Direct visualization and characterization of Cxcr2 signaling as a mediator of systemic neutrophil reaction to a local infection.
Keywords: innate immunity, leukocyte biology, cell migration
Abstract
Neutrophils are the first line of defense against tissue damage and are rapidly mobilized to sites of bacterial infection. However, the signals that regulate neutrophil recruitment are not well defined. Here, using photolabel-enabled fate mapping in zebrafish larvae, we show that localized otic infection with Pseudomonas aeruginosa induces systemic activation and mobilization of neutrophils from the CHT through Cxcr2 signaling. We have cloned the zebrafish Cxcr1 and Cxcr2 receptors and show that Cxcr2 functions as a Cxcl8 receptor in live zebrafish. With the use of morpholino-mediated depletion, we show that infection-induced neutrophil mobilization from the CHT is mediated by Cxcr2 but not Cxcr1. By contrast, Cxcr2 depletion does not affect neutrophil recruitment to the chemoattractant LTB4. Taken together, our findings identify Cxcl8-Cxcr2 signaling as an infection-induced long-range cue that mediates neutrophil motility and mobilization from hematopoietic tissues, positioning Cxcr2 as a critical pathway that mediates infection-induced systemic activation of neutrophils.
Introduction
Neutrophils are the first line of defense against bacterial infection and can be rapidly recruited to sites of infection. In response to localized infection, neutrophils and other phagocytes are recruited from local tissues to mediate host defense. In addition, under some “emergency” conditions, localized infection can induce long-range recruitment of neutrophils from hematopoietic tissue [1], including the bone marrow in humans and the CHT in zebrafish larvae, which are the primary sites of neutrophil production [2, 3]. Under baseline conditions, <2% of the total mature neutrophils are in the circulation [4], and they can be mobilized rapidly from hematopoietic tissue. In zebrafish larvae, the majority of neutrophils are in the CHT, with few circulating neutrophils [5]. However, the underlying molecular mechanisms that mediate long-range neutrophil mobilization are not well understood.
Human neutrophils express CXCR2 and the closely related receptor CXCR1. CXCR2 has been implicated in the pathogenesis of a variety of inflammatory disorders, including acute lung injury, chronic obstructive pulmonary disease, asthma, cystic fibrosis, sepsis, and reperfusion injury [6]. Recently, inhibition of CXCR2 was shown to suppress inflammation-driven and spontaneous tumorigenesis in several mouse models of neoplasia [7]. CXCL8 is the most potent CXCR2 ligand in humans [8], but a peptide fragment derived from collagen also attracts neutrophils through CXCR2 and CXCR1 [9]. CXCL8 and related cytokines are produced in several tissues upon infection or tissue injury and are speculated to mediate local neutrophil accumulation [10]. Rodents do not have the Cxcl8 gene, and mouse neutrophils lack CXCR1 [6]. The major CXCR2 ligands in mice are keratinocyte-derived chemokine and MIP-2. Cxcr2−/− mice have a profound defect in neutrophil emigration to sites of inflammation and delayed wound healing [11, 12]. Cxcr2−/− neutrophils show reduced adhesion to the endothelium and impaired infiltration to the injury core in a murine model of sterile inflammation [13].
The role of CXCR2 in neutrophil mobilization from hematopoietic tissue has been investigated recently. MIP-2 induces neutrophil mobilization from the bone marrow when administered exogenously [14]. CXCR2 antagonizes CXCR4 activity and is required for neutrophil trafficking from murine bone marrow to the peripheral blood at steady state [15]. However, during emergency mobilization, antibodies neutralizing CXCR2 do not affect neutrophil mobilization from the bone marrow during polymicrobial sepsis [16]. As bacterial products can activate and recruit neutrophils directly and thus, mask the requirement of CXCR2 under this extreme condition, we decided to investigate the relevance of CXCR2 to systemic neutrophil activation and neutrophil mobilization during a localized bacterial infection.
Similar to human, zebrafish express the Cxcl8 chemokine and both Cxcl8 receptors, Cxcr1 and Cxcr2 [17]. In addition, a second Cxcl8 lineage has been identified in zebrafish [18]. Therefore, zebrafish represent a strong model system to study the in vivo contributions of Cxcl8-Cxcr1/2 signaling compared with murine models that lack CXCL8 and neutrophil expression of CXCR1. Here, we observed rapid and robust mobilization of neutrophils out of hematopoietic tissue upon a local Pseudomonas infection in zebrafish. We have cloned zebrafish Cxcr1 and Cxcr2 genes and show that Cxcr2 but not Cxcr1 mediates neutrophil responses to this localized bacterial infection, implicating Cxcl8-Cxcr2 as a key long-range cue that mediates neutrophil systemic activation and recruitment induced by infection.
MATERIALS AND METHODS
Zebrafish maintenance and drug treatment
Adult AB fish, including Tg lines Tg(mpx:dendra2) [19] and Tg(fli1a:gfp) [20], were maintained in accordance with the University of Wisconsin-Madison Research Animal Resources Center (Madison, WI, USA). For live-imaging or wounding assays, larvae were anesthetized in E3 containing 0.2 mg/ml Tricaine (ethyl 3-aminobenzoate; Sigma-Aldrich, St. Louis, MO, USA). To prevent pigment formation, some larvae were maintained in E3 containing 0.2 mM N-phenylthiourea (Sigma-Aldrich). Where indicated, larvae were pretreated with 32.5 μM LY294002 (Calbiochem, EMD Millipore, Billerica, MA, USA) [21] or 5 μM SB225002 (Calbiochem, EMD Millipore) in E3 with 1% DMSO. The following experiments were performed in E3 supplemented with indicated drugs for indicated periods.
Otic injection, LTB4 bath assay, and Sudan Black staining
PAK strain—WT or expressing a constitutive plasmid-encoded red fluorescent mCherry protein (pMKB1::mCherry)—was prepared as described previously [22]. Injection in the otic vesicle was performed as described [23], using ∼2500 CFU, unless specified otherwise. Recombinant zebrafish Cxcl8 (XP_001342606.2) was purified as in ref. [24]. Cxcl8 (30 nM), LTB4 (30 nM), or PBS was coinjected with 0.1% phenol red (Sigma-Aldrich) to monitor the injection. LTB4 bath assay was performed as described [25]. Briefly, LTB4 was added directly to E3 at a final concentration of 30 nM for 30 min. Neutrophils in fixed larvae were stained with Sudan Black as described previously [26].
qRT-PCR
RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). First-strand cDNA was then prepared using the SuperScript III First-Strand synthesis system (Invitrogen, Life Technologies, Carlsbad, CA, USA), with 4 ng RNA as template. cDNA was diluted 1:20 for each PCR reaction. qPCR was performed in triplicate with the reference nlp32 [27] or Cxcl8 [17] primers, which were checked to have similar efficiency, and fold change was determined using the ΔΔ comparative threshold method.
