lean mice with increased metabolic control. Cell Metab. 2011;14:219–230. doi: 10.1016/j.cmet.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellafante E, Murzilli S, Salvatore L, Latorre D, Villani G, Moschetta A. Hepatic-specific activation of peroxisome proliferator-activated receptor gamma coactivator-1beta protects against steatohepatitis. Hepatology. 2013;57:1343–1356. doi: 10.1002/hep.26222. [DOI] [PubMed] [Google Scholar]
- 13.Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L, Housset C, Capeau J, Girard J, Guillou H, Postic C. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berglund ED, Lustig DG, Baheza RA, Hasenour CM, Lee-Young RS, Donahue EP, Lynes SE, Swift LL, Charron MJ, Damon BM, Wasserman DH. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes. 2011;60:2720–2729. doi: 10.2337/db11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Berglund ED, Vianna CR, Donato J, Jr., Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu SY, Mashek DG. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J Lipid Res. 2010;51:3270–3280. doi: 10.1194/jlr.M009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr., Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 22.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012 doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, Tuncman G, Hotamisligil GS. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Jr., Xiong Y, Daniel KW, Floering L, Collins S. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem. 2005;280:42731–42737. doi: 10.1074/jbc.M506223200. [DOI] [PubMed] [Google Scholar]
- 27.Caron S, Samanez CH, Dehondt H, Ploton M, Briand O, Lien F, Dorchies E, Dumont J, Postic C, Cariou B, Lefebvre P, Staels B. The Farnesoid X Receptor Inhibits the Transcriptional Activity of the Carbohydrate Response Element Binding Protein in Human Hepatocytes -- R2. Mol Cell Biol. 2013 doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casado M, Vallet VS, Kahn A, Vaulont S. Essential role in vivo of upstream stimulatory factors for a normal dietary response of the fatty acid synthase gene in the liver. J Biol Chem. 1999;274:2009–2013. doi: 10.1074/jbc.274.4.2009. [DOI] [PubMed] [Google Scholar]
- 29.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 30.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest. 2005;115:3217–3227. doi: 10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Sheng L, Shen H, Zhao Y, Wang S, Brink R, Rui L. Hepatic TRAF2 regulates glucose metabolism through enhancing glucagon responses. Diabetes. 2012;61:566–573. doi: 10.2337/db11-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O’Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 40.Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 41.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 43.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–964. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 46.Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 47.Dentin R, Tomas-Cobos L, Foufelle F, Leopold J, Girard J, Postic C, Ferre P. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. Journal of hepatology. 2012;56:199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004;24:7435–7443. doi: 10.1128/MCB.24.17.7435-7443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D, Hsiao JJ, Zhang D, Iwasaki T, Stark R, Flannery C, Kahn M, Carmean CM, Yu XX, Murray SF, Bhanot S, Monia BP, Cline GW, Samuel VT, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 2009;10:499–506. doi: 10.1016/j.cmet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A. 2009;106:11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escande C, Chini CC, Nin V, Dykhouse KM, Novak CM, Levine J, van Deursen J, Gores GJ, Chen J, Lou Z, Chini EN. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fafalios A, Ma J, Tan X, Stoops J, Luo J, Defrances MC, Zarnegar R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat Med. 2011;17:1577–1584. doi: 10.1038/nm.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299:E384–393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, Singh I, Yeldandi AV, Rao MS, Maeda N, Reddy JK. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem. 1996;271:24698–24710. doi: 10.1074/jbc.271.40.24698. [DOI] [PubMed] [Google Scholar]
