Abstract
Context
In older adults with multiple conditions, medications may not impart the same benefits seen in patients who are younger or without multimorbidity. Furthermore, medications given for one condition may adversely affect other outcomes. β-Blocker use with coexisting cardiovascular disease (CVD) and chronic obstructive pulmonary disease (COPD) is such a situation.
Objective
To determine the effect of β-blocker use on cardiac and pulmonary outcomes and mortality in older adults with coexisting COPD and CVD.
Design, Setting, Participants
The study included 1062 participants who were members of the 2004–2007 Medicare Current Beneficiary Survey cohorts, a nationally representative sample of Medicare beneficiaries. Study criteria included age over 65 years plus coexisting CVD and COPD/asthma. Follow-up occurred through 2009. We determined the association between β-blocker use and the outcomes with propensity score-adjusted and covariate-adjusted Cox proportional hazards.
Main Outcome Measures
The 3 outcomes were major cardiac events, pulmonary events, and all-cause mortality.
Results
Half of the participants used β-blockers. During follow-up, 179 participants experienced a major cardiac event; 389 participants experienced a major pulmonary event; and 255 participants died. Each participant could have experienced any ≥1 of these events. The hazard ratio for β-blocker use was 1.18 [95% confidence interval (CI), 0.85–1.62] for cardiac events, 0.91 (95% CI, 0.73–1.12) for pulmonary events, and 0.87 (95% CI, 0.67–1.13) for death.
Conclusion
In this population of older adults, β-blockers did not seem to affect occurrence of cardiac or pulmonary events or death in those with CVD and COPD.
Keywords: multiple chronic conditions, multimorbidity, chronic obstructive pulmonary disease, cardiovascular disease, β-blocker, cardiac events, pulmonary events, COPD, CVD, coronary artery disease
As older adults accumulate diseases and conditions, they meet criteria for an increasing number of disease guidelines with resultant accumulation of multiple medications.1 It is unclear whether over time, and in the face of multiple diseases and medications, each medication conveys benefits that outweigh harms.1,2 This is a particular concern in situations when a drug may impart harm to a coexisting condition. β-blocker use in individuals with coexisting coronary or vascular disease (CVD) and chronic obstructive pulmonary disease (COPD) is such a situation.
Current American Heart Association/American College of Cardiology (AHA/ACC) guidelines note that β-blockers should be used for 3 years after the initial event in all patients who have had a myocardial infarction (MI) or acute coronary syndrome.3 Guidelines further recommend continuing β-blockers indefinitely in patients with left ventricular dysfunction while noting it is also deemed reasonable to continue them in those with normal left ventricular function. These recommendations are based on results from randomized clinical trials (RCTs) from which older individuals with multiple conditions, particularly COPD or asthma, were largely excluded.4,5 The noncommittal statement that β-blockers “may be considered as chronic therapy for all other patients with other CVD” was because of the lack of RCT evidence in any population.3 No specific mention was made of patients who have both CVD and COPD.
Two recent observational studies have suggested no benefits to long-term β-blocker use. In the REACH study of 21,860 propensity-matched adults with CVD (mean age ± SD, 68.5 ± 10.0) followed for up to 4 years (median follow-up = 44 mo), no difference in the composite outcome (fatal and nonfatal MI and nonfatal stroke) was observed for β-blockers users and nonusers.6 In an observational study from 25 hospitals in the Osaka region of Japan, 5628 adults (age, 64.7 ± 11.8 years) with MI were treated with primary percutaneous coronary intervention. With a median follow-up of nearly 4 years, no difference was observed in all-cause death, fatal MI, or non-MI death.7 Unfortunately, these trials make no specific mention of patients with both CVD and COPD.
β-blockers, particularly nonselective ones, may increase airway hyperresponsiveness and compete with β2-agonists, thus theoretically increasing risk of adverse pulmonary outcomes, such as exacerbations.8,9 Observational studies suggest a survival benefit with β-blocker use in individuals with COPD and CVD, but these studies included few older participants.10–14 One study involving individuals with a mean age of 75 years found a survival benefit with β-blocker in the year following an MI but not in the subgroup receiving β2 agonists or with severe COPD.10 The benefits and harms of β-blockers in older adults with coexisting CVD and COPD remains relatively unexplored.