RACE
5′-RACE was performed using the SMARTer RACE cDNA amplification kit (Clontech, Mountain View, CA, USA). Briefly, total RNA was extracted from adult whole-kidney marrow, and 5′-RACE-ready cDNA was made, according to the manufacturer's instruction. Cxcr1-specific product was amplified using Cxcr1-specific reverse primer, Cxcr1 359 reverse (5′-CGACAGCAAGCGCCGGTTGGAGCCGA-3′). Cxcr2-specific product was amplified using nest PCR with two nested reverse primers: Cxcr2 470 reverse (5′-AAGAGGGCGGCGCACAGCCACACCAACG-3′) and Cxcr2 267 reverse (5′-GATGGTGCCGAACGGCCAGTGACCGGC-3′). PCR products were purified and cloned into TOPO vector (TOPO TA Cloning Kit Dual Promoter; Invitrogen, Life Technologies). Both sequences were mapped to Danio rerio strain Tuebingen chromosome 9 genomic scaffold zv9_scaffold1227 (NW_003039861.2).
Cells and transfection
HEK293Ts were cultured in DMEM (Invitrogen, Life Technologies) containing 10% FCS (HyClone, Logan, UT, USA) and were transfected with pDendra2-N (Evrogen, Moscow, Russia), pDendra2-N-zCxcr2, or pDendra2-N-zCxcr1 using Lipofectamine 2000 (Invitrogen, Life Technologies). Cells were replated on coverslips, fixed, stained with Phalloidin-Alexa568 and DAPI, and mounted. Fluorescence images were taken with a laser-scanning confocal microscope [21]. Single planes at the cell edge are shown.
FACS and RT-PCR
Dissociated cells of Tg(mpx:dendra2) at 3 dpf were sorted by FACS, as described [28]. RNA was extracted using the RNeasy Mini Kit (Qiagen). RT-PCR was performed using the OneStep RT-PCR kit (Qiagen), according to the manufacturer's instruction with an annealing temperature of 55° and amplification of 40 cycles. Primers to amplify mpx [22], myoD, and ef1α [28] were described previously. Primers for Cxcr1 and Cxcr2 were as follows: Cxcr1 forward: 5′-CGGCAATGTCGCGTGCAAGC-3′; Cxcr1 reverse: 5′-GGCCACAACCACGGCTACGA-3′; Cxcr2 forward: 5′-TCCTTGCCCGGAGACCGTGA-3′; and Cxcr2 reverse: 5′-ATGGTGCCGAACGGCCAGTG-3′.
MO and mRNA microinjection
All MOs were purchased from Gene Tools (Philomath, OR, USA), resuspended in distilled water, and stored at room temperature at a concentration of 1 mM. MOs (3 nl) were injected into the yolk of one-cell stage embryo at concentrations indicated below: 100 μM Cxcr2 MO targeting the ATG region (ACTCTGTAGTAGCAGTTTCCATGTT); 75 μM Cxcr1 MO targeting the exon 1-intron 1 boundary (TGTCAGGATACTAAACTTACCAGTC). Efficient MO-mediated knockdown was confirmed with RT-PCR using mRNA extracted from 3 dpf morphant. For mRNA overexpression, a pCS2+ plasmid containing zebrafish Cxcr2-GFP was linearized with NotI and transcribed with an SP6 message machine kit (Ambion, Life Technologies, Grand Island, NY, USA). mRNA solution (3 nl; 100 ng/μl) was injected alone or together with Cxcr2 MO into the cytoplasm of one-cell stage embryos.
WISH
WISH with 3 dpf larvae was performed according to established protocol using 55° hybridization temperature [29]. The DNA template for Cxcl8 was amplified using a pCS2+ plasmid containing the Cxcl8 cDNA as template. T7 promoter was attached to the 3′ of the coding sequence of Cxcl8 with the following primers: Cxcl8 in situ forward: 5′-ATGACCAGCAAAATCATTTCAGTGTGTGTTATTG-3′; and Cxcl8 in situ reverse: 5′-GATCACTAATACGACTCACTATAGGGAGATCATGGTTTTCTGTTGACAATGATCCTATCAATGATC-3′.
To construct the template for Cxcl8 sense-probe control, the T7 promoter was attached to the 5′ of the coding sequence of Cxcl8.
Live imaging, photoconversion, and image quantification
Larvae at 3 dpf were settled on a custom-made, glass-bottom dish. Time-lapse fluorescence images were acquired with a laser-scanning confocal microscope (FluoView FV1000; Olympus, Center Valley, PA, USA) or a fluorescence-dissecting microscope, as described [19]. Photoconversion was performed using the laser-scanning confocal microscope, according to established methods [18]. Zebrafish larvae with photolabeled neutrophils were infected and put on the fluorescent-dissecting microscope right after infection, but the movies start a few minutes postinfection. To quantify circulating neutrophils, photolabeled neutrophils that circulate through the posterior cardinal vein were scored in individual, 3-min movies with 3-s intervals. Neutrophil speed was quantified using ImageJ software (NIH, Bethesda, MD, USA) plug-in MTrackJ [30]. Kymography was performed as described [5]. Supplemental Movies 1–4 (and see Figs. 1A, B, and D, and 4B) were taken by stereofluorescence. Supplemental Movie 5 (and see Figs. 1F, 3C, 6D, and 7B, D, F, and H) was taken by confocal microscopy. (See Figs. 2A, C, and E, 4D and F, 5B and D, and 6A, which were taken with a camera connected to a stereomicroscope.)
Figure 1. Localized otic infection with PAK strain induces neutrophil mobilization out of the CHT.
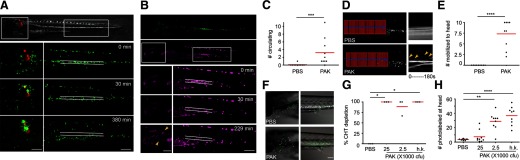
(A) PAK (pMKB1::mCherry) was injected into the otic vesicle of Tg(mpx:dendra2) at 3 dpf. Still images of green neutrophils in the head and CHT (both boxed and enlarged) at indicated time postinfection are shown. Lines indicate the location of the CHT where neutrophils were aggregated. Note the dispersion of neutrophils upon infection. Original scale bars: 100 μm. (B–H) Neutrophils in the CHT were photoconverted to red fluorescence in Tg(mpx:dendra2) at 3 dpf. (B) PAK was injected into the otic vesicle. Still images of photolabeled neutrophils in the head and CHT (both boxed and enlarged) at indicated times postinfection are shown. Lines indicate the location of the CHT where neutrophils were photolabeled originally. Note the dispersion of photolabeled neutrophils in the CHT. Arrowheads indicate some of the photoconverted neutrophils that were mobilized to the head. Original scale bars: 100 μm. (C) Relative numbers of circulating, photolabeled neutrophils by 2 hpi with PAK or PBS. Total numbers of photolabeled neutrophils seen circulating through the posterior cardinal vein in a 3-min movie with 3-s intervals are shown; n = 10 each. ***P < 0.001, two-tailed Mann-Whitney test. (D) Kymography of representative movies used to do quantification in C. Fluorescence signal in boxed regions was stacked vertically into a one-dimensional line at each time-point. The lines were then combined horizontally in time sequence. Kymography of 3-min movies with 3-s intervals is shown. Horizontal lines indicate autofluorescent pigments, which are stationary throughout the movie. Arrowheads indicate the appearance of neutrophils circulating in the posterior cardinal vein. (E) Numbers of photolabeled neutrophils that have reached the head (boxed regions in B) by 2 hpi with PAK or PBS control; n = 9 each. ****P < 0.0001, two-tailed Mann-Whitney test. (F) Representative images of neutrophils in the head and the CHT, 1 dpi with PAK or PBS control. Original scale bar: 100 μm. (G) Percentages of larvae from three separate experiments that were completely depleted of photolabeled neutrophils in the CHT by 1 dpi with PBS, PAK at 25,000, 2500 CFU, or h.k. (bacteria number equivalent to 25,000 CFU). *P < 0.05, Kruskal-Wallis test, followed by Dunn's multiple comparison test. (H) Quantification of photolabeled neutrophils at the head by 1 dpi with PBS, PAK at 25,000, 2500 CFU, or h.k. (bacteria number equivalent to 25,000 CFU); n = 9 each. **P < 0.01; ****P < 0.0001, Kruskal-Wallis test, followed by Dunn's multiple comparison test.