- 58.Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944. doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Jr., Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 62.Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guinez C, Filhoulaud G, Rayah-Benhamed F, Marmier S, Dubuquoy C, Dentin R, Moldes M, Burnol AF, Yang X, Lefebvre T, Girard J, Postic C. OGlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, Dimarchi RD, Tschop MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453–1463. doi: 10.2337/db12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan KL, Denu JM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol. 2010;24:1605–1614. doi: 10.1210/me.2010-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, Tamai Y, Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He L, Sabet A, Djedjos S, Miller R, Sun XJ, Hussain MA, Radovick S, Wondisford FE. Metformin and Insulin Suppress Hepatic Gluconeogenesis through Phosphorylation of CREB Binding Protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 75.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 76.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr., Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-Adiponectin-Ceramide Axis Controls Energy Expenditure and Insulin Action in Mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 80.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, Zhu Y, Borensztajn J, Reddy JK. Sustained activation of PPARalpha by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26:628–638. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291:E358–364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 84.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metabolism. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10:168–174. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 89.Inoue Y, Inoue J, Lambert G, Yim SH, Gonzalez FJ. Disruption of hepatic C/EBPalpha results in impaired glucose tolerance and age-dependent hepatosteatosis. J Biol Chem. 2004;279:44740–44748. doi: 10.1074/jbc.M405177200. [DOI] [PubMed] [Google Scholar]
- 90.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 91.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 92.Jelen S, Gena P, Lebeck J, Rojek A, Praetorius J, Frokiaer J, Fenton RA, Nielsen S, Calamita G, Rutzler M. Aquaporin-9 and urea transporter-A gene deletions affect urea transmembrane passage in murine hepatocytes. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G1279–1287. doi: 10.1152/ajpgi.00153.2012. [DOI] [PubMed] [Google Scholar]
- 93.Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes & metabolism journal. 2012;36:328–335. doi: 10.4093/dmj.2012.36.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E46–54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006;397:417–425. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 97.Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q, Xiong Y, Guan KL, Zhao S. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci U S A. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes, obesity & metabolism. 2010;12(Suppl 2):99–107. doi: 10.1111/j.1463-1326.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, Fan Y, Giannoukakis N, Gramignoli R, Strom S, Ringquist S, Dong HH. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 108.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim YD, Kim YH, Tadi S, Yu JH, Yim YH, Jeoung NH, Shong M, Hennighausen L, Harris RA, Lee IK, Lee CH, Choi HS. Metformin inhibits growth hormone-mediated hepatic PDK4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes. 2012;61:2484–2494. doi: 10.2337/db11-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim YD, Li T, Ahn SW, Kim DK, Lee JM, Hwang SL, Kim YH, Lee CH, Lee IK, Chiang JY, Choi HS. Orphan nuclear receptor small heterodimer partner negatively regulates growth hormone-mediated induction of hepatic gluconeogenesis through inhibition of signal transducer and activator of transcription 5 (STAT5) transactivation. J Biol Chem. 2012;287:37098–37108. doi: 10.1074/jbc.M112.339887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kimura T, Christoffels VM, Chowdhury S, Iwase K, Matsuzaki H, Mori M, Lamers WH, Darlington GJ, Takiguchi M. Hypoglycemia-associated hyperammonemia caused by impaired expression of ornithine cycle enzyme genes in C/EBPalpha knockout mice. J Biol Chem. 1998;273:27505–27510. doi: 10.1074/jbc.273.42.27505. [DOI] [PubMed] [Google Scholar]
- 112.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008;27:1017–1028. doi: 10.1038/emboj.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 116.Kotoulas OB, Kalamidas SA, Kondomerkos DJ. Glycogen autophagy. Microsc Res Tech. 2004;64:10–20. doi: 10.1002/jemt.20046. [DOI] [PubMed] [Google Scholar]
- 117.Kulozik P, Jones A, Mattijssen F, Rose AJ, Reimann A, Strzoda D, Kleinsorg S, Raupp C, Kleinschmidt J, Muller-Decker K, Wahli W, Sticht C, Gretz N, von Loeffelholz C, Stockmann M, Pfeiffer A, Stohr S, Dallinga-Thie GM, Nawroth PP, Berriel Diaz M, Herzig S. Hepatic deficiency in transcriptional cofactor TBL1 promotes liver steatosis and hypertriglyceridemia. Cell Metab. 2011;13:389–400. doi: 10.1016/j.cmet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 118.Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 119.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 120.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 121.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Le Lay J, Tuteja G, White P, Dhir R, Ahima R, Kaestner KH. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 2009;10:55–62. doi: 10.1016/j.cmet.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med. 2011;17:812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, Gonzalez FJ, Xie W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139:653–663. doi: 10.1053/j.gastro.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee JM, Lee YK, Mamrosh JL, Busby SA, Griffin PR, Pathak MC, Ortlund EA, Moore DD. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS, Hong S, Park KG, Lee IK, Choi CS, Hanson RW, Choi HS, Koo SH. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11:331–339. doi: 10.1016/j.cmet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee YJ, Ko EH, Kim JE, Kim E, Lee H, Choi H, Yu JH, Kim HJ, Seong JK, Kim KS, Kim JW. Nuclear receptor PPARgamma-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci U S A. 2012;109:13656–13661. doi: 10.1073/pnas.1203218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemke U, Krones-Herzig A, Berriel Diaz M, Narvekar P, Ziegler A, Vegiopoulos A, Cato AC, Bohl S, Klingmuller U, Screaton RA, Muller-Decker K, Kersten S, Herzig S. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab. 2008;8:212–223. doi: 10.1016/j.cmet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 132.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 134.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 135.Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, Cline GW, Shulman GI, Coleman RA. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem. 2009;284:27816–27826. doi: 10.1074/jbc.M109.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li S, Liu C, Li N, Hao T, Han T, Hill DE, Vidal M, Lin JD. Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab. 2008;8:105–117. doi: 10.1016/j.cmet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes. 2004;53:410–417. doi: 10.2337/diabetes.53.2.410. [DOI] [PubMed] [Google Scholar]
- 141.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 142.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 143.Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, Lu T, Bao J, Han D, Sack MN, Finkel T. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4:ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu S, Croniger C, Arizmendi C, Harada-Shiba M, Ren J, Poli V, Hanson RW, Friedman JE. Hypoglycemia and impaired hepatic glucose production in mice with a deletion of the C/EBPbeta gene. J Clin Invest. 1999;103:207–213. doi: 10.1172/JCI4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu S, Hatano B, Zhao M, Yen CC, Kang K, Reilly SM, Gangl MR, Gorgun C, Balschi JA, Ntambi JM, Lee CH. Role of peroxisome proliferator-activated receptor {delta}/{beta} in hepatic metabolic regulation. J Biol Chem. 2011;286:1237–1247. doi: 10.1074/jbc.M110.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, Yan C, Wang Y, Nakagawa Y, Nerio N, Anghel A, Lutfy K, Friedman TC. Liver X receptor agonist T0901317 inhibition of glucocorticoid receptor expression in hepatocytes may contribute to the amelioration of diabetic syndrome in db/db mice. Endocrinology. 2006;147:5061–5068. doi: 10.1210/en.2006-0243. [DOI] [PubMed] [Google Scholar]
- 148.Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The Glucagon Receptor Is Required for the Adaptive Metabolic Response to Fasting. Cell Metabolism. 2008;8:359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Louet JF, Chopra AR, Sagen JV, An J, York B, Tannour-Louet M, Saha PK, Stevens RD, Wenner BR, Ilkayeva OR, Bain JR, Zhou S, DeMayo F, Xu J, Newgard CB, O’Malley BW. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab. 2010;12:606–618. doi: 10.1016/j.cmet.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012 doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu Y, Xiong X, Wang X, Zhang Z, Li J, Shi G, Yang J, Zhang H, Ning G, Li X. Yin Yang 1 promotes hepatic gluconeogenesis through upregulation of glucocorticoid receptor. Diabetes. 2013;62:1064–1073. doi: 10.2337/db12-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lustig Y, Ruas JL, Estall JL, Lo JC, Devarakonda S, Laznik D, Choi JH, Ono H, Olsen JV, Spiegelman BM. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. Genes Dev. 2011;25:1232–1244. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mao T, Shao M, Qiu Y, Huang J, Zhang Y, Song B, Wang Q, Jiang L, Liu Y, Han JD, Cao P, Li J, Gao X, Rui L, Qi L, Li W. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc Natl Acad Sci U S A. 2011;108:15852–15857. doi: 10.1073/pnas.1107394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 157.Matsukuma KE, Bennett MK, Huang J, Wang L, Gil G, Osborne TF. Coordinated control of bile acids and lipogenesis through FXR-dependent regulation of fatty acid synthase. J Lipid Res. 2006;47:2754–2761. doi: 10.1194/jlr.M600342-JLR200. [DOI] [PubMed] [Google Scholar]