Among persons 65 years and older, almost 60% take at least 5 medications and almost 20% take at least 10.15 Risk of adverse effects increases 10% with each medication.16 Evidence of benefit and absence of harm should guide decision making in older adults with multiple conditions to reduce medication burden and risk of adverse medication effects. Only if benefits outweigh harms is the use of β-blockers in older adults with CVD and COPD warranted. Given the impracticality of employing RCTs to determine the benefits and harms of every treatment in older adults with multiple coexisting conditions, we have to rely on observational data to inform medication decision making. Inclusion of well-characterized nationally representative cohorts, control of confounding factors, attenuation of indication and contraindication biases, and determination of benefits and harms among key clinical subgroups are important to evaluating medication effects in observational studies.17–23 We determined the effect of β-blocker use on CVD and COPD outcomes in a nationally representative cohort of older adults with coexisting COPD and CVD.
METHODS
Study Sample
The study population included Medicare Current Beneficiary Survey (MCBS) participants who were enrolled during 2004–2007.24 MCBS is a nationally representative sample of Medicare beneficiaries obtained using stratified multi-stage sampling from the Centers for Medicare and Medicaid Services (CMS) enrollment file. MCBS employs a rolling cohort design where each fall, a new cohort of participants are enrolled and followed for up to 4 years (follow-up data until 2009). MCBS participants eligible for this study sample were aged 65 years and older and had CVD as well as either COPD or asthma. Because of the lack of health claims in the MCBS data files, Medicare Advantage beneficiaries (Medicare Part C), including those enrolled in HMOs and PPOs, were excluded from the current study.
A combination of self-report, medication data and Medicare hospital, outpatient, physician, or skilled nursing facility claims data were used to identify eligible participants and was agreed upon by consensus of 4 coauthors (D.S.H.L., C.P.G., J.A.D., and M.E.T.). CVD included any history of MI, angina, other acute/subacute/or chronic ischemic heart disease, or peripheral vascular disease. COPD criteria included any of chronic bronchitis, emphysema, COPD, asthma, or use of any of β-adrenergic bronchodilators, anticholinergic bronchodilators, combination bronchodilators, or inhaled corticosteroids. Upon enrollment, claims data were retrospectively gathered for the preceding 9 months. Preenrollment information and the first year of follow-up were used to define eligibility.
A total of 20,236 beneficiaries were enrolled in MCBS during 2004–2007 and of these participants, a total of 16,542 were aged 65 and over. Of these, we excluded: 3425 participants who were either lacking health claims at baseline because of Medicare Advantage use, 974 who did not have medication data available, and 2049 who were non-respondents at baseline. Of the remaining 10,094 participants, a total of 3385 met the criteria for CVD and 1062 met the criteria for both CVD and COPD. Thus, 1062 MCBS participants constituted this study subset.
Descriptive Data
Baseline sociodemographic, medical, behavioral, and functional data were obtained from the cost and use annual in-person interviews, and from Medicare hospital, outpatient, physician, and skilled nursing home claims data. Medical, behavioral, and functional data included several self-reported health conditions, medication insurance coverage, self-perceived health, smoking status, body mass index, falls in the past year, depression, defined by a claim for depression25 or self-reported depression plus loss of interest; cognition; activities of daily living (ADLs); and physical function. Cognitive impairment or dementia was considered present if there was a claim for dementia or cognitive disorder25 or self-reported memory loss plus either trouble concentrating or difficulty making decisions that interfered with ADL. Basic ADL (BADL) dependency was defined as not performing independently one or more of: walking, transferring, dressing, bathing, eating, and toileting. Instrumental ADLs (IADLs) dependency was defined as not performing independently one or more of: using the telephone, light housework, heavy housework, preparing meals, shopping, and paying bills. Physical function was defined by the amount of difficulty (1 = no difficulty to 5 = unable to do) with stooping, lifting, extending arms, handling objects, and walking ¼ mile; physical function scores ranged from 5 to 25. The Elixhauser comorbidity scale was computed based on the ICD-9 codes from claims data excluding cardiac and pulmonary conditions.25
Medication Use Data
Prescription medication data were obtained by direct observation during in-person interviews. Interviews occurred every 4 months and participants were asked to record their drug purchases and save their medicine containers to aid their recall. Medications were identified by therapeutic name and class codes. Starting in 2006, Medicare part D was implemented; however, to keep medication use consistent in all enrolled cohorts, part D information was not used.