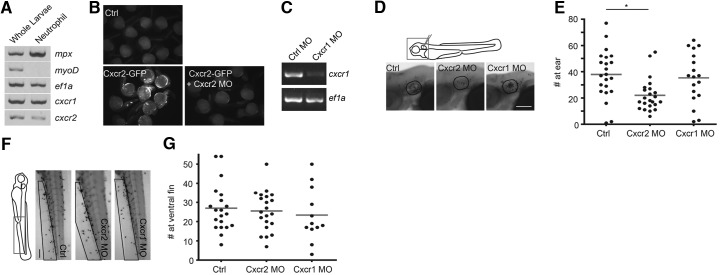
Figure 4. Cxcr2, but not Cxcr1, is involved in neutrophil recruitment to PAK otic infection.
(A) Dissociated cells of Tg(mpx:dendra2) at 3 dpf were sorted by FACS to enrich for Dendra2-positive neutrophils. RT-PCR of mpx (neutrophil marker), myoD (muscle cell maker), ef1α (housekeeping), Cxcr1, and Cxcr2 was performed using RNA extracted from whole larvae or sorted neutrophils. (B) Zebrafish Cxcr2-GFP mRNA was injected into zebrafish eggs at one-cell stage alone or in combination with Cxcr2 MO. Representative images of uninjected embryos (Ctrl), injected with Cxcr2-GFP mRNA alone (Cxcr2-GFP) or plus Cxcr2 MO (Cxcr2-GFP+Cxcr2 MO) at 1 dpf indicated efficient suppression of Cxcr2 translation with Cxcr2 MO. (C) WT AB fish were injected with buffer or Cxcr1 MO at one-cell stage. Total RNA was extracted at 3 dpf, and RT-PCR of ef1α and Cxcr1 was performed. (D and E) WT AB fish were injected with buffer, Cxcr2 MO, or Cxcr1 MO at one-cell stage. At 3 dpf, larvae were injected with PAK into the otic vesicle. Neutrophils recruited to the ear (outlined regions in D) at 1 hpi were quantified; n = 22, 23, and 18, respectively. *P < 0.05, Kruskal-Wallis test, followed by Dunn's multiple comparison test. Original scale bar: 100 μm. (F and G) WT AB fish were injected with buffer, Cxcr2 MO, or Cxcr1 MO at one-cell stage. At 3 dpf, larvae were bathed in 30 nM LTB4 for 30 min, and neutrophils recruited to the ventral fin (boxed regions in F) were quantified; n = 20, 21, and 12, respectively. Original scale bar: 100 μm.
Figure 3. Cloning of zebrafish Cxcr2 receptor.
(A) Cartoon of the arrangement of the Cxcr2 gene. Box, Exon; line, intron; arrow, translation start site (ATG). (B) Alignment of human and zebrafish Cxcr2 proteins. Seven transmembrane domains are indicated with Roman numerals. Note the truncation of zebrafish Cxcr2 protein at the N-terminus. H. sapiens, Homo sapiens. (C) HEK cells were transfected with plasmids coding Dendra2, Cxcr2-Dendra2, or Cxcr1-Dendra2. Cells were stained with DAPI and Phalloidin-Alexa568. Representative confocal images were taken 1 day post-transfection. Original scale bar: 10 μm.
Figure 6. Cxcr2 knockdown alters neutrophil homeostasis at steady state.
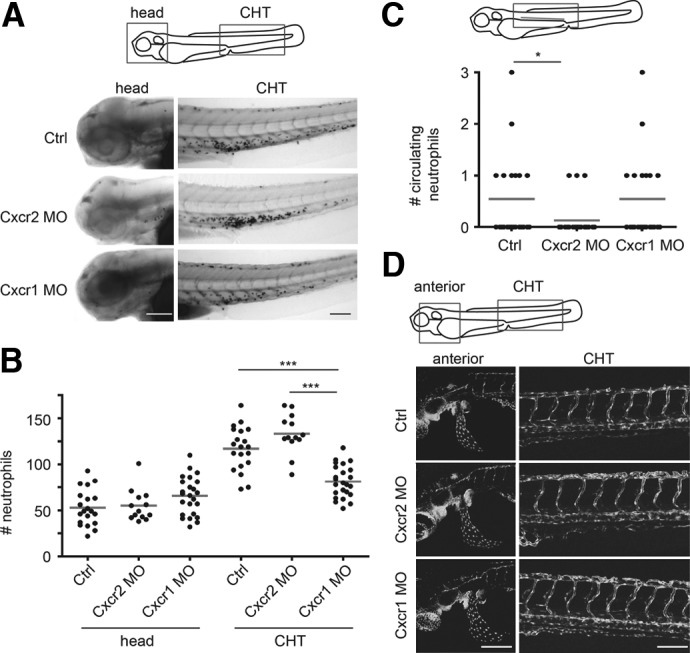
(A and B) Ctrl, Cxcr2, or Cxcr1 morphants were fixed at 3 dpf and stained with Sudan black, and neutrophil numbers in the head or the CHT were quantified; n = 20, 13, 24, 20, 13, and 24, respectively. ***P < 0.001, Kruskal-Wallis test, followed by Dunn's multiple comparison test. Original scale bars: 100 μm. (C) Quantification of neutrophils circulating through indicated posterior cardinal vein within 3 min in 2 dpf Ctrl, Cxcr2, or Cxcr1 morphants in Tg(mpx:dendra2) background; n = 24 each. *P < 0.05, Kruskal-Wallis test, followed by Dunn's multiple comparison test. (D) Embryos of Tg(fli1a:GFP) were injected with buffer (Ctrl) or Cxcr2 or Cxcr1 MO at one-cell stage. Representative images of the vasculature in the head, yolk sac, and trunk are shown. Original scale bars: 100 μm.
