Abstract
Active vitamin D metabolites 1,25-dihydroxyvitamin D2 [1,25-(OH)2-D2; derived from ergocalciferol] and D3 [1,25-(OH)2-D3; derived from cholecalciferol] are found in low levels in the circulation and require a very sensitive method for measurement. Radioimmunoassay (RIA) has been the method of choice, but it lacks the specificity needed to distinguish between 1,25-(OH)2-D2 and -D3whereas liquid chromatography-tandem mass spectrometry (LC/MS/MS) methods have the advantage of high specificity and sensitivity. Here, we compare a new derivative for ionizing 1,25-(OH)2-D to enhance the signal and provide the most sensitive assay for measuring vitamin D. We used the Amplifex diene method of derivatizing prior to LC/MS/MS and compared it to the standard RIA method and the 4-phenyl-1,2,4-triazole-3,5-dione (PTAD) method of derivatizing prior to LC/MS/MS. In the evaluation of 20 human serum samples, all methods correlated strongly across the upper levels of the standard 1,25-(OH)2-D2 and - D3 ranges (Amplifex and RIA, pc = 0.97; Amplifex and PTAD, pc = 0.96) but less strongly on the lower levels of the standard range (Amplifex and RIA, pc = 0.81; Amplifex and PTAD, pc = 0.65) suggesting differences in the sensitivities between the assays. The Amplifex method was determined to be more sensitive than the PTAD method, as peak areas were significantly higher for the Amplifex method and provided for a 10 fold higher signal-to-noise ratio than PTAD. Therefore, the Amplifex LC/MS/MS method is the most sensitive and specific method available for measuring 1,25-(OH)2-D2 and -D3 while using the smallest sample volume.
1. Introduction
Vitamin D was discovered in cod liver oil by E. McCollum and M. Davis at the University of Wisconsin in 1922 [1]. Subsequent research demonstrated that vitamin D2 and vitamin D3 have significant biological activity in their 1,25-dihydroxylated forms upon binding to the vitamin D receptor.
Vitamin D was initially recognized for its ability to prevent bone mineralization impairments, such as osteomalacia in adults and rickets in children. This effect is due to enhanced calcium and phosphorus absorption from the intestine and subsequent deposition in bone matrix and tooth enamel. Calcium homeostasis is also critical for muscle and nervous system functioning, with bone as its principal storage site. Recently, vitamin D has been implicated in cancer, cardiovascular disease, cognition and emotional health, and innate and adaptive immune function, though conclusive evidence for most of these various effects is lacking. Vitamin D toxicity, which clinically manifests as hypercalcemia, resulting in the calcification of soft tissues and other problems, is infrequent, but the popular trend of using vitamin D supplements increases this risk [2].
Vitamin D3 is synthesized in the skin upon exposure to UVB light from the sun or ingested in food or supplements. Vitamin D2 is of plant origin, whereas D3 is derived from animals. Vitamin D3 is carried in the bloodstream to the liver, where it is metabolized to calcidiol (25-(OH)-D3) and then converted, primarily by the kidneys, to its active form calcitriol [1,25-(OH)2 -D3], which is a strong ligand of the vitamin D receptor in target tissues.
Research and clinical practice related to vitamin D has been hampered because of difficulty measuring its active forms in serum. Clinicians rely on the measurement of 25-(OH)-D2 and/or -D3 because of their relative abundance in serum and long half-life, but they have little biological activity. The bioactive, dihydroxylated forms are present at very low concentrations (i.e., low pg/mL) in blood. Radioimmunoassay (RIA), high-pressure liquid chromatography with ultraviolet detection (HPLC-UV), and liquid chromatography (LC) tandem mass spectrometry (LC/MS) have been employed, but conventional methods lack sufficient accuracy, specificity, sensitivity, and repeatability [3].