β-blocker medications were classified as selective (acebutolol, atenolol, betaxolol, bisoprolol, esmolol, nebivolol, metoprolol), nonselective (levobunolol, metipranolol, nadolol, propranolol, sotalol, timolol), and nonselective β-blocker agent with α-blocking properties (carvedilol and labetalol). Classification of β-blocker users and nonusers was based on interviews during the first year of follow-up.
Outcomes
The 3 outcomes were major cardiac events, major pulmonary events, and all-cause mortality. The outcomes were ascertained for 3 years, during years 2–4; the criteria to identify these outcomes were agreed upon by 4 coauthors (D.S.H.L., C.P.G., J.A.D, and M.E.T.).24 Major cardiac events were ascertained from hospital claims data during follow-up and included diagnosis codes for acute coronary syndrome (acute MI or unstable angina) or procedure codes relating to cardiac revascularization procedures including coronary artery bypass graft, cardiac angioplasty, cardiac stent, or insertion of intra-aortic balloon assist. Major pulmonary events, also ascertained from hospital claims data during follow-up, included diagnosis codes related to exacerbations or complications of COPD or asthma (ie, bronchitis, emphysema, asthma, bronchiectasis, and respiratory failure) or procedure codes for endotracheal intubation, plication of emphysematous bleb, or lung volume reduction surgery. Death was ascertained from 3 years of Medicare vital status data.
Statistical Analysis
Baseline characteristics of the study population and distributions of β-blocker use during follow-up were summarized using means and SDs or frequency and percentages, as appropriate.
To control for confounding by indication, we estimated a propensity score (PS) using a logistic regression model with β-blocker use as the dependent variable. A propensity score is the conditional probability of treatment based on a set of participant characteristics at baseline. Baseline for both β-blocker users and nonusers is considered as the 9 months of preenrollment and the first year of follow-up (up to the end of year 1). Of the demographic and health variables selected to characterize our study population, those variables associated with any of the outcomes or β-blocker use and any of the outcomes were included in the PS model (listed in Table 1, n = 32 variables).26–30 To assess proper PS model specification, and its subsequent utility in controlling for the differences between β-blocker use and nonuse, we regressed each covariate on β-blocker use, adjusting for the PS (Table 1).26,29,30,31
TABLE 1.
Baseline Characteristics of β-Blocker Users and Non-β-Blocker Users*
| Characteristics | β-Blocker Users, N=531 |
β-Blocker Nonusers, N=531 |
Unadjusted P† | PS-adjusted P‡ |
|---|---|---|---|---|
| Age, mean (SD), y§ | 77.1 (7.0) | 77.7 (7.2) | 0.166 | 0.988 |
| Male sex§ | 261 (49.2) | 251 (47.3) | 0.539 | 0.982 |
| Nonwhite race§ | 62 (11.7) | 54 (10.2) | 0.432 | 0.970 |
| Hispanic ethnicity | 22 (4.1) | 36 (6.8) | 0.061 | 0.110 |
| Currently married§ | 266 (50.1) | 260 (49.0) | 0.713 | 0.978 |