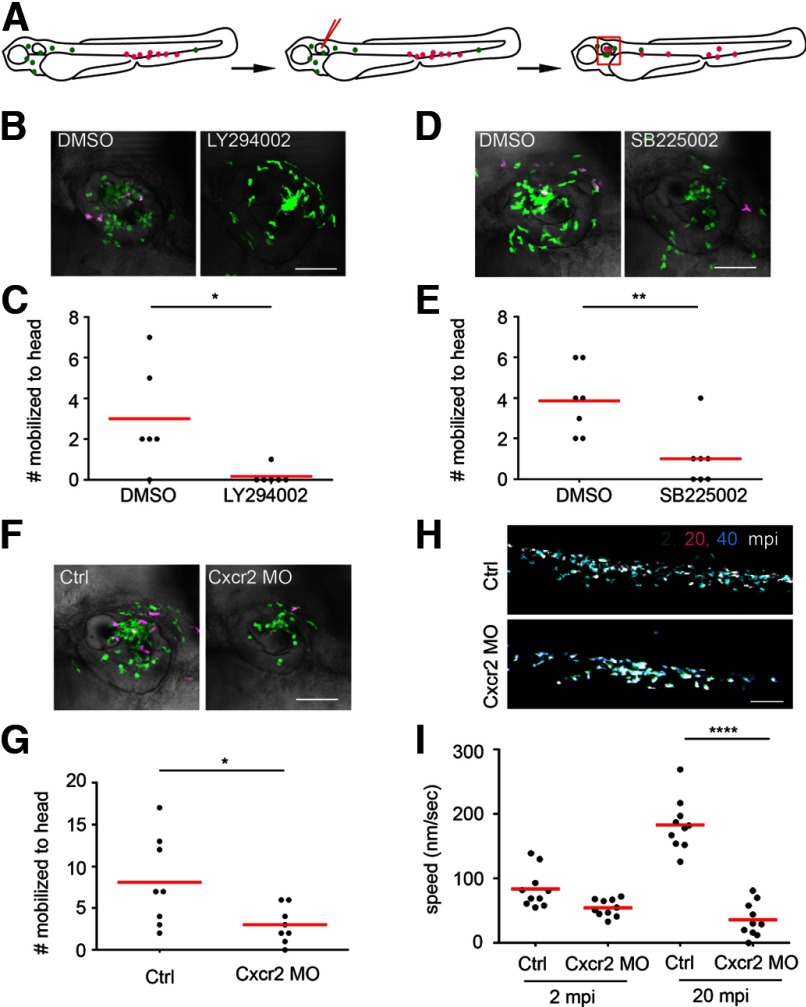
Figure 7. Neutrophils from the CHT are recruited to the infected ear via a Cxcr2-dependent pathway.
(A) Illustration of experimental procedures in B–G. Neutrophils in the CHT were photoconverted to red fluorescence in Tg(mpx:dendra2) at 3 dpf. The larvae were then injected with PAK in the ear. Photolabeled neutrophils that were mobilized to the head (as in the boxed region illustrated in Fig. 1A) at 1 hpi were quantified. (B and C) The larvae were treated with DMSO or 32.5 μM LY294002, 1 h prior to and postinfection; n = 6 each. *P = 0.0152, two-tailed Mann-Whitney test. Original scale bar: 50 μm. (D and E) The larvae were treated with DMSO or 5 μM SB225002, 1 h prior to and postinfection; n = 7 each. **P=0.0052, two-tailed Mann-Whitney test. Original scale bar: 50 μm. (F and G) Tg(mpx:dendra2) embryos were injected with buffer (Ctrl) or Cxcr2 MO at one-cell stage and then processed as described in A; n = 8 each. *P = 0.0277, two-tailed Mann-Whitney test. Original scale bar: 50 μm. (H) Tg(mpx:dendra2) embryos were injected with buffer (Ctrl) or Cxcr2 MO at one-cell stage. Larvae were injected at 3 dpf with PAK in the otic vesicle. Neutrophil locations in the CHT at 2 (green), 20 (magenta), and 40 (cyan) min postinfection were overlayed. Neutrophil location remains relatively stationary during the first 20 mpi; however, neutrophils move out of their original location 40 mpi in control fish but not in Cxcr2 morphants. Original scale bar: 100 μm. (I) Neutrophil motility at the CHT, 2 or 20 min postinfection, in control and Cxcr2 morphants was quantified. Results were from 10 individual neutrophils from two independent movies for each condition. ****P < 0.0001, Kruskal-Wallis test, followed by Dunn's multiple comparison test.
Figure 2. Cxcr2 inhibitor SB225002 impairs neutrophil recruitment to PAK otic infection.
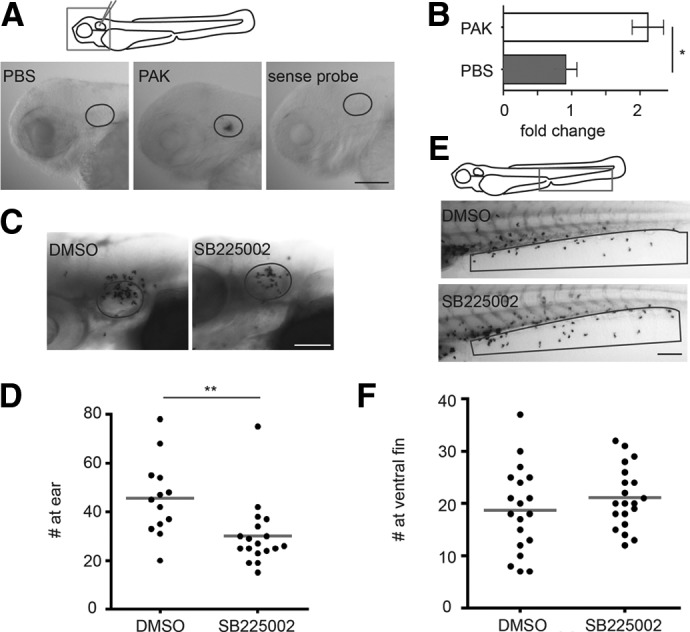
(A and B) PBS or PAK was injected into the otic vesicle of 3 dpf WT AB larvae. (A) Larvae were fixed at 1 hpi, and WISH hybridization was performed with Cxcl8 antisense probe. The position of the ear is outlined. PAK-injected larvae were also probed with the Cxcl8 sense probe as a control. Original scale bar: 100 μm. (B) Total RNA was extracted 1 hpi and reverse-transcribed into cDNA. The expression level of Cxcl8 was determined by real-time qPCR. Mean ± sd of three independent experiments was shown. Values were normalized with uninjected siblings. *P < 0.01, two-tailed Mann-Whitney test. (C and D) WT AB zebrafish larvae at 3 dpf were treated with DMSO or 5 μM SB225002 for 1 h, followed by otic infection with PAK. Larvae were kept in SB225002 and fixed 1 hpi, and neutrophils recruited to the ear (outlined regions in C) were quantified; n = 13 and 18, respectively. **P < 0.01, two-tailed Mann-Whitney test. Original scale bar: 100 μm. (E and F) WT AB zebrafish larvae at 3 dpf were treated with DMSO or 5 μM SB225002 for 1 h, followed by bathing in 30 nM LTB4. Larvae were fixed 30 min postaddition of LTB4, and neutrophils recruited to the ventral fin (boxed region in E) were quantified; n = 19 and 20, respectively. Original scale bar: 100 μm.
