“The only true wisdom is in knowing you know nothing.”
Socrates
In this issue of Mayo Clinic Proceedings, 2 complementary papers review recent advances in the contemporary therapy of melanoma1 and provide a speculative synthesis2 on the similarities between the mechanism protecting the fetus from an immune response by the mother and the evasion of host responses by metastatic melanoma. The first of these articles, by Shah and Dronca,1 predicts a bright future for academicians and clinicians who will be responsible for evaluating and clinically applying multiple new strategies proposed to treat this devastating disease. That treatment will likely involve a flurry of new molecules and treatment modalities that are currently being developed.1 These novel patient- and tumor-specific therapies are expected to target the hallmark features of cancer that promote cancer formation and progression (Table).3 The second article, by Enninga et al,2 discusses multiple mechanisms used by the placenta to protect itself and the fetus from the mother’s immune attack. Mechanisms similar to these can potentially be used by metastatic melanoma to evade the host response and immunotherapy.2 The multiplicity of potential mechanisms listed2 will secure the academic future for years to come as we attempt to resolve new methods for targeting immune modulation as a treatment for patients with melanoma.
TABLE.
| Hallmark of melanoma | Target—agent (examples) |
|---|---|
| Sustaining proliferative signaling | BRAF—vemurafenib, dabrafenib |
| MEK—trametinib | |
| C-KIT—imatanib | |
| Receptor kinases—nilotinib, lapatinib NMPRTase—APO866 |
|
| Evading growth suppressors | Not defined yet |
| Activating invasion and metastasis | Not defined yet |
| Enabling replicative immortality | hTERT—BIBR1532 or nucleoside analogues |
| Inducing angiogenesis | VEGF—bevacizumab, ranibizumab |
| Resisting cell death | Not defined yet |
| Escape from immune surveillance/immune destruction | CTLA-4—ipilimumab PD-1—MK3475 or nivolumab |
| Other mechanisms of immunosuppression, including systemic immunosuppression | Not defined yet |
| Altered melanogenic activityc | Tyrosinase inhibitors, melatonin |
| Tumor promoting inflammation | Not defined yet |
| Genome instability and mutation | Not defined yet |
| Regulating cellular energetics | Mitochondria respiration—oligomycin Proteosomes—bortezomib |
BRAF = proto-oncogene B-Raf; CTLA-4 = cytotoxic T-lymphocyte antigen 4; hTERT = human telomerase reverse transcriptase; MEK = mitogen-activated protein kinase; NMPRTase = nicotinamide phosphoribosyltransferase; PD-1/PD-L1 = programed cell death protein/PD-ligand; VEGF = vascular endothelial growth factor.
Based on data from Cell3 and Nat Rev Clin Oncol6 and on http://clinicaltrials.gov.
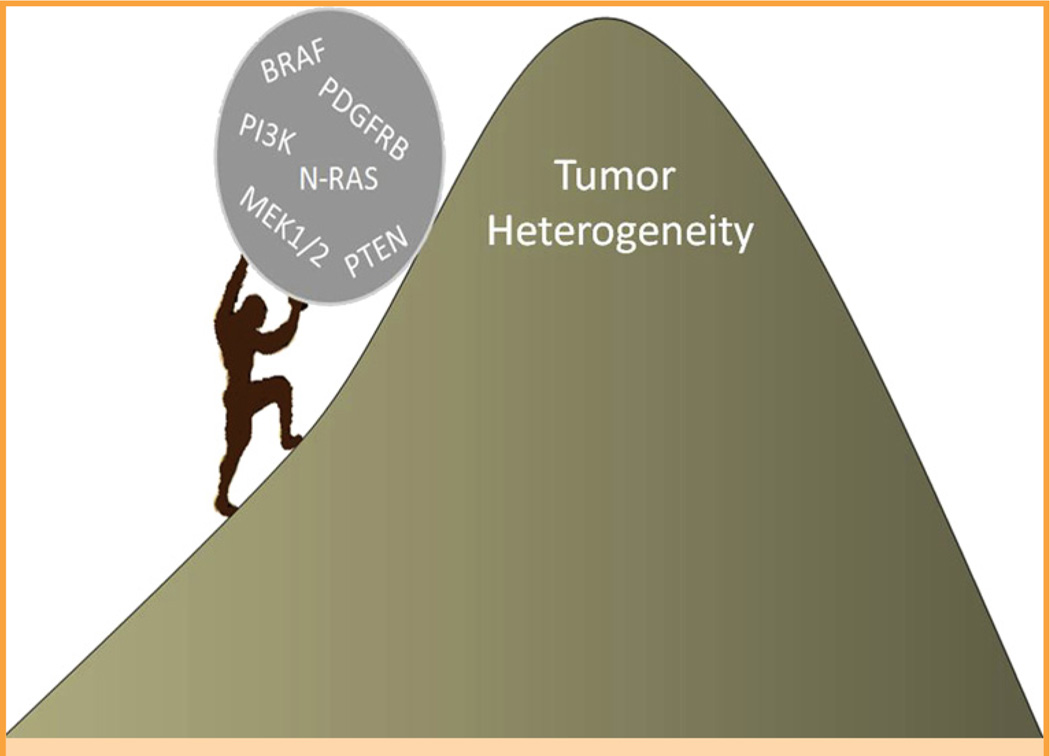