| High school education§ | 137 (25.8) | 168 (31.6) | 0.036 | 0.995 |
| Currently employed§ | 27 (5.1) | 38 (7.2) | 0.161 | 0.943 |
| Income <$25,000§ | 329 (62.0) | 320 (60.3) | 0.571 | 0.990 |
| Perceived health fair or poor§ | 408 (76.8) | 359 (67.6) | <0.001 | 0.993 |
| Current smoker | 89 (16.8) | 94 (17.7) | 0.685 | 0.736 |
| Body mass index§ | 0.024 | 0.979 | ||
| <25 | 239 (45.0) | 288 (54.2) | ||
| 25–29 | 155 (29.2) | 118 (22.2) | ||
| ≥30 | 137 (25.8) | 125 (23.5) | ||
| Prescription drug insurance | 361 (68.0) | 377 (71.0) | 0.286 | 0.217 |
| Dependent in any BADLs§ | 271 (51.0) | 249 (46.9) | 0.178 | 0.998 |
| Dependent on any IADLs§ | 354 (66.7) | 333 (62.7) | 0.178 | 0.974 |
| Physical functioning score (range, 5–25), mean (SD)§ | 12.6 (5.3) | 11.7 (4.9) | 0.002 | 0.999 |
| Health limits social activities most or all the time§,∥ | 293 (55.2) | 255 (48.0) | 0.020 | 0.986 |
| Vision impairment§ | 63 (11.9) | 70 (13.2) | 0.517 | 0.941 |
| Hearing impairment | 246 (46.3) | 246 (46.3) | 1.000 | 0.625 |
| Urinary incontinence§ | 109 (20.5) | 94 (17.7) | 0.242 | 0.978 |
| Fell in past year | 40 (7.5) | 43 (8.1) | 0.732 | 0.365 |
| Dementia or cognitive impairment§,∥ | 107 (20.2) | 113 (21.3) | 0.650 | 0.985 |
| Depression | 132 (24.9) | 115 (21.7) | 0.217 | 0.498 |
| Prior MI§ | 250 (47.1) | 150 (28.3) | <0.001 | 0.975 |
| PVD§ | 316 (59.5) | 298 (56.1) | 0.264 | 0.990 |
| Diabetes§ | 264 (49.7) | 238 (44.8) | 0.110 | 0.981 |
| Hypertension§ | 520 (97.9) | 495 (93.2) | <0.001 | 0.837 |
| Heart failure§,∥ | 326 (61.4) | 258 (48.6) | <0.001 | 0.980 |
| Prior stroke§ | 126 (23.7) | 83 (15.6) | 0.001 | 0.944 |
| ESRD§,r | 154 (29.0) | 110 (20.7) | 0.002 | 0.958 |
| No. Elixhauser comorbidities, mean (SD)§,∥ | 7.8 (3.6) | 6.9 (3.5) | <0.001 | 0.969 |
| Cardiac and pulmonary medications | ||||
| ACE inhibitor/ARB§,∥ | 334 (62.9) | 255 (48.0) | <0.001 | 0.970 |
| Aldosterone§ | 50 (9.4) | 28 (5.3) | 0.011 | 0.983 |
| Calcium channel blocker§ | 184 (34.7) | 173 (32.6) | 0.475 | 0.958 |
| Diuretic§ | 344 (64.8) | 237 (44.6) | <0.001 | 0.991 |
| Home oxygen§ | 81 (15.3) | 102 (19.2) | 0.089 | 0.987 |
| Potassium | 19 (3.6) | 21 (4.0) | 0.747 | 0.304 |
| Statin† | 316 (59.5) | 211 (39.7) | <0.001 | 0.981 |
| Vasodilator§ | 69 (13.0) | 30 (5.7) | <0.001 | 0.931 |
| No. non-β-blocker medications, mean (SD)§,∥ | 11.7 (6.7) | 10.0 (6.0) | <0.001 | 0.996 |
Data are presented as n (%) unless otherwise indicated.
Derived from individual logistic models, with each characteristic being the sole predictor and the binary indicator for β-blocker use as the dependent variable.
A continuous propensity score was added to each bivariate logistic model above.
These variables (total = 32) were included as covariates in the propensity score model.
Associated with cardiac or pulmonary event or death during follow-up; included as a covariate in the outcome models.
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker; BADL, basic activities of daily living; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; IADL, instrumental activities of daily living; MI, myocardial infarction; PVD, peripheral vascular disease.