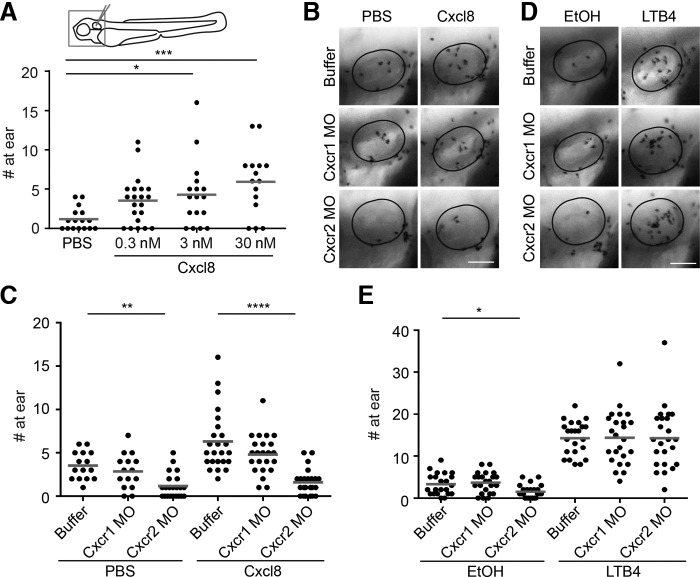
Figure 5. Cxcr2 depletion specifically impairs neutrophil recruitment to purified zebrafish Cxcl8 but not to LTB4.
(A) WT AB larvae were injected at 3 dpf into the ear cavity with 1 nl PBS control or purified zebrafish Cxcl8 at concentrations of 0.3, 3, and 30 nM. Larvae were fixed 45 min postinjection and stained with Sudan Black. Neutrophils recruited to the ear were quantified. *P < 0.05; ***P < 0.001, Kruskal-Wallis test, followed by Dunn's multiple comparison test. (B and C) Ctrl, Cxcr1, or Cxcr2 morphants were injected at 3 dpf into the ear cavity with 1 nl PBS control or 30 nM zebrafish Cxcl8. Larvae were fixed 45 min postinjection and stained with Sudan Black. Neutrophils recruited to the ear (outlined in B) were quantified; n = 18, 15, 22, 24, 23, and 23, respectively. **P < 0.01; ****P < 0.0001, Kruskal-Wallis test, followed by Dunn's multiple comparison test. Original scale bar: 50 μm. (D and E) Ctrl, Cxcr1, or Cxcr2 morphants were injected at 3 dpf into the ear cavity with 1 nl 0.001% EtOH or 30 nM LTB4 in PBS. Larvae were fixed 45 min postinjection and stained with Sudan Black. Neutrophils recruited to the ear (outlined in D) were quantified; n = 22, 23, 23, 23, 24, and 24, respectively. *P < 0.05, Kruskal-Wallis test, followed by Dunn's multiple comparison test. Original scale bar: 50 μm.
Statistical analysis
We performed statistical analysis using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Comparisons between two groups were performed with two-tailed Mann-Whitney test. Comparisons among three groups were performed with Kruskal-Wallis test, followed by Dunn's multiple comparison test. One representative experiment from three independent experiments, which all showed similar statistical significance, was shown.
RESULTS
Localized otic infection with the PAK strain induces neutrophil mobilization out of the CHT
To determine whether systemic mobilization of neutrophils occurs with localized bacterial infection in zebrafish larvae, we photolabeled neutrophils in hematopoietic tissue using the Tg line, Tg(mpx:dendra2). The CHT in zebrafish larvae has a hematopoietic function similar to the fetal liver in mammals [31]. The PAK strain, labeled with red fluorescence [PAK(pBK1:mCherry), 2500 CFU], was injected into the otic vesicle of zebrafish larvae at 3 dpf. Within the first few minutes, green neutrophils resident in the head were rapidly recruited to the infected ear, representing local recruitment. In addition, neutrophils in the CHT increased their motility by 15 min and dispersed from the CHT, ∼30 min postinoculation (Fig. 1A and Supplemental Movie 1). To determine whether neutrophils in the CHT were also recruited to the infected ear, CHT neutrophils were photolabeled using photoconversion of the Dendra2 protein to red fluorescence [19] (Fig. 1B). Upon PAK injection, but not PBS control injection, neutrophils in the CHT were mobilized swiftly into the caudal/posterior cardinal vein (Fig. 1C and D and Supplemental Movie 2). The majority of these neutrophils exited the vasculature at the head from 30 min postinfection onward and were recruited to the infected ear (Fig. 1B and E and Supplemental Movie 2). The fate of photolabeled neutrophils was tracked for 1 additional day. Almost all photolabeled neutrophils mobilized out of the CHT and dispersed randomly in the head (Fig. 1F) compared with control fish, where photolabeled neutrophils remained in the CHT. The newly formed green neutrophils remained in the CHT in infected fish, probably as a result of the clearance of the infection at this later time-point. Injecting heat killed bacteria (h.k.) or different doses of bacteria induced similar phenotypes (Fig. 1G). Interestingly, fewer photolabeled neutrophils were present in the head region and none in the CHT 1 day after injection with a higher dose of PAK (25,000 CFU; Fig. 1H), which may have been the result of neutrophil death, as observed previously by Hall et al. [3] after Salmonella injection in the hindbrain ventricle. Taken together, our results indicate that neutrophils in the CHT are activated promptly and respond to a localized infection at a distal site.
Cxcr2 inhibitor SB225002 impairs neutrophil recruitment to PAK otic infection
To understand the underlying molecular mechanisms that lead to long-range neutrophil mobilization, we first investigated the role of Cxcr2 signaling, a well-characterized pathway that mediates neutrophil mobilization out of hematopoietic tissue upon G-CSF stimulation in mice. We first determined whether the expression of the ligand Cxcl8 was up-regulated during infection. WISH of 3 dpf larvae detected a robust and localized Cxcl8 mRNA expression by 1 hpi in the PAK-injected ear compared with PBS control (Fig. 2A). Consistently, a twofold up-regulation of Cxcl8 mRNA was detected by qRT-PCR in PAK-infected whole larvae compared with PBS-injected control (Fig. 2B). To determine whether Cxcl8-Cxcr2 signaling is involved in neutrophil responses to infection, we first used a selective nonpeptide inhibitor of human CXCR2, SB225002 [32]. One caveat is that this inhibitor would also inhibit the closely related CXCL8 receptor, CXCR1, at higher doses. Treatment with SB225002 at 5 μM significantly reduced the number of neutrophils recruited to ear infection by 1 hpi (Fig. 2C and D). Meanwhile, a similar treatment did not inhibit neutrophil random motility (data not shown) or neutrophil response to exogenously supplied LTB4 (Fig. 2E and F) [25], indicating that SB225002 specifically inhibits neutrophil responses to PAK infection but not neutrophil motility or signaling induced by LTB4.
Cxcr2, but not Cxcr1, is involved in neutrophil recruitment to PAK otic infection
To further characterize the Cxcl8-Cxcr2 signaling axis, we then cloned the zebrafish Cxcr2 gene. Protein alignment indicates that human and zebrafish Cxcr2 proteins are well-conserved, except that zebrafish Cxcr2 is truncated at the N-terminus (Fig. 3B), a region known to contribute to plasma membrane localization of the receptor. To confirm the annotated sequence, we performed 5′-RACE and mapped an additional exon ∼2600 nt upstream of the present start codon; however, no additional coding sequence was discovered (Fig. 3A; detailed sequence in the Supplemental data). We also performed 5′-RACE to clone the closely related zebrafish Cxcr1. Similarly, two exons separated by an intron of ∼2600 nt were identified (detailed sequence in Supplemental data). As plasma membrane localization is critical for GPCRs to interact with extracellular ligands, we next examined the cellular localization of Cxcr1 and Cxcr2 (Fig. 3C). When transiently expressed in HEK293 cells, Dendra2 localized throughout the cytosol. Cxcr1-Dendra2 localized to the plasma membrane and intracellular organelles, although Cxcr2-Dendra primarily localized at intracellular membranes, a clear plasma membrane localization is also observed.
