Abstract
Rationale and Objectives
The aim of this study was to develop and compare two methods for quantification of metabolite concentrations in human skeletal muscle using phased-array receiver coils at 3 Tesla.
Materials and Methods
Water suppressed and un-suppressed spectra were recorded from the quadriceps muscle (vastus medialis) in 8 healthy adult volunteers, and from a calibration phantom containing 69 mM/L N-acetyl aspartate. Using the phantom replacement technique, trimethylamine specifically [TMA] and creatine [Cr] concentrations were estimated, and compared to those values obtained by using the water reference method.
Results
Quadriceps [TMA] concentrations were 9.5 ± 2.4 and 9.6 ± 4.1 mmol/kg wet weight using the phantom replacement and water referencing methods respectively, while [Cr] concentrations were 26.8 ± 12.2 and 24.1 ± 5.3 mmol/kg wet weight respectively.
Conclusions
Reasonable agreement between water referencing and phantom replacement methods was found, although for [Cr] variation was significantly higher for the phantom replacement technique. The relative advantages and disadvantages of each approach are discussed.
Keywords: MR Spectroscopy, Muscle, Quantitation, Phantom Replacement
INTRODUCTION
There is increasing interest in the use of proton magnetic resonance spectroscopy (MRS) in the musculoskeletal (MSK) system both for research and clinical investigation [1, 2]. Traditionally, results from in vivo MRS have been expressed as ratios of metabolite levels, but this approach may be misleading if all metabolite levels in the spectrum are changed relative to normal tissue. Therefore, it is generally preferable to estimate individual metabolite concentrations using spectral quantitation techniques. For MRS in the brain, spectral quantitation techniques using a variety of principles are now well-established [3]. However, there have been few quantitative MRS studies in the MSK system [4–7], and the design of quantitation techniques for MSK MRS presents additional challenges, in that the presence of lipid compartments within the region-of-interest (ROI) needs to be carefully considered. In addition, phased-array receiver coils are increasingly being used for MSK MRS, and quantitation methods designed for use with single-channel transmit-receive coils (e.g. [8]) require further modifications for use with phased-array coils [9].
The most commonly used approach to quantifying metabolite signals uses a reference MRS signal as a standard [3], although other approaches have been explored, such as the ‘ERETIC’ method which uses an electronically-generated reference signal [10, 11]. The reference signal may be ‘internal’, i.e. from the same region of interest as the metabolites to be determined [12, 13], or it may be external to the region of interest, most commonly a standard sample placed adjacent to the subject [14]. A third option is the ‘phantom replacement’ method, for which the reference sample is scanned separately from the in vivo study [8, 15]. Each method has its own advantages and disadvantages. The internal reference method assumes that a signal is present in the spectrum (from the same ROI as the compounds to be quantified) that originates from a molecule of stable, known concentration. While the internal referencing method is simple in its implementation, relatively insensitive to inhomogeneities of the B0 and B1 fields, and requires no or little additional scan time, its most obvious limitation is that the concentration of the reference compound may not be accurately known. For example, in vivo water is often used as the concentration reference, and this may not be constant between subjects or regions within the MSK system. The external reference method requires the collection of a spectrum from an external calibration sample placed next to the subject during the same scanning session; while the concentration of the reference compound is precisely known with the external referencing method, the disadvantages of this method include its sensitivity to inhomogeneities of the B0 and B1 fields, the additional scanner time required while the patient is in the magnet, and the possible deleterious effects on the in vivo B0 field homogeneity due to the magnetic susceptibility effects of the external sample.
The phantom replacement method combines some of the advantages of internal and external referencing, by utilizing a phantom reference of known concentration; however, the phantom is scanned in a separate session. The advantages of this method include the lack of need for additional patient scan time, the known reference concentration, and the absence of potentially deleterious magnetic susceptibility effects [8]. Nevertheless, despite its advantages, the method remains sensitive to B1 inhomogeneity and variable radiofrequency coil loading [9]. For transmit-receive coils, the coil loading can be estimated, and corrected for, using the reciprocity theorem [8], and this may be extended for use with receive-only phased array coils by comparing the relative sensitivities of each element of the receive array to that of the transmit coil [9, 16, 17].