For each outcome, the association between β-blocker use and time to first outcome event was examined using a Cox proportional hazards model controlling for fixed-in-time confounding.31,32 Events were determined during the 3 years of follow-up (years 2–4) for both β-blocker users and non-users. Participants who did not experience an outcome event were censored at the time of loss to follow-up, the end of follow-up, or death for the cardiac and pulmonary event analyses. We first fit a bivariate model that included β-blocker use as the sole predictor (unadjusted model). To account for potential indication or selection bias, we then constructed a PS-adjusted model in which we added the PS as a continuous variable to the unadjusted model, along with the year of enrollment and additional confounding variables. To assess the independent effect of β-blockers, as well as check the robustness of the PS model results, we then created a covariate-adjusted model in which we added the year of enrollment and additional confounding covariates to the un-adjusted model. Covariates were considered confounders if they changed the magnitude of the estimate hazard ratio (HR) by >10%. Model diagnostics were performed and the proportional hazard assumptions were not violated in any model.
Because death was a relatively common outcome (24.3% of β-blocker nonusers died vs. 23.7% of users), we used proportional hazard competing risk analyses as described by Fine and Gray34 to determine the association between β-blocker use and cardiac or pulmonary events, accounting for the death rate. Competing risk models were unadjusted, covariate-adjusted, and PS-adjusted.
Statistical tests were conducted at the 0.05 (2-tailed) level of significance. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). The association between β-blocker use versus nonuse on outcomes are presented as HRs with corresponding 95% confidence intervals (CIs). Cumulative hazard plots are also provided for the purpose of graphically displaying the relationship between β-blocker use and time to first event for each outcome.
RESULTS
The mean age of our 1062 participants was 77.4 (± 7.1) years, 512 (48.2%) were male individuals. Among this cohort with coexisting CVD and COPD, 400 (37.7%) had a prior MI. By happenstance, exactly 50% of the participants used a β-blocker. Among the 531 β-blocker users, 385 (72.5%) used a cardioselective β-blocker, 23 (4.3%) had a nonselective β-blocker, 98 (18.5%) had a β-blocker with α-blocking properties, and 25 (4.7%) multiple β-blocker agents. Nonusers differed from β-blocker users in several characteristics as shown in Table 1. Using the PS as a continuous variable, we were able to balance the differences between β-blocker users and nonusers, as shown by the PS-adjusted P-values. The mean and the PS range for β-blocker users was 0.58 ( ± 0.19) and 0.14–0.94, and was 0.42 ( ± 0.18) and 0.05–0.93 for nonusers; the propensity score range indicates good overlap and comparability between users and nonusers.
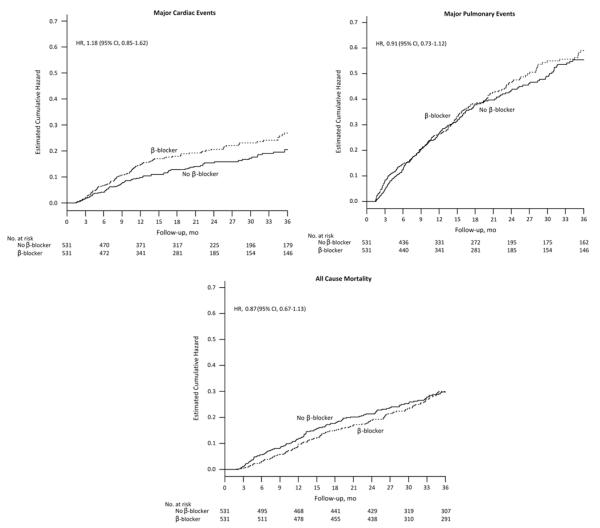
The cumulative hazard plots for cardiac and pulmonary events and death are shown in Figure 1; these display the total amount of risk (hazard) for each event accumulated put to each time point. Over 3 years of follow-up, 179 participants experienced a major cardiac event; 102 were among β-blocker users, and 77 were among nonusers. The PS-adjusted HR for β-blocker use was 1.18 (95% CI, 0.85–1.62) for cardiac events (Table 2). Among the 389 participants who experienced a major pulmonary event, 199 were β-blocker users. The PS-adjusted HR for β-blocker use was 0.91 (95% CI, 0.73–1.12) for pulmonary events. Of the 255 participants who died over the 3 years, 126 were β-blocker users. The PS-adjusted HR for β-blocker use was 0.87 (0.67, 1.13) for death. In each of these outcomes, the covariate-adjusted HR produced similar results. After accounting for rates of death in the competing risk analysis, the PS-adjusted HR for β-blocker use was nearly unchanged, 1.22 (95% CI, 0.87–1.71) for cardiac events and 0.91 (95% CI, 0.73–1.13) for pulmonary events.