After mapping the exon-intron structures of Cxcr1 and Cxcr2 genes, we also confirmed that Cxcr2 and Cxcr1 are expressed in zebrafish neutrophils using RT-PCR with mRNA extracted from FACS-sorted neutrophils (Fig. 4A) [28]. MOs against each mRNA were then designed to test the contribution of Cxcr1 and Cxcr2 during neutrophil response to bacterial infection. Because of the limited number of exons, we could only use a translation blocking MO against Cxcr2. To confirm the efficiency of this MO, we coinjected it with mRNA encoding Cxcr2-GFP into embryos at the one-cell stage. Overexpression of Cxcr2-GFP alone led to developmental defects (smaller, curved embryos) and clusters of bright GFP-positive cells. Coinjection with Cxcr2 MO prevented the expression of Cxcr2-GFP, indicating sufficient suppression of Cxcr2 translation by the Cxcr2 MO (Fig. 4B). Meanwhile, Cxcr1 knockdown was achieved with a splice-blocking MO, as confirmed by the reduced amount of correctly spliced Cxcr1 mRNA (Fig. 4C). Cxcr2 MO, but not Cxcr1 MO, significantly inhibited neutrophil recruitment to PAK otic infection (Fig. 4D and E and Supplemental Movie 3), indicating that Cxcr2 is a major functional receptor mediating neutrophil responses to infection in zebrafish larvae. In contrast, neither Cxcr2 nor Cxcr1 MO inhibited neutrophil responses to LTB4 (Fig. 4F and G). We next confirmed that Cxcr2 was indeed the Cxcl8 receptor. Injecting increasing amounts of purified zebrafish Cxcl8 into the otic cavity resulted in increasing neutrophil recruitment to the ear (Fig. 5A). The Cxcr2 MO, but not Cxcr1 MO, significantly reduced the number of neutrophils recruited to the Cxcl8-injected ear (Fig. 5B and C), indicating that Cxcr2 is a major Cxcl8 receptor in vivo. In contrast, neutrophil recruitment to LTB4, injected in the ear, was normal in Cxcr1 and Cxcr2 morphants (Fig. 5D and E), excluding general defects in recruitment of head-resident neutrophils in the morphants.
Cxcr2 knockdown alters neutrophil homeostasis at steady state
To exclude the possibility that the phenotype associated with Cxcr2 MO is a result of defects in neutrophil development, we investigated neutrophil homeostasis in Cxcr2 morphants. Neutrophil numbers in the head mesenchyme were similar among control, Cxcr2, and Cxcr1 morphants. Cxcr2 morphants had a slight but not significant increase in the number of neutrophils in the CHT (Fig. 6A and B). In contrast, Cxcr1 morphants had fewer neutrophils in the CHT, which may indicate a nonspecific toxicity of the Cxcr1 MO or that Cxcr1 is important for neutrophil development in the CHT. The number of circulating neutrophils in the intact larvae was reduced in Cxcr2 but not Cxcr1 morphants (Fig. 6C and Supplemental Movie 4), indicating that Cxcr2 is required for neutrophils to enter the vasculature under steady-state conditions. In addition, as Cxcl8 was previously shown to regulate the development of the vasculature [33], we next investigated the structure of the vascular system in Cxcr1 and Cxcr2 morphants using Tg(fli1a:gfp) larvae. We observed that vascular development appeared normal in Cxcr1 and Cxcr2 morphants (Fig. 6D).
Neutrophils from the CHT are recruited to the infected ear via a Cxcr2-dependent pathway
To determine whether Cxcr2 is involved in long-range neutrophil mobilization from the CHT in response to a localized infection, we photoconverted neutrophils in the CHT to red fluorescence and then quantified the number of photolabeled neutrophils that mobilized to the head at 1 hpi (Fig. 7A). The PI3K inhibitor LY294002 significantly reduced the number of photolabeled neutrophils recruited to the head (Fig. 7B and C). The CXCR2 inhibitor SB225002 also impaired this long-range neutrophil mobilization (Fig. 7D and E). Accordingly, the Cxcr2 MO significantly inhibited the mobilization of neutrophils from the CHT (Fig. 7F and G). There were also fewer (although this was not statistically significant) photolabeled neutrophils in the head region in the Cxcr1 morphants (data not shown), which may have resulted from the reduced number of neutrophils in the CHT. In addition, we observed reduced motility of neutrophils in the CHT after PAK otic infection in the Cxcr2 morphants (Fig. 7H and I and Supplemental Movie 5), implicating Cxcr2 signaling in the systemic activation of neutrophils induced by local infection.
DISCUSSION
Here, we have cloned zebrafish Cxcr1 and Cxcr2. We have found that both receptors are expressed in zebrafish neutrophils, whereas Cxcr2 but not Cxcr1 mediates neutrophil recruitment to a localized infection. On the other hand, Cxcr2 does not affect neutrophil motility induced by LTB4 or development of the zebrafish vasculature. We have also shown that Cxcr2 is a functional Cxcl8 receptor in zebrafish. Taken together, our data provide the first functional characterization of Cxcr2 in zebrafish and identify a novel role for Cxcr2 in the long-range mobilization of neutrophils to a localized bacterial infection.
We report the first noninvasive, real-time observation of neutrophil mobilization from the hematopoietic tissue to a distal infection site in an intact whole organism. The mobilization induced by a localized infection is robust, as almost all photolabeled neutrophils in the CHT were mobilized within 1 dpi. Although not a focus of our study, newly generated neutrophils repopulate the CHT, providing an ideal model to study emergency granulopoiesis [3]. Emergency mobilization of neutrophils from the CHT to a distal infection is a multistep process: neutrophils in the hematopoietic tissue first mobilize into the peripheral blood and then emigrate into the inflamed site. We found that reduced numbers of neutrophils from the CHT trafficked to the infected ear in Cxcr2 morphants (Fig. 6), indicating that Cxcr2 signaling is a major mediator of this long-range mobilization. It has been shown previously in a sterile injury model in mice that the Cxcr2 ligand MIP-2 forms a gradient in the vasculature adjacent to the damaged tissue and mediates neutrophil crawling on the endothelium, which facilitates neutrophil emigration and infiltration into the core of tissue damage [13]. A snapshot at 1 hpi did not detect increased numbers of neutrophils accumulating in the vasculature in Cxcr2 morphants (data not shown), excluding the possibility of a defect in neutrophil emigration alone. Therefore, Cxcr2 possibly regulates both neutrophil mobilization out of the CHT and their emigration out of the vasculature. We only observed a partial inhibition of total mobilization, possibly as MOs only mediated transient and partial knockdown. Also, for this reason, we focused on acute mobilization (1 hpi) rather than on a more persistent (overnight) response. In addition, Cxcr1 might partially compensate for the loss of Cxcr2 function. Unfortunately, the coinjection of Cxcr1 and Cxcr2 MOs led to severe developmental defects (data not shown) that prohibited the feasibility of testing this hypothesis with the currently available tools.