In this manuscript, two approaches for quantitative MRS in the MSK system are compared, namely the internal reference method using the tissue water signal, and the phantom replacement method. Spectra from the quadriceps muscle (vastus medialis) of eight healthy volunteer subjects were quantified using both approaches and compared. The ERETIC method was not used in the current study, because it involves special hardware not available on standard clinical MRI scanners, and also may be sensitive to variable receiver coil loading, which is also difficult to measure in clinical phased-array coils.
METHODS
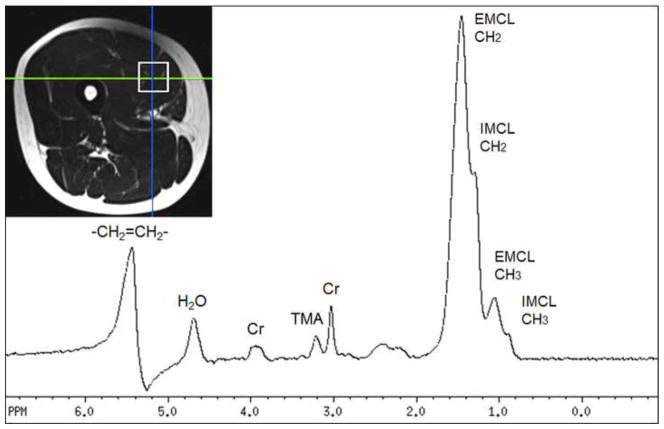
Eight healthy normal subjects were recruited for MRS (4 female, age 32 ± 4 years, mean ± standard deviation, range 25–37 years). Written informed consent was obtained from all subjects after institutional review board approval had been obtained. MR images and spectra were acquired on a 3T scanner (Magnetom Trio, Siemens Medical Solutions, Inc., Malvern, PA) using a four-element ‘body matrix’ receiver coil and a circularly polarized (CP) body transmit coil. Axial T2 weighted anatomic images (TR/TE 2886 ms/100 ms, FOV 20cm, slice thickness 6mm, acquisition time 4 minutes) were collected to provide a guide for spectroscopy voxel localization within the right quadriceps muscle. Specifically, the voxel was carefully positioned in the vastus medialis muscle with attention to avoid blood vessels, subcutaneous and other fat, and the femur bone (Figure 1). For each voxel, a single voxel Point-REsolved Spectroscopy Sequence (PRESS), TR 2 s; TE 135 ms, voxel size 2×2×4 cm (16 cm3), 128 averages, acquisition time 4 min 20s) spectrum was acquired with a 4-pulse CHESS water-suppression scheme[18], followed by two acquisitions without water suppression (16 averages, scan time 40 sec), one collected with ‘body matrix’ receive and the other with the CP-transmit coil used as receive. Prior to data collection, field homogeneity was optimized using linear, manual shimming. For each experiment, the transmitter voltage (V) required for a 90° pulse was recorded.
Figure 1.
T2-weighted MRI showing voxel location used for MRS in one subject, and the corresponding water suppressed spectrum from that region. Signals assigned to unsaturated fats (-CH2=CH2-), water (H2O), trimethylamines (TMA), creatine (Cr - both CH3 and CH2 groups at 3.0 and 3.9 ppm, respectively), extra- and intra-myocellular lipids respectively (EMCL and IMCL).
In vivo water and metabolite T1 and T2 relaxation times were also determined in six human subjects, as well as in the phantom used for quantitation (see below). T1 and T2 values were estimated by fitting signal intensities recorded as a function of TR and TE using standard equations . TR was varied from 530ms to 20s, and TE from 30ms to 500ms.
Spectral peak areas were determined using AMARES[19] method in the jMRUI [20] software package. Spectra were manually zero and first order phase corrected, eddy-current corrected[21] and 5 Hz exponential line broadening applied. The spectra were fitted to Lorentzian line shape by AMARES. Full details of the AMARES method can be found in reference [19].