FIGURE 1.
Cumulative hazard plots for cardiac and pulmonary events and all-cause mortality by β-blocker use. Cumulative hazard plots display the total amount of risk (hazard) for each event accumulated up to each time point. The hazard ratios provided were estimated using Cox proportional hazard models adjusted by propensity score and year of entry for all models, with the addition of the total number of Elixhauser comorbidities to the COPD model and heart failure to the cardiac and all-cause mortality models. Follow-up was 3 years. Variables included in the propensity score are noted in Table 1. CI indicates confidence intervals; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
TABLE 2.
Major Cardiac and Pulmonary Events and All-cause Mortality According to β-blocker Use Among MCBS Cohort Members With CVD and COPD (N = 1062)*
| HR (95% CI) |
|||
|---|---|---|---|
| Outcome (n=no. events) | Unadjusted | PS-adjusted‡,§ | Covariate-adjusted† |
| Cardiac event (n = 179) | 1.35 (1.00–1.81) | 1.18 (0.85–1.62) | 1.12 (0.83–1.52) |
| Pulmonary event (n = 389) | 1.05 (0.86–1.29) | 0.91 (0.73–1.12)∥ | 0.86 (0.71–1.06)∥ |
| Death (n = 255) | 0.97 (0.76–1.23) | 0.87 (0.67–1.13) | 0.82 (0.63–1.07)¶ |
The HRs for β-blocker use were created with Cox proportional hazards analysis. The referent groups were β-blocker nonusers.
All covariate models are controlled by entry year. Cardiac model additionally includes: heart failure, ACE/ARB use, and Elixhauser comorbidities. Pulmonary model includes: total non β-blocker medications, heart failure, and Elixhauser comorbidities. Mortality model includes: Elixhauser comorbidities, total number of non β-blocker medications, prior MI, cognitive impairment, limited social activities, ESRD, heart failure, and ACE/ARB use.
Variables used to generate the propensity score are marked in Table 1.
All PS models are controlled by PS and entry year. Pulmonary PS model additionally includes: physical function and Elixhauser comorbidities. Mortality model includes: cognitive impairment.
One outlier removed; outlier did not have event.
Two outliers removed; outliers did not have event.
CI indicates confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HR, hazard ratio; MCBS, Medicare Current Beneficiary Survey; PS, propensity score.
DISCUSSION
The likelihood of both benefit and harm is important in determining the appropriateness of medication use in older adults with multiple chronic conditions. In this nationally representative cohort of older adults with coexisting CVD and COPD, β-blockers did not adversely affect pulmonary outcomes, but there was also no observed beneficial effect on cardiac outcomes or mortality. Cohort members may represent survivors who have lower risk of the outcomes compared with individuals who died earlier. Moreover, the use of other cardioprotective drugs, such as statins, diuretics, and ACEI, was greater among β-blocker users than nonusers, perhaps lessening any protective cardiac effect of β-blockers.
Similar results showing no significant cardiac or mortality benefit for β-blocker users were seen recently in 2 observational studies, the REACH and STEMI studies. The REACH study included 21,860 propensity score-matched participants, 45 years or older with CVD.6 The STEMI study included 3846 propensity score-matched percutaneous coronary intervention patients followed for nearly 4 years (age, 64.7 ± 11.8 years).7 Neither study showed that β-blockers improved cardiac outcomes or mortality, and the results appear to extend to this older cohort with coexisting COPD. This current study also provides the additional information that there is no overall β-blocker effect for pulmonary events either.
Previous studies suggesting a benefit of β-blockers in individuals with coexisting CVD and COPD involved a younger population.11–14,35 In the current study of older adults, factors such as functional and cognitive status were determinants of receiving β-blockers and of experiencing the outcomes. Previous studies did not account for the fact that more functional and cognitively intact individuals may be more likely to receive β-blockers and to experience better outcomes.
Although we accounted for many factors that affect the risk of major cardiac events, we lacked prognostic factors such as measures of left ventricular ejection fraction.4 The possibility that β-blocker users were at higher risk for subsequent cardiac events compared to nonusers cannot be excluded. Studies following older adults beyond 1-year post-MI, that account for left ventricular ejection fraction and other prognostic factors, are required to confirm or refute the current findings.