Unfortunately, the immediate future is not so bright for these patients because currently available or developing treatment strategies are able to prolong life for only a few months before the disease relapses, leading to death. Any modest improvements in overall survival typically occur during periods in which the therapies, which are very expensive, produce toxic adverse effects.1,4–6 Tumor progression often occurs because of the evolution of melanoma biology, which, in turn, produces multiple resistant mechanisms that hinder the efficacy of both new and older therapies. The resistance mechanisms include, but are not limited to, preexisting or developing intratumor heterogeneity, alterations in tumor microenvironment, and the ability of tumor to generate an immunosuppressive environment. At a genetic level, the tumor may develop intrinsic resistance to proto-oncogene B-Raf (BRAF) gene inhibitors, develop additional BRAF or neuro-blastoma RAS viral oncogene homolog mutations, and misregulate downstream mitogen-activated protein kinase kinases, beta-type platelet-derived growth factor–phosphatidylinositide 3-kinase/AKT or epidermal growth factor receptor–SRC family kinase–signal transducer and activator of transcription 3 pathways, or other growth factor (Figure), cytokine, and metabolic pathways.1,2,6–8 The issues raised in both papers1,2 are extremely important and deserve further consideration, as discussed herein.
FIGURE 1.
Current approaches to treating metastatic melanoma resemble “Sisyphus work” from Greek mythology. Similar to Sisyphus, it will be problematic to reach the pinnacle where targeted cure is achieved because clonal heterogeneity will overcome the drug effects in most patients with metastatic melanoma. BRAF = proto-oncogene B-Raf; MEK1/2 = mitogen-activated protein kinase kinase 1 or 2; N-RAS = neuroblastoma RAS viral oncogene homolog; PDGFRB = beta-type platelet-derived growth factor; PI3K = phosphatidylinositide 3-kinase; PTEN = phosphatase and tensin homolog.
Immune-Based Therapy Has Its Limitations Owing to the Tumor’s Ability to Generate an Immunosuppressive Environment
Complementary to the information reported by Enninga et al,2 one should realize that melanoma cells in or without cooperation from the stroma can produce a large spectrum of immunosuppressant agents, such as immunosuppressive growth factors, cytokines, proopiomelanocortin (POMC) peptides (including adrenocorticotropin hormone [ACTH], α-melanocyte-stimulating hormone, (α-MSH) and β-endorphin9), glucocorticoids,10 and lymphotoxic intermediates of melanogenesis (defined later herein).11,12
The POMC System
High expression of POMC in melanomas positively correlates with tumor progression.13,14 Noteworthy, POMC-derived melanocortins are recognized for their immunosuppressive properties, but they also serve as anti-apoptotic, mitogenic, and melanogenic factors in human melanocytes (acting through activation of the MC1 receptor).12,15 Although the cell’s synthesis of melanin is considered a marker of differentiation, intermediates of melanogenesis are highly immunosuppressive or selectively toxic toward immune cells.11,16 Therefore, pathologic expression of POMC in melanoma can contribute to tumor progression, identifying POMC as a facultative and context-dependent tumor facilitator.