Phantom Replacement Method
In addition to the scans described above, an additional measurement was performed in a reference phantom consisted of a 3.8 liter cylindrical bottle containing a solution of 69 mM/L N-Acetyl Aspartate (NAA). The same voxel size was used both in vivo and in the reference phantom. Molar metabolite concentrations [M], where M is either creatine (Cr) or trimethylamines (TMA, primarily carnitine (Ctn) [22]), were calculated from
| (1) |
where the subscripts refer to the scans performed in vivo (i) or in the reference phantom (r).[P] is the molar concentration of the reference phantom (69 mM/L NAA), S is the signal intensity (e.g. spectral peak area as determined by AMARES), n is the number of protons contributing to the peak, k is a term to account for T1 and T2 relaxation effects, TA is a measure of the phased-array coil loading, and CFvol is a correction term to account for the lipid composition of the voxel. Since metabolites are believed to only be present in the aqueous fraction of the voxel volume, CFvol was calculated from the water/lipid ratio observed in the unsuppressed spectrum, corrected for relaxation time effects, and the proton molar concentration of water and lipid respectively [7]. Molar concentrations were converted to mmol/kg wet weight by dividing by muscle tissue density, which was assumed to be 1.05 gm/mL [23]. As mentioned above, while coil-loading may be directly estimated from the transmitter amplitude required for a 90° pulse in the case of transmit-receive coils, this cannot be done for receive-only phased-arrays. Instead, a ‘virtual’ transmitter amplitude (TA) for the phased-array is estimated from a knowledge of the CP-transmit coil voltage (V) required for a 90° pulses and a measure of the relative signal intensities (e.g. the tissue or phantom water signal) of the CP-coil and the matrix phased array [17]. The relative sensitivity may be measured directly from two scans, one using the phased-array coil (SPA), and the other using the CP-coil (SCP), for receive.
| (2) |
| (3) |
where ViCP and VrCP are the circularly-polarized transmitter voltages for the in vivo and phantom reference scans respectively. In order to minimize the effects of possible inhomogeneity of the transmit B1 field produced by the CP-coil, the voxel is in the reference phantom was placed in a similar position in the magnet to the position of the voxel in the in vivo spectra.
The relaxation time correction factors are calculated from ki = exp(−TE/T2i) × (1−exp(−TR/T1i) and kr = exp(−TE/T2r) × (1−exp(−TR/T1r), respectively, where T1i and T2i are the longitudinal and transverse relaxation times of the metabolites in vivo, and T1r and T2r the corresponding relaxation times of the reference phantom NAA signal.
The CFvol is defined in equation (4):
| (4) |
In the above equation, with subscripts of water and lipid, Vwater and Vlipid are the volume of muscle and lipid in the voxel respectively, Iwater and Ilipid are the intensities of unsuppressed water and lipid signals, Ki_water and Ki_lipid are the in vivo relaxation decay factors for water and lipid. Nwater and Nlipid represent the molar concentrations of the water and lipid respectively.
Internal Reference Method
In vivo metabolite concentrations [M] were also calculated using the muscle water signal as an internal intensity reference, using the methodology described previously (7), according to the equation (5)
| (5) |
where all measurements are performed in vivo using the phased-array receive coil. SPA(M) or the metabolites signal intensity, was determined from the water-suppressed scan, while SPA(H2O) or the water signal intensity, was determined from the scan without water suppression. [H2O] was 55556 mM/L, and the water content factor WC was assumed to be 77% [23]. nH2O, nM are the number of protons in water and metabolites. kH2O and kM account for T1 and T2 relaxation effects of water and metabolites respectively.
In addition to the in vivo experiments, both quantitation techniques were validated with measurements on two phantom solutions of known concentration (aqueous phosphocholine, 5mM/L and 10mM/L).