- 158.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 159.Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr., Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matsuzaka T, Atsumi A, Matsumori R, Nie T, Shinozaki H, Suzuki-Kemuriyama N, Kuba M, Nakagawa Y, Ishii K, Shimada M, Kobayashi K, Yatoh S, Takahashi A, Takekoshi K, Sone H, Yahagi N, Suzuki H, Murata S, Nakamuta M, Yamada N, Shimano H. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology. 2012;56:2199–2208. doi: 10.1002/hep.25932. [DOI] [PubMed] [Google Scholar]
- 162.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merla G, Howald C, Antonarakis SE, Reymond A. The subcellular localization of the ChoRE-binding protein, encoded by the Williams-Beuren syndrome critical region gene 14, is regulated by 14-3-3. Human molecular genetics. 2004;13:1505–1514. doi: 10.1093/hmg/ddh163. [DOI] [PubMed] [Google Scholar]
- 164.Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619–624. doi: 10.1172/JCI2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 166.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 168.Molusky MM, Li S, Ma D, Yu L, Lin JD. Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and diurnal glucose metabolism through 11beta-hydroxysteroid dehydrogenase 1. Diabetes. 2012;61:1025–1035. doi: 10.2337/db11-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP Pathway Is Essential for Developing Diabetic Fatty Liver and Carbohydrate-Induced Hypertriglyceridemia in Animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, Lopez-Vicario C, Barak Y, Arroyo V, Claria J. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyteand macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 171.Morris DL, Cho KW, Zhou Y, Rui L. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes. 2009;58:2039–2047. doi: 10.2337/db08-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 174.Mueller KM, Themanns M, Friedbichler K, Kornfeld JW, Esterbauer H, Tuckermann JP, Moriggl R. Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development. Molecular and cellular endocrinology. 2012;361:1–11. doi: 10.1016/j.mce.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mutel E, Abdul-Wahed A, Ramamonjisoa N, Stefanutti A, Houberdon I, Cavassila S, Pilleul F, Beuf O, Gautier-Stein A, Penhoat A, Mithieux G, Rajas F. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. Journal of hepatology. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 176.Nader N, Ng SS, Wang Y, Abel BS, Chrousos GP, Kino T. Liver x receptors regulate the transcriptional activity of the glucocorticoid receptor: implications for the carbohydrate metabolism. PLoS One. 2012;7:e26751. doi: 10.1371/journal.pone.0026751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Loffler MG, Cresswell J, Yu XX, Murray SF, Bhanot S, Monia BP, Bogan JS, Samuel V, Shulman GI. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 181.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44:1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 182.Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ning J, Hong T, Ward A, Pi J, Liu Z, Liu HY, Cao W. Constitutive role for IRE1alpha-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program. Endocrinology. 2011;152:2247–2255. doi: 10.1210/en.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 186.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 187.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 188.Okar DA, Manzano A, Navarro-Sabate A, Riera L, Bartrons R, Lange AJ. PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem Sci. 2001;26:30–35. doi: 10.1016/s0968-0004(00)01699-6. [DOI] [PubMed] [Google Scholar]
- 189.Oosterveer MH, Mataki C, Yamamoto H, Harach T, Moullan N, van Dijk TH, Ayuso E, Bosch F, Postic C, Groen AK, Auwerx J, Schoonjans K. LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J Clin Invest. 2012;122:2817–2826. doi: 10.1172/JCI62368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Opherk C, Tronche F, Kellendonk C, Kohlmuller D, Schulze A, Schmid W, Schutz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004;18:1346–1353. doi: 10.1210/me.2003-0283. [DOI] [PubMed] [Google Scholar]
- 191.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, Wronska A, Chen BX, Marks AR, Fukamizu A, Backs J, Singer HA, Yates JR, 3rd, Accili D, Tabas I. Calcium Signaling through CaMKII Regulates Hepatic Glucose Production in Fasting and Obesity. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 194.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends in endocrinology and metabolism: TEM. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012 doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 196.Parker JC, Andrews KM, Allen MR, Stock JL, McNeish JD. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun. 2002;290:839–843. doi: 10.1006/bbrc.2001.6265. [DOI] [PubMed] [Google Scholar]