The lack of pulmonary harm may be a result of nearly two thirds of the participants using a cardioselective β-blocker; the theoretical harms are associated with nonselective β-blockers causing airway hyperreactivity.8,9 Although we were not able to test for the association of nonselective β-blockers because of the small number of participants using a nonselective β-blocker, caution should still be used for non-selective β-blocker use in patients with COPD. In a study by Düngen et al, older adults randomized to carvedilol (a non-selective β-blocker agent with α-blocking properties) showed more adverse pulmonary events compared with bisoprolol (a cardioselective β-blocker).36
This study has strengths and limitations. This nationally representative cohort enhances the generalizability of prior results observing a lack of benefit of β-blockers for CVD and mortality. These results can be extended to the older adult population with concomitant CVD and COPD.22 The well-characterized cohort allowed us to account for a wide range of factors, including function and cognition, which affected both the propensity to receive β-blockers and to experience the outcomes. This finding confirms earlier reports that cognition and function are potent determinants of outcomes in older adults with cardiac disease.33 We used propensity score adjustment and covariate adjustment to account for biases and confounding inherent in observation studies.30 Findings were similar with each method, suggesting robust results irrespective of adjustment method. However, we cannot exclude the possibility of additional unmeasured confounders. Although not detected in the current study, we cannot exclude the possibility of an increased risk of pulmonary outcomes with β-blockers in the subset with severe COPD. The results of this current study match other observation studies, but there is the possibility of a lack of power because of low sample size or variability. β-blocker use was defined as prevalent use; therefore, prior use information was not known. Outcomes in prevalent user may be different than users starting a β-blocker for the first time (as in what happens in RCTs). For example, those with increased pulmonary outcomes may have already stopped using a β-blocker prior to initiation of this study. Although this cohort is nationally representative, there are several factors that may have contributed to selection bias. First, individuals with severe disease or functional disability may have declined participation in MCBS. Secondly, because of the lack of health claims data for participants in Medicare Advantage plans, including HMO and PPO, these were excluded from this analysis. As for any observational study, we cannot infer a causal relationship for these findings.
The main study implication is that β-blockers may not confer the same cardiac and survival benefits in older adults with CVD and COPD as seen in younger adults. Further, taking into account the recent results in the REACH study, consideration should be given to whether β-blockers are warranted in older adults with COPD and CVD beyond the first year post-MI. This recommendation does not conflict with AHA/ACC guidelines.3 The long-term effects of β-blockers after MI in older adults with COPD should be determined before recommending indefinite use of β-blockers post-MI. The inherent difficulty in disentangling risk from treatment effect may necessitate an RCT in this subgroup. In the meantime, we cannot assume the same benefit as seen in younger adults, with or without COPD, in whom most current evidence was obtained. It is important to point out that this current study does not address other conditions for which a β-blocker may be indicated, such as heart failure.
Determining whether benefits outweigh harms provides an evidence-based approach to polypharmacy in older adults with multiple health conditions. Medications causing greater harm than benefit should be stopped, as should medications without evidence of benefit. We studied the effect of one medication in older adults with one common combination of coexisting conditions. There are many similar situations which will require investigation to inform clinical decision making for the growing population of older adults with multiple conditions who currently receive multiple medications.