RESULTS
An example spectrum and ROI from one volunteer are shown in Figure 1. 3T muscle T1 and T2 relaxation times were estimated to be: T1 (sec) TMA 1.37 ± 0.07, Cr 1.73 ± 0.32, H2O 1.64 ± 0.04: T2 (msec) TMA 134 ± 16, Cr 177 ± 11, H2O 35 ± 2.6. For the NAA standard solution T1 was estimated to be 1.68 sec and T2 1.10 sec.
For the two phosphocholine solutions (5 and 10 mM/L), the phantom replacement method gave concentration values of 5.0±0.3 and 9.3±0.4 mM/L respectively, while the water reference method gave concentration values of 4.9±0.3 and 11.1±0.9 mM/L. Quadriceps [TMA] concentrations were 9.5 ± 2.4 and 9.6 ± 4.1 mmol/kg wet weight using the phantom replacement and water referencing methods respectively, while [Cr] concentrations were 26.8 ± 12.2 and 24.1 ± 5.3 mmol/kg wet weight respectively. The 1/CFvol correction factor was 0.97 ± 0.01, indicating that only 3% of the voxel volume was occupied by lipids on average.
Cr and TMA concentration values were also examined for correlations with each other using linear regression analysis; for the phantom replacement method, the Pearson coefficient (R2) was 0.519, while for the water referencing method it was 0.452.
DISCUSSION
This study illustrates that quantitative proton MRS of the musculoskeletal system is feasible, and that two different metabolite quantitation techniques (water referencing and the phantom replacement method) produce similar results. There have been relatively few prior quantitative studies of proton MRS of muscle [24]. The Cr concentrations determined here (24–28 mmol/kg wet weight) are in a similar range to results determined by MRS (e.g. 36.2 ± 5 mmol/kg wet weight [4]) or by traditional biochemical analysis (range 21–35 mmol/kg wet weight [4]). Muscle Ctn concentration values (the primary constituent of the TMA peak) have previously been estimated to range from 4 to 12 mmol/kg wet weight [25, 26], covering the range of the TMA values found in this study (~9.5 ± 4 mmol/kg wet weight) and also similar to prior determinations by MRS [7, 27].
There was a larger variability for the Cr concentration quantified by the phantom replacement method than that quantified by the internal water reference method. Therefore, in this study in normal volunteers, or for studies of exercise physiology, the internal reference method may be preferable for [Cr] determination. However, an important limitation of the internal reference method is that in diseased conditions, the in vivo water content (as well as water T1 and T2 relaxation times) may vary from that found in normal tissue. Water content and relaxation times may also change over time with disease progression, or with age. Water content may also change on a day to day basis in response to variable exercise. While the effects of changes in water relaxation times can be minimized by the use of long TR and short TE, there is no simple way to account for changes in water concentration. For this reason, the phantom replacement method may be considered as an alternative method for the study of metabolite concentrations in musculoskeletal diseases where water content is likely to be abnormal (e.g. tumors, chronic myopathies).
The reason for the increased variability in Cr concentration by the phantom replacement method is unclear, and at present remains unexplained. One source of quantitation error with the phantom replacement technique (which would apply to both Cr and TMA) is that the B1 transmit field produced by the CP coil is not completely homogeneous, and therefore B1 may vary from one subject location to another. Variations in the transmit B1 field will effect both the signal excited, and also the virtual transmitter amplitude measurement (equation 2). The internal reference method is largely insensitive to mis-calibration of transmitter B1, so long as both the water-suppressed and un-suppressed reference signals are affected equally. A future refinement of the phantom replacement method (not done in the current study) would need to include transmit B1 mapping methods to account for transmit B1 inhomogeneity, which may help reduce variability.
Standard deviations of [Cr] were also quite large, even when using the internal reference method, which should be insensitive to variations in the transmit B1 field (22%). One possible explanation for this might be variable muscle fiber orientation with respect to the main magnetic field from subject to subject. It has been previously shown that residual dipolar couplings may exist for Cr in muscle fibers, giving complicated multiple patterns in the spectrum which are orientation dependent[28]. Since the Cr peak was fit as a singlet at 3.0 ppm in the current study, the presence of any residual dipolar couplings would result in the underestimation of the true Cr concentration. However, inspection of all spectra in this study failed to reveal any triplet structure for the Cr peak, perhaps indicating that the muscle fiber bundle orientation in this study was (by chance) relatively close to the ‘magic angle’, where the couplings are zero.