In our experimental setup, neutrophils in the CHT displayed increased motility, 15–30 min postbacterial otic infection and were recruited to the infected ear from 40 min onward. It is possible that inflammatory mediators produced locally at the site of tissue injury/infection are released into the vasculature to mediate this systemic response. Cxcl8 has all the traits to be the mediator that can activate neutrophils in this short time-frame. 1) We have observed that Cxcl8 mRNA expression is up-regulated shortly after infection (Fig. 2). 2) In mammals, it was demonstrated that neutrophil mobilization peaks within a few minutes of CXCL8 administration [34]. 3) Upon transplantation of cells expressing a Cxcl8-mCherry fusion protein in the rostral part of the larvae, the secreted protein is present on the vasculature as far as the CHT [24]. This observation indicates that locally produced Cxcl8 is released into the blood [35], and presented on the endothelial surface, allowing it to mediate the systemic response. 4) Knocking down Cxcl8 receptor Cxcr2 reduced neutrophil mobilization. However, injection of rCxcl8 into the otic vesicle did not induce significant mobilization of neutrophils from the CHT to the injected ear (data not shown), which could be a result of quick diffusion of Cxcl8 out of the otic vesicle, whereas during infection, a local and sustained Cxcl8 production likely occurs. Unfortunately, Cxcl8 knockdown results in a leaky vasculature [33]; therefore, we were not able to show a definitive role for Cxcl8 in the infection-induced systemic activation of neutrophils. In addition, we cannot rule out the possibility that other mediators [36], such as LTB4, complement component C5a, TNF-α, or bacterial products, also play a Cxcr2-independent role in this process.
Similar to our observations, increased neutrophil motility was observed during neutrophil mobilization upon systemic G-CSF administration, using intravital two-photon microscopy in murine bone marrow [37]. Hence, a correlation between neutrophil motility in hematopoietic tissue and mobilization has been proposed, but no direct link has been established. Interestingly, in Cxcr2 morphants, neutrophil mobilization still happens in larvae, in which neutrophils in the CHT display minimal velocity (Supplemental Movie 3). Together with our previous observations in a zebrafish leukocyte adhesion deficiency model showing that neutrophils expressing a dominant-negative Rac2 protein increasingly mobilize to the periphery, despite loss of motility [5], these data indicate that motility and mobilization are separable. In addition, as Cxcr2 is expressed in many tissues, including leukocytes, endothelium, and epithelial cells [17], it remains to be determined whether Cxcr2 regulates neutrophil homeostasis through neutrophil-intrinsic mechanisms. It is well known that the human CXCL8-CXCR2 axis in endothelial cells drives angiogenesis [38]. Although zebrafish Cxcl8 lacks the angiogenic Glu-Leu-Arg motif [17], it is conceivable that alterations in the endothelium would facilitate neutrophil mobilization, independent of neutrophil-intrinsic Cxcr2 signaling. We are actively generating neutrophil-specific Cxcr2 knockout lines and rescue lines to test this possibility.
What are other mechanisms that drive neutrophil mobilization out of hematopoietic tissue? G-CSF is an essential regulator of neutrophil mobilization from the bone marrow to the periphery [4]. However, previous studies focused on the roles of G-CSF in neutrophil mobilization upon infection, have yielded conflicting results. G-CSF−/− mice mount a profound and sustained neutrophilia following systemic Candida albicans infection [39]. Also, the acute granulocyte infiltration into the peritoneal cavity was normal in Listeria monocytogenes infection [40]. In contrast, G-CSF−/− mice infected i.v. with L. monocytogenes demonstrated reduced neutrophil recruitment into the blood compared with WT littermates [41]. Neutrophil mobilization from the bone marrow in response to pulmonary P. aeruginosa infection is impaired in G-CSFR−/− mice [42]. It is possible that different pathogens or routes of infection evoke different cellular responses that drive neutrophil mobilization via distinct signaling mechanisms. We have observed that G-CSF in zebrafish is up-regulated quickly after ear infection (data not shown). Our future studies will determine whether G-CSF is required for neutrophil mobilization with localized PAK infection. In murine models, CXCR2 is required for G-CSF-induced neutrophil mobilization [37]. It is also intriguing to test whether G-CSF and Cxcr2 are similarly required for neutrophil mobilization with diverse immune challenges.
In summary, here, we provide the first in vivo observation and characterization of the kinetics of neutrophil recruitment from hematopoietic tissue to a distal, localized bacterial infection. We have cloned the zebrafish Cxcr1 and Cxcr2 receptors and characterized their function during neutrophil response to infection. We have further demonstrated that Cxcr2 mediates the systemic recruitment of neutrophils from the hematopoietic tissue. Given the central role of Cxcr2 in regulating neutrophil homeostasis under steady-state and emergency conditions, we expect that our work will aid in the characterization of the mechanisms that regulate neutrophil activation, a fundamental component of the innate immune system.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. National Institutes of Health to A.H. (GM074827) and National Science Foundation to A.H. (EFRI-1136903).
The authors thank Drs. Damien Maurin and Hugues Lortat-Jacob (Institut de Biologie Structurale, CNRS, Grenoble, France) for the generous gift of purified zebrafish Cxcl8 and lab members for zebrafish maintenance and useful suggestions.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- −/−
- deficient
- CHT
- caudal hematopoietic tissue
- dpf
- days postfertilization
- dpi
- day postinjection
- E3
- embryo medium
- HEK
- human embryonic kidney
- h.k.