Steroidogenesis and the Hypothalamic-Pituitary-Adrenal Axis
Melanoma cells also have the capability to produce corticosterone and cortisol, a property shared by normal melanocytes and other skin cells (as previously reviewed by Slominski et al17). Interestingly, this production can be stimulated by locally expressed corticotropin-releasing factor (CRF) and POMC-derived ACTH (as previously reviewed by Slominski et al18). This hypothalamic-pituitary-adrenal (HPA)–like axis operates in the skin to attenuate damage imposed by environmental factors and to prevent autoimmune attack against the skin due to damage-induced exposure of cutaneous antigens.17,18 The misplaced or deregulated HPA-like organization or its elements can also operate under pathologic conditions, such as melanoma, to generate an immunosuppressive and protumor environment that, in turn, facilitates melanoma growth and progression.18
Although the previously mentioned neuroendocrine regulatory pathways are operating on the local, skin, and melanoma levels,17,18 there also is a strong possibility for concomitant systemic immunosuppressive activity. Specifically, melanoma-derived CRF or CRF-like peptides, interleukin-1, IL-6, tumor necrosis factor α, and ACTH could either activate the pituitary-adrenal axis or directly affect the adrenal cortex, leading to systemic immunosuppression.9,18 Thus, the factors listed previously herein would affect not only tumor environment but also systemic homeostasis through activation of the central HPA axis, setting the body into a permanent stress response mode.
The previously mentioned possibilities have typically not been considered by mainstream melanoma researchers and clinicians. However, these operative mechanisms could confound and complicate translational research and therapeutic approaches. To wit, these local and global endocrine-immune mechanisms should be accounted for when designing a comprehensive melanoma treatment program.
Neuroendocrine Mediators and Stress Hormones
Cutaneous melanomas are products of malignant transformation of melanocytes, which are of neural crest origin; therefore, it is not surprising that they have the capability to produce neurotransmitters and hormones and express the corresponding receptors.12 This opens a Pandora’s box of possibilities for how melanomas can autoregulate their phenotype and how they can interact with a variety of host factors on the local and systemic levels.12 Noteworthy, melanoma cells can produce and metabolize catecholamines, serotonin, melatonin, and hormones of the hypothalamic-pituitary-thyroid axis, as well as a variety of other neurotransmitters.9,11,12,17 Thus, melanomas are empowered with a variety of stress mediators and hormones through which they can control local and systemic immune responses. The corresponding panel of receptors12 and metabolizing enzymes, cytochromes especially,19 will facilitate tumor plasticity and survival in response to changes in their environment.
Concluding Remarks on Immunomodulation
The previously mentioned possibilities for regulating local and systemic immune responses by tumor-derived hormones, neurotransmitters, or cytokines acting on central neuroendocrine axes, with a possibility of resetting the body homeostasis by melanoma at stage III (regional metastasis) or IV (distal metastasis) disease (eg, by activation of the HPA axis by tumor-derived factors or stimulation of adrenergic activity by levodopa released from melanotic melanoma), have not been considered previously. However, such complex interactions would likely complicate any translational research or therapeutic approaches and have to be taken into account when designing a comprehensive melanoma treatment program.