The positive linear correlations between TMA and Cr, observed with both quantitation methodologies, suggest that the concentrations of the two compounds are linked in normal subjects, and may reflect quantities such as muscle cellular density. However, another possibility that cannot be entirely ruled out, is that some unknown systematic quantitation error may be affecting both peaks equally.
An additional complication in MSK spectroscopy (compared to that performed in the central nervous system) is that the localized region of interest contains appreciable amounts of both water and lipids. Since we do not expect any metabolites to be present in the lipid compartments of the voxel, when using the phantom replacement technique (with a phantom containing no lipids), the effective voxel volume (i.e the water-containing volumes) will not be identical between the phantom and the in vivo voxel. For this reason, it is important to apply a correction factor, based on measurements of the water/lipid ratio from the spectra recorded without water suppression [7]. Although the lipid correction factor (CFvol) was close to 1 in the current study in muscle of healthy subjects (and with the voxel carefully placed to avoid as much as possible any visible fat signal on MRI), this factor may be more significant in studying disease conditions with elevated lipid signals, or when the voxel location contain appreciable amounts of extramyocellular lipids.
In summary, two methods are presented for quantitative analysis of proton MRS of the musculoskeletal system. Both methods give similar results and are in general concordance with prior literature concentration values in normal subjects. The phantom replacement method, adapted for use with phased-array receive coils, may be preferable to the water reference method in disease states, since it requires no assumption for constant water content. Patient scan time between the two methods is almost the same (the phantom replacement necessitates one extra water measurement with the CP coil as receive although this is of almost negligible duration (just a few seconds)). Depending on the stability of the MR system, relative occasional measurements of the reference phantom are needed (e.g. once every one to two weeks). However, care has to be taken to ensure that appropriate lipid content correction factors are used, and that the transmit B1 levels are as homogeneous as possible (or determined using B1 mapping). The methods described here are feasible for routine MRS of the MSK system, and should be readily applicable to MRS of other organ systems throughout the body, as well as for use with MR spectroscopic imaging [9].
Highlights.
Two MRS quantitation methods were compared in healthy human muscle at 3T in vivo
They are based on phantom replacement and internal water reference respectively
Both methods measured comparable concentrations for muscle creatine and TMA
In healthy muscle the water reference method showed slightly lower variability
In pathological conditions phantom replacement method may be more appropriate
Acknowledgments
Grant Support: Supported in part by NIH P41RR015241, R01CA125258, and Siemens Medical Solutions, General Electric Radiology Research Fellowship (GERRAF), The William M.G. Gatewood M.D. Fellowship, The SCBT/MR Young Investigator Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boesch C. Musculoskeletal spectroscopy. J Magn Reson Imaging. 2007;25(2):321–38. doi: 10.1002/jmri.20806. [DOI] [PubMed] [Google Scholar]
- 2.Fayad LM, et al. Musculoskeletal tumors: how to use anatomic, functional, and metabolic MR techniques. Radiology. 2012;265(2):340–56. doi: 10.1148/radiol.12111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen JF, et al. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240(2):318–32. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- 4.Bottomley PA, Lee Y, Weiss RG. Total creatine in muscle: imaging and quantification with proton MR spectroscopy. Radiology. 1997;204(2):403–10. doi: 10.1148/radiology.204.2.9240527. [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir MS, et al. Absolute quantification of carnosine in human calf muscle by proton magnetic resonance spectroscopy. Phys Med Biol. 2007;52(23):6781–94. doi: 10.1088/0031-9155/52/23/001. [DOI] [PubMed] [Google Scholar]