- heat-killed
- hpi
- hour postinjection
- LTB4
- leukotriene B4
- MO
- morpholino oligonucleotides
- PAK
- Pseudomonas aeruginosa K
- qRT-PCR
- quantitative RT-PCR
- Tg
- transgenic
- WISH
- whole-mount in situ hybridization
AUTHORSHIP
A.H. and Q.D. designed the research, analyzed the data, prepared the figures, and wrote the manuscript. M.S. and P.H. provided advice on research design and reviewed the manuscript. M.S. performed ear injection with purified zebrafish Cxcl8 in Cxcr1 and Cxcr2 morphants. J.M.G. performed FACS for neutrophils. D.A.B. performed cell-culture work, and Q.D performed experiments.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Furze R. C., Rankin S. M. (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christopher M. J., Link D. C. (2007) Regulation of neutrophil homeostasis. Curr. Opin. Hematol. 14, 3–8 [DOI] [PubMed] [Google Scholar]
- 3. Hall C. J., Flores M. V., Oehlers S. H., Sanderson L. E., Lam E. Y., Crosier K. E., Crosier P. S. (2012) Infection-responsive expansion of the hematopoietic stem and progenitor cell compartment in zebrafish is dependent upon inducible nitric oxide. Cell Stem Cell 10, 198–209 [DOI] [PubMed] [Google Scholar]
- 4. Semerad C. L., Liu F., Gregory A. D., Stumpf K., Link D. C. (2002) G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17, 413–423 [DOI] [PubMed] [Google Scholar]
- 5. Deng Q., Yoo S. K., Cavnar P. J., Green J. M., Huttenlocher A. (2011) Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev. Cell 21, 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stadtmann A., Zarbock A. (2012) CXCR2: from bench to bedside. Front. Immunol. 3, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamieson T., Clarke M., Steele C. W., Samuel M. S., Neumann J., Jung A., Huels D., Olson M. F., Das S., Nibbs R. J., Sansom O. J. (2012) Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 122, 3127–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Damme J., Decock B., Conings R., Lenaerts J. P., Opdenakker G., Billiau A. (1989) The chemotactic activity for granulocytes produced by virally infected fibroblasts is identical to monocyte-derived interleukin 8. Eur. J. Immunol. 19, 1189–1194 [DOI] [PubMed] [Google Scholar]
- 9. Weathington N. M., van Houwelingen A. H., Noerager B. D., Jackson P. L., Kraneveld A. D., Galin F. S., Folkerts G., Nijkamp F. P., Blalock J. E. (2006) A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 12, 317–323 [DOI] [PubMed] [Google Scholar]
- 10. Baggiolini M., Clark-Lewis I. (1992) Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 307, 97–101 [DOI] [PubMed] [Google Scholar]
- 11. Cacalano G., Lee J., Kikly K., Ryan A. M., Pitts-Meek S., Hultgren B., Wood W. I., Moore M. W. (1994) Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265, 682–684 [DOI] [PubMed] [Google Scholar]
- 12. Devalaraja R. M., Nanney L. B., Du J., Qian Q., Yu Y., Devalaraja M. N., Richmond A. (2000) Delayed wound healing in CXCR2 knockout mice. J. Invest. Dermatol. 115, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonald B., Pittman K., Menezes G. B., Hirota S. A., Slaba I., Waterhouse C. C., Beck P. L., Muruve D. A., Kubes P. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 [DOI] [PubMed] [Google Scholar]
- 14. Burdon P. C., Martin C., Rankin S. M. (2005) The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood 105, 2543–2548 [DOI] [PubMed] [Google Scholar]
- 15. Eash K. J., Greenbaum A. M., Gopalan P. K., Link D. C. (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delano M. J., Kelly-Scumpia K. M., Thayer T. C., Winfield R. D., Scumpia P. O., Cuenca A. G., Harrington P. B., O'Malley K. A., Warner E., Gabrilovich S., Mathews C. E., Laface D., Heyworth P. G., Ramphal R., Strieter R. M., Moldawer L. L., Efron P. A. (2011) Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J. Immunol. 187, 911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oehlers S. H., Flores M. V., Hall C. J., O'Toole R., Swift S., Crosier K. E., Crosier P. S. (2010) Expression of zebrafish Cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev. Comp. Immunol. 34, 352–359 [DOI] [PubMed] [Google Scholar]
- 18. Van der Aa L. M., Chadzinska M., Tijhaar E., Boudinot P., Verburg-van Kemenade B. M. (2010) CXCL8 chemokines in teleost fish: two lineages with distinct expression profiles during early phases of inflammation. PLoS One 5, e12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoo S. K., Huttenlocher A. (2011) Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawson N. D., Weinstein B. M. (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- 21. Yoo S. K., Deng Q., Cavnar P. J., Wu Y. I., Hahn K. M., Huttenlocher A. (2010) Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 18, 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng Q., Harvie E. A., Huttenlocher A. (2012) Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell Microbiol. 14, 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levraud J. P., Colucci-Guyon E., Redd M. J., Lutfalla G., Herbomel P. (2008) In vivo analysis of zebrafish innate immunity. Methods Mol. Biol. 415, 337–363 [DOI] [PubMed] [Google Scholar]
- 24. Sarris M., Masson J. B., Maurin D., Van der Aa L. M., Boudinot P., Lortat-Jacob H., Herbomel P. (2012) Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan-bound gradients. Curr. Biol. 22, 2375–2382 [DOI] [PubMed] [Google Scholar]
- 25. Yoo S. K., Starnes T. W., Deng Q., Huttenlocher A. (2011) Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Guyader D., Redd M. J., Colucci-Guyon E., Murayama E., Kissa K., Briolat V., Mordelet E., Zapata A., Shinomiya H., Herbomel P. (2008) Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111, 132–141 [DOI] [PubMed] [Google Scholar]
- 27. Green J., Taylor J. J., Hindes A., Johnson S. L., Goldsmith M. I. (2009) A gain of function mutation causing skeletal overgrowth in the rapunzel mutant. Dev. Biol. 334, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walters K. B., Green J. M., Surfus J. C., Yoo S. K., Huttenlocher A. (2010) Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood 116, 2803–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thisse C., Thisse B. (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- 30. Meijering E., Dzyubachyk O., Smal I. (2012) Methods for cell and particle tracking. Methods Enzymol. 504, 183–200 [DOI] [PubMed] [Google Scholar]
- 31. Murayama E., Kissa K., Zapata A., Mordelet E., Briolat V., Lin H. F., Handin R. I., Herbomel P. (2006) Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975 [DOI] [PubMed] [Google Scholar]
- 32. White J. R., Lee J. M., Young P. R., Hertzberg R. P., Jurewicz A. J., Chaikin M. A., Widdowson K., Foley J. J., Martin L. D., Griswold D. E., Sarau H. M. (1998) Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 273, 10095–10098 [DOI] [PubMed] [Google Scholar]
- 33. Stoll S. J., Bartsch S., Augustin H. G., Kroll J. (2011) The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circ. Res. 108, 1367–1377 [DOI] [PubMed] [Google Scholar]
- 34. Hechtman D. H., Cybulsky M. I., Fuchs H. J., Baker J. B., Gimbrone M. A., Jr. (1991) Intravascular IL-8 inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J. Immunol. 147, 883–892 [PubMed] [Google Scholar]
- 35. Middleton J., Patterson A. M., Gardner L., Schmutz C., Ashton B. A. (2002) Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood 100, 3853–3860 [DOI] [PubMed] [Google Scholar]
- 36. Summers C., Rankin S. M., Condliffe A. M., Singh N., Peters A. M., Chilvers E. R. (2010) Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohler A., De Filippo K., Hasenberg M., van den Brandt C., Nye E., Hosking M. P., Lane T. E., Mann L., Ransohoff R. M., Hauser A. E., Winter O., Schraven B., Geiger H., Hogg N., Gunzer M. (2011) G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117, 4349–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heidemann J., et al. (2003) Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J. Biol. Chem. 278, 8508–8515 [DOI] [PubMed] [Google Scholar]
- 39. Basu S., Hodgson G., Zhang H. H., Katz M., Quilici C., Dunn A. R. (2000) “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood 95, 3725–3733 [PubMed] [Google Scholar]
- 40. Zhan Y., Lieschke G. J., Grail D., Dunn A. R., Cheers C. (1998) Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood 91, 863–869 [PubMed] [Google Scholar]
- 41. Lieschke G. J., Grail D., Hodgson G., Metcalf D., Stanley E., Cheers C., Fowler K. J., Basu S., Zhan Y. F., Dunn A. R. (1994) Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84, 1737–1746 [PubMed] [Google Scholar]
- 42. Gregory A. D., Hogue L. A., Ferkol T. W., Link D. C. (2007) Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood 109, 3235–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.