Melanin and Melanogenesis: A Double-Edged Sword
Melanin Pigment
The main differentiated function of melanocyte is to produce melanin through a tightly regulated multistep biochemical process called melanogenesis.11,12 This process is highly controlled and takes place within the boundaries of specialized membrane-bound organelles, the melanosomes. Melanin pigment protects normal melanocytes and the epidermis from a variety of insults by acting as a light filter, radioprotector, and scavenger of free radicals, metal cations, and many potentially toxic chemicals.12,17 These properties under physiologic conditions are beneficial to the skin. However, they make melanotic melanomas relatively resistant to chemotherapy, radiotherapy, and phototherapy because of the radioprotective and scavenging capabilities of melanin. (Note that melanomas can occur in both melanotic, ie, melanin-producing, and nonmelanotic forms.)
Melanogenesis
The active process of melanogenesis generates reactive oxygen species as well as quinone and semiquinone intermediates that display cytotoxic, genotoxic, or mutagenic activities and act as potent immunosuppressants. The immunosuppression is best illustrated ex vivo by shutting off T- and B-cell immune activities or the selective lymphotoxic effects of levodopa or products of its autoxidation (as reported or reviewed by Slominski et al11,12,16,20). In melanotic melanoma cells, the process of melanin synthesis is deregulated, affecting the behavior of not only melanoma cells but also its surrounding environment.14 The net effect is cytotoxicity to surrounding tissues (but not to melanoma cells), mutagenesis in melanoma cells, and almost complete local immunosuppression (as previously reviewed11,12,20). This inhibits the host responses and promotes tumor progression. In support of the latter, recent studies have shown that melanogenesis shortens overall and disease-free survival in patients with metastatic melanoma.21 The immunosuppressive field generated by intermediates of melanogenesis will attenuate any type of immunotherapy against melanoma. Note that intermediates of melanogenesis can enter the circulation and have systemic effects, depending on the activity of the pathway and the tumoral volume (as previously reviewed11,12,20). A clinical example of the latter is the general melanosis that can develop in some patients with metastatic melanotic melanoma.
Clinical Implications
Therefore, uncontrolled melanogenesis should have a role, and perhaps a critical role, in tumor progression and, together with melanin pigment, will attenuate radiotherapy, chemotherapy, and phototherapy as well as immunotherapy. An important clinical challenge is how to overcome these negative effects because melanogenesis-related proteins are highly immunogenic and are currently being considered as targets for immunotherapy.
Solutions to the Problem
The scale of the problems listed previously herein and as reviewed by Shah and Dronca1 and Enninga et al2 requires comprehensive and educated solutions. These solutions, to be broadly applied, must also remediate the skyrocketing costs of new therapies already being implemented or proposed.1,6,7
Academic Solutions
Such an approach requires serious investments in melanoma research extending far beyond the mainstream approaches outlined in previous reports.1,6,7 Specifically, the new challenges outlined by Enninga et al,2 and further extended in this editorial, have to be properly addressed. The major challenge to be solved is how to attenuate melanoma’s ability to rewire local and systemic homeostatic responses through action on established neuroendocrine axes, in turn securing tumor survival and growth to the detriment of the host during disease stages III and IV.
Patient Expectations
Patients with melanoma who have stage III or IV disease, armed with knowledge of recent advances in melanoma therapy, expect effective and relatively nontoxic treatment that can adequately extend overall survival without serious adverse effects. Unfortunately, such expectations are not being met by current therapies; eg, presently, single or combined therapies can trigger severe toxic effects.22 Furthermore, only a limited number of contemporary targeted agents improve overall survival,2 whereas most have an effect on the response rate or time to tumor progression, with the effectiveness duration limited because of acquired resistance.23 The endocrine, immune, and melanogenesis mechanisms discussed previously herein, plus tumor cell heterogeneity, cancer stem cell models, and genetic instability, likely account for melanoma’s treatment resistance and recurrence. Oncologic therapy is moving irreversibly toward personalized therapy that benefits selected patients in whom the tumor exhibits treatment-actionable markers. These individual molecular markers (eg, gene signatures) will permit better risk definition and treatment prediction, which will help avoid so-called wasted toxicity and allow patients to live longer and healthier lives owing to personalized and targeted treatment of their cancers. Therefore, economical and educated solutions are needed urgently that counteract other pathways of tumor progression, such as escape from immune surveillance, hormonal regulation, genomic instability, and autocrine and paracrine factors that enable evasion of growth suppressors. Some suggestions follow.