- 6.Baguet A, et al. Important role of muscle carnosine in rowing performance. J Appl Physiol. 2010;109(4):1096–101. doi: 10.1152/japplphysiol.00141.2010. [DOI] [PubMed] [Google Scholar]
- 7.Fayad LM, et al. A feasibility study of quantitative molecular characterization of musculoskeletal lesions by proton MR spectroscopy at 3 T. AJR Am J Roentgenol. 2010;195(1):W69–75. doi: 10.2214/AJR.09.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soher BJ, et al. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35(3):356–63. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- 9.Bonekamp D, et al. Quantitative SENSE-MRSI of the human brain. Magn Reson Imaging. 2010;28(3):305–13. doi: 10.1016/j.mri.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzer-Schweizer S, et al. In-vivo assessment of tissue metabolite levels using 1H MRS and the Electric REference To access In vivo Concentrations (ERETIC) method. NMR Biomed. 2010;23(4):406–13. doi: 10.1002/nbm.1476. [DOI] [PubMed] [Google Scholar]
- 11.Akoka S, Barantin L, Trierweiler M. Concentration Measurement by Proton NMR Using the ERETIC Method. Anal Chem. 1999;71(13):2554–7. doi: 10.1021/ac981422i. [DOI] [PubMed] [Google Scholar]
- 12.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–37. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 13.Barker PB, et al. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6(1):89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- 14.Danielsen ER, Michaelis T, Ross BD. Three methods of calibration in quantitative proton MR spectroscopy. J Magn Reson B. 1995;106(3):287–91. doi: 10.1006/jmrb.1995.1046. [DOI] [PubMed] [Google Scholar]
- 15.Buchli R, Boesiger P. Comparison of methods for the determination of absolute metabolite concentrations in human muscles by 31P MRS. Magn Reson Med. 1993;30(5):552–8. doi: 10.1002/mrm.1910300505. [DOI] [PubMed] [Google Scholar]
- 16.Jost G, Harting I, Heiland S. Quantitative single-voxel spectroscopy: the reciprocity principle for receive-only head coils. J Magn Reson Imaging. 2005;21 (1):66–71. doi: 10.1002/jmri.20236. [DOI] [PubMed] [Google Scholar]
- 17.Natt O, et al. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med. 2005;53(1):3–8. doi: 10.1002/mrm.20337. [DOI] [PubMed] [Google Scholar]
- 18.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 19.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 20.Naressi A, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12(2–3):141–52. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 21.Ordidge RJ, Cresshull ID. The correction of transient B0 field shifts following the application of pulsed gradients by phase correction in the time domain Y1 - 1986 Y2 - 8. Journal of Magnetic Resonance. 1986;69(1) [Google Scholar]
- 22.Ren J, Sherry AD, Malloy CR. 1H MRS of intramyocellular lipids in soleus muscle at 7 T: spectral simplification by using long echo times without water suppression. Magn Reson Med. 2010;64(3):662–71. doi: 10.1002/mrm.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38(11):2317–20. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Subhawong TK, et al. 1H Magnetic Resonance Spectroscopy Findings in Idiopathic Inflammatory Myopathies at 3 T: Feasibility and First Results. Invest Radiol. 2013;48(7):509–16. doi: 10.1097/RLI.0b013e3182823562. [DOI] [PubMed] [Google Scholar]
- 25.Vukovich MD, Costill DL, Fink WJ. Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise. Med Sci Sports Exerc. 1994;26(9):1122–9. [PubMed] [Google Scholar]
- 26.Bohmer T, Bergrem H, Eiklid K. Carnitine deficiency induced during intermittent haemodialysis for renal failure. Lancet. 1978;1(8056):126–8. doi: 10.1016/s0140-6736(78)90422-1. [DOI] [PubMed] [Google Scholar]
- 27.Fayad LM, et al. Quantification of muscle choline concentrations by proton MR spectroscopy at 3 T: technical feasibility. AJR Am J Roentgenol. 2010;194(1):W73–9. doi: 10.2214/AJR.09.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermathen P, Boesch C, Kreis R. Mapping fiber orientation in human muscle by proton MR spectroscopic imaging. Magn Reson Med. 2003;49(3):424–32. doi: 10.1002/mrm.10396. [DOI] [PubMed] [Google Scholar]