- 197.Patel R, Patel M, Tsai R, Lin V, Bookout AL, Zhang Y, Magomedova L, Li T, Chan JF, Budd C, Mangelsdorf DJ, Cummins CL. LXRbeta is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in mice. J Clin Invest. 2011;121:431–441. doi: 10.1172/JCI41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 200.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knockouts using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 202.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 205.Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–1117. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 208.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 209.Ramadoss P, Unger-Smith NE, Lam FS, Hollenberg AN. STAT3 targets the regulatory regions of gluconeogenic genes in vivo. Mol Endocrinol. 2009;23:827–837. doi: 10.1210/me.2008-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD, Neal D, Williams PE, Lautz M, Mari A, Cherrington AD, Edgerton DS. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest. 2011;121:3713–3723. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G852–858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 212.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 213.Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 217.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 Block Insulin Signaling by Ubiquitin-mediated Degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 219.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sakiyama H, Wynn RM, Lee WR, Fukasawa M, Mizuguchi H, Gardner KH, Repa JJ, Uyeda K. Regulation of nuclear import/export of carbohydrate response element-binding protein (ChREBP): interaction of an alpha-helix of ChREBP with the 14-3-3 proteins and regulation by phosphorylation. J Biol Chem. 2008;283:24899–24908. doi: 10.1074/jbc.M804308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 224.Sanderson LM, Degenhardt T, Koppen A, Kalkhoven E, Desvergne B, Muller M, Kersten S. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol Cell Biol. 2009;29:6257–6267. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B, Shaw MM, Groop L, Caprio S. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781–789. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, Roper M, Fischer SJ, Lowe E, Orlicky DJ, McManaman JL, Palmer C, Gitomer WL, Huang W, O’Doherty RM, Becker TC, Klemm DJ, Jensen DR, Pulawa LK, Eckel RH, Friedman JE. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem. 2007;282:15717–15729. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sekine K, Chen YR, Kojima N, Ogata K, Fukamizu A, Miyajima A. Foxo1 links insulin signaling to C/EBPalpha and regulates gluconeogenesis during liver development. EMBO J. 2007;26:3607–3615. doi: 10.1038/sj.emboj.7601784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 231.Seyer P, Vallois D, Poitry-Yamate C, Schutz F, Metref S, Tarussio D, Maechler P, Staels B, Lanz B, Grueter R, Decaris J, Turner S, da Costa A, Preitner F, Minehira K, Foretz M, Thorens B. Hepatic glucose sensing is required to preserve beta cell glucose competence. J Clin Invest. 2013;123:1662–1676. doi: 10.1172/JCI65538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol. 2000;20:6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sheng L, Zhou Y, Chen Z, Ren D, Cho KW, Jiang L, Shen H, Sasaki Y, Rui L. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med. 2012 doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 238.Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest. 2004;113:1571–1581. doi: 10.1172/JCI20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Lichtman AH, Iwawaki T, Glimcher LH, Lee AH. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012;16:487–499. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sos BC, Harris C, Nordstrom SM, Tran JL, Balazs M, Caplazi P, Febbraio M, Applegate MA, Wagner KU, Weiss EJ. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011;121:1412–1423. doi: 10.1172/JCI42894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 242.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, Qi L, Shi H, McKenzie AN, Lee CH. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 245.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G837–850. doi: 10.1152/ajpgi.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Su Y, Lam TK, He W, Pocai A, Bryan J, Aguilar-Bryan L, Gutierrez-Juarez R. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes. 2012;61:85–93. doi: 10.2337/db11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16:588–600. doi: 10.1016/j.cmet.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism. Trends in endocrinology and metabolism: TEM. 2011;22:458–466. doi: 10.1016/j.tem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 250.Tobin KA, Ulven SM, Schuster GU, Steineger HH, Andresen SM, Gustafsson JA, Nebb HI. Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J Biol Chem. 2002;277:10691–10697. doi: 10.1074/jbc.M109771200. [DOI] [PubMed] [Google Scholar]