Inhibition of Melanogenesis
There are several inhibitors of melanogenesis that are approved by the US Food and Drug Administration and available for immediate use as adjuvants.12,16 As discussed previously herein, an inhibition of melanogenesis should improve efficacy of immunotherapy, radiotherapy, or, perhaps, chemotherapy, defining new and realistic approaches to treat this devastating disease.
Use of Melatonin and Vitamin D as Adjuvants
Because melatonin and vitamin D have anticancer and protective properties (as reviewed previously17,24,25), they can be used as adjuvants during any type of therapy for stage III or IV melanoma. Moreover, because melatonin is nontoxic and the toxicity of vitamin D lies in its calcemic effect that can be easily monitored, both compounds can be used in stage I and II disease as prophylactic agents against recurrent or progressing disease.
Conclusion
Comprehensive research on neuroimmunoendocrine communication between melanoma and the host is required to develop successful melanoma therapy. An economical solution for now is inclusion of melatonin and vitamin D in any treatment protocols and inhibition of melanogenesis to sensitize the tumor against targeted therapies.
Acknowledgments
We thank Dianne Kovacic, MD, fellow in dermatopathology, University of Tennessee Health Sciences Center, Memphis, TN, for preparation of the Figure 1.
Grant Support: This study was supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants 2R06AR052190 and 1R01AR056666-01A2) and the West Clinic Cancer Foundation (A.S.).
Contributor Information
Andrzej T. Slominski, Department of Pathology and Laboratory Medicine, Department of Medicine, University of Tennessee Health Sciences Center, Memphis, TN.
J. Andrew Carlson, Department of Pathology and Laboratory Medicine, Albany Medical College, Albany, NY.
REFERENCES
- 1.Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc. 2014 doi: 10.1016/j.mayocp.2014.02.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enninga EAL, Holtan SJ, Creedon DJ, et al. Immunomodulatory effects of sex hormones: requirements for pregnancy and relevance in melanoma. Mayo Clin Proc. 2014 doi: 10.1016/j.mayocp.2014.01.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Dummer R, Gutzmer R, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14(8):733–740. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 6.Schadendorf D, Hauschild A. Melanoma in 2013: melanoma: the run of success continues. Nat Rev Clin Oncol. 2014;11(2):75–76. doi: 10.1038/nrclinonc.2013.246. [DOI] [PubMed] [Google Scholar]
- 7.Kwong LN, Davies MA. Targeted therapy for melanoma: rational combinatorial approaches. Oncogene. 2014;33(1):1–9. doi: 10.1038/onc.2013.34. [DOI] [PubMed] [Google Scholar]
- 8.Straussman R, Morikawa T, Shee K, et al. Tumour microenvironment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 13.Funasaka Y, Sato H, Chakraborty AK, Ohashi A, Chrousos GP, Ichihashi M. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc. 1999;4(2):105–109. doi: 10.1038/sj.jidsp.5640192. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Wortsman J, Mazurkiewicz JE, et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122(6):658–666. [PubMed] [Google Scholar]
- 15.Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126(9):1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124(6):1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34(6):827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski AT, Zmijewski MA, Semak I, et al. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014;14(1):77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: selective review and hypothesis. Anticancer Res. 1998;18(58):3709–3715. [PubMed] [Google Scholar]
- 21.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44(10):2071–2074. doi: 10.1016/j.humpath.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson DB, Wallender EK, Cohen DN, et al. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1:373. doi: 10.1158/2326-6066.CIR-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasparini G, Longo R. The paradigm of personalized therapy in oncology. Expert Opin Ther Targets. 2012;16(suppl 1):S7–S16. doi: 10.1517/14728222.2011.637921. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]