Acknowledgments
Supported by R21HS019446 from the Agency for Healthcare Research and Quality and by the Yale Pepper Center (P30 AG021342) from the National Institute on Aging. D.S.H.L. was funded by training grant T32AG019134 from the National Institute on Aging and the American Society for Clinical Pharmacology New Investigator Award. D.S.H.L. and S.M. were funded by the Oregon Medical Research Foundation New Investigator Grant.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple diseases. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 2.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011. update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, López-Sendón J, Lopez de Sa E, et al. GRACE Investigators. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare National Coverage Decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Gabriel Steg PG, Deedwania P, et al. Beta-blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani D, Yasukhiko S, Suna S, et al. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cordiol. 2013;1111:457–464. doi: 10.1016/j.amjcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 8.van der Woude HJ, Zaagsma J, Postma DS, et al. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest. 2005;127:818–824. doi: 10.1378/chest.127.3.818. [DOI] [PubMed] [Google Scholar]
- 9.Chang CL, Mills GD, McLachlan JD, et al. Cardio-selective and non-selective beta-blockers in chronic obstructive pulmonary disease: effects on bronchodilator response and exercise. Intern Med J. 2010;40:193–200. doi: 10.1111/j.1445-5994.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Radford MJ, Wang Y, et al. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol. 2001;37:1950–1956. doi: 10.1016/s0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- 11.Brooks TW, Creekmore FM, Young DC, et al. Rates of hospitalizations and emergency department visits in patients with asthma and chronic obstructive pulmonary disease taking beta-blockers. Pharmacotherapy. 2007;27:684–690. doi: 10.1592/phco.27.5.684. [DOI] [PubMed] [Google Scholar]
- 12.Rutten FH, Zuithoff NPA, Hak E, et al. Beta–blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2010;170:880–887. doi: 10.1001/archinternmed.2010.112. [DOI] [PubMed] [Google Scholar]
- 13.van Gestel YR, Hoeks SE, Sin DD, et al. Impact of cardioselective beta-blockers on mortality in patients with chronic obstructive pulmonary disease and atherosclerosis. Am J Respir Crit Care Med. 2008;78:695–700. doi: 10.1164/rccm.200803-384OC. [DOI] [PubMed] [Google Scholar]
- 14.Short PM, Lipworth SIW, Elder DIH, et al. Effect of β blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. Br Med J. 2011;342:d2549. doi: 10.1136/bmj.d2549. doi:10.1136/bmj.d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell AA, Kaufman DW, Rosenberg L. [Accessed January 23, 2012];Patterns of medication use in the United States. A Report from the Slone Survey (Internet) 2006 Available at: http://www.bu.edu/slone/SloneSurvey/SloneSurvey.htm.
- 16.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Siscovick DS. Minimizing bias due to confounding by indication in comparative effectiveness research: the importance of restriction. JAMA. 2010;304:897–898. doi: 10.1001/jama.2010.1205. [DOI] [PubMed] [Google Scholar]
- 18.Vandenbrouke JP. When are observational studies as credible as randomized trials? Lancet. 2004;363:1728–1731. doi: 10.1016/S0140-6736(04)16261-2. [DOI] [PubMed] [Google Scholar]
- 19.Laupacis A, Mamdani M. Observational studies of treatment effectiveness: some cautions. Ann Intern Med. 2004;140:923–924. doi: 10.7326/0003-4819-140-11-200406010-00014. [DOI] [PubMed] [Google Scholar]
- 20.Giordano SH, Kuo YF, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Am Cancer Soc. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson EB. Evidence, guidelines, performance incentives, complexity, and old people: a clinician’s dilemma. J Am Geriatr Soc. 2009;57:353–354. doi: 10.1111/j.1532-5415.2008.02125.x. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed November 11, 2011];Methods guide for effectiveness and comparative effectiveness reviews. Available at: http://www.effectivehealthcare.ahrq.gov.
- 24.Medicare Current Beneficiary Survey [Accessed November 11, 2011]; Available at: http://www.cms.hhs.gov/apps/mcbs/overview.asp.
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD 10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Leon AC, Hedeker D, Teres JJ. A mixed-effect propensity adjustment for effectiveness analyses of ordered categorical doses. Stat Med. 2007;26:110–123. doi: 10.1002/sim.2042. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 28.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. A critical appraisal of propensity score matching in the medical literature from 1996 to 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 31.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc.; Cary, NC: 1995. [Google Scholar]
- 32.Hosmer DWJ, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley & Sons Inc.; New York: 1999. [Google Scholar]
- 33.Chaudhry SI, Wang Y, Gill TM, et al. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 35.Freemantle N, Cleland J, Young P, et al. β-Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Düngen HD, Apostolovic S, Inkrot S, et al. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBISELD trial. Eur J Heart Fail. 2011;13:670–680. doi: 10.1093/eurjhf/hfr020. [DOI] [PMC free article] [PubMed] [Google Scholar]