- 251.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, Newgard CB, Shiota M. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in zucker diabetic fatty rats. Diabetes. 2009;58:78–86. doi: 10.2337/db08-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 256.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Molecular and Cellular Biology. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Molecular and cellular endocrinology. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 258.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, Vance DE, Tzoneva M, Hart AC, Naar AM. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, Israelian K, Westphal CH, Rodgers JT, Shioda T, Elson SL, Mulligan P, Najafi-Shoushtari H, Black JC, Thakur JK, Kadyk LC, Whetstine JR, Mostoslavsky R, Puigserver P, Li X, Dyson NJ, Hart AC, Naar AM. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 262.Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 263.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, Hedrick S, Vera L, Montminy M. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012 doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Warne JP, Alemi F, Reed AS, Varonin JM, Chan H, Piper ML, Mullin ME, Myers MG, Jr., Corvera CU, Xu AW. Impairment of central leptin-mediated PI3K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab. 2011;14:791–803. doi: 10.1016/j.cmet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 268.Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol. 1989;257:E108–117. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- 269.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wei D, Tao R, Zhang Y, White MF, Dong XC. Feedback regulation of hepatic gluconeogenesis through modulation of SHP/Nr0b2 gene expression by Sirt1 and FoxO1. Am J Physiol Endocrinol Metab. 2011;300:E312–320. doi: 10.1152/ajpendo.00524.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287:10301–10315. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 273.Winkler R, Benz V, Clemenz M, Bloch M, Foryst-Ludwig A, Wardat S, Witte N, Trappiel M, Namsolleck P, Mai K, Spranger J, Matthias G, Roloff T, Truee O, Kappert K, Schupp M, Matthias P, Kintscher U. Histone deacetylase 6 (HDAC6) is an essential modifier of glucocorticoid-induced hepatic gluconeogenesis. Diabetes. 2012;61:513–523. doi: 10.2337/db11-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wong RH, Chang I, Hudak CS, Hyun S, Kwan HY, Sul HS. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell. 2009;136:1056–1072. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wu JW, Wang SP, Casavant S, Moreau A, Yang GS, Mitchell GA. Fasting Energy Homeostasis in Mice with Adipose Deficiency of Desnutrin/Adipose Triglyceride Lipase. Endocrinology. 2012 doi: 10.1210/en.2011-1518. [DOI] [PubMed] [Google Scholar]
- 276.Wu Z, Jiao P, Huang X, Feng B, Feng Y, Yang S, Hwang P, Du J, Nie Y, Xiao G, Xu H. MAPK phosphatase-3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice. J Clin Invest. 2010;120:3901–3911. doi: 10.1172/JCI43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xi L, Xiao C, Bandsma RH, Naples M, Adeli K, Lewis GF. C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen-activated protein kinases. Hepatology. 2011;53:127–135. doi: 10.1002/hep.24011. [DOI] [PubMed] [Google Scholar]
- 278.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Xu E, Charbonneau A, Rolland Y, Bellmann K, Pao L, Siminovitch KA, Neel BG, Beauchemin N, Marette A. Hepatocyte-specific Ptpn6 deletion protects from obesity-linked hepatic insulin resistance. Diabetes. 2012;61:1949–1958. doi: 10.2337/db11-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 282.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 285.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci U S A. 2007;104:17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang T, Wang S, Lin Y, Xu W, Ye D, Xiong Y, Zhao S, Guan KL. Acetylation negatively regulates glycogen phosphorylase by recruiting protein phosphatase 1. Cell Metab. 2012;15:75–87. doi: 10.1016/j.cmet.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhao LF, Iwasaki Y, Nishiyama M, Taguchi T, Tsugita M, Okazaki M, Nakayama S, Kambayashi M, Fujimoto S, Hashimoto K, Murao K, Terada Y. Liver X receptor alpha is involved in the transcriptional regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Diabetes. 2012;61:1062–1071. doi: 10.2337/db11-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhou XY, Shibusawa N, Naik K, Porras D, Temple K, Ou H, Kaihara K, Roe MW, Brady MJ, Wondisford FE. Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat Med. 2004;10:633–637. doi: 10.1038/nm1050. [DOI] [PubMed] [Google Scholar]
- 296.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Fisher SJ, White MF, Biddinger SB, Ozcan U. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]