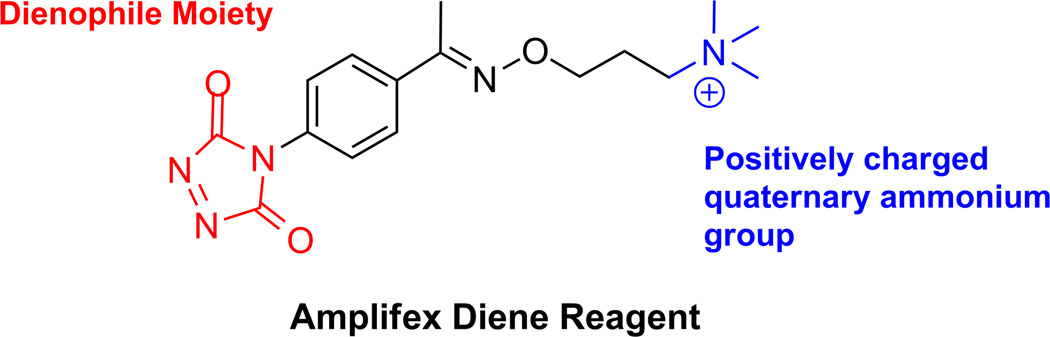
LC/MS/MS has become the method of choice for 1,25-(OH)2-D2 and -D3 analysis due to its sensitivity and repeatability [4]. The active form of vitamin D circulates in much lower levels than 25-OH-D [5]. Though 25-OH-D is in the ng/mL range, 1,25-(OH)2-D2 and -D3 are in the low pg/mL range, necessitating sensitive RIA or LC/MS/MS methods. However, even LC/MS/MS methods are limited in sensitivity for 1,25-(OH)2-D2 and -D3 due to their low concentrations in the circulation and their lipophilic nature [6]. Radioreceptor assays or RIA are sensitive measurements, but they require extensive pretreatments due to antibody cross-reactivity with vitamin D metabolites, and these methods do not separate the two isoforms of 1,25(OH)2-D without chromatography (6). Similarly, HPLC-UV lacks the sensitivity for the low levels of 1,25-(OH)2-D2 and -D3. Although the LC/MS/MS methods are considered the ‘gold standard’ for measuring active forms of vitamin D, their use remains a challenge due to the poor ionization efficiency caused by a lack of ionizable polar groups [4]. Derivatizing techniques have been developed to enhance the detection response of the poorly ionizable compounds [6]. Though several derivatizing agents have been reported, only 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), a representative Cookson-type reagent, is readily available commercially for laboratory use and has demonstrated the ability to allow analysis of 1,25-(OH)2-D2 and -D3 [2]. The addition of PTAD derivatization to multiple solid phase extraction techniques has greatly improved sensitivity and decreased sample size; however, the lower limits of detection could be improved [6]. Recently, a new reagent for derivatizing 1,25-(OH)2-D2 and -D3 was developed specifically for LC/MS/MS: Amplifex diene (AB SCIEX). Unlike other 1,2,4-triazoline-3,5-dione (TAD)-based reagents, Amplifex is optimized for MS/MS analysis due to its positively charged end group and activated dienophile moiety (Fig. 1). This reagent should show increased sensitivity and selectivity due to enhanced ionization.
Figure 1.
Chemical structure of Amplifex diene reagent used to derivatize 1,25-(OH)2-D3 and -D2. Amplifex improves ionization and enhances sensitivity for molecules that have a cis-diene system.
Our goals were to: (a) determine the most accurate and sensitive method for measuring 1,25-(OH)2-D2 and -D3(b) test a new derivatizing compound for 1,25-(OH)2-D2 and -D3(c) compare this compound to the currently available derivatizing agent, PTAD, using the same mass spectrometric analysis and the established RIA methodology for quantification, and (d) provide a testing site for AB Sciex’s new vitamin D derivatizer, which is not yet commercially available. We predict that the Amplifex diene reagent will provide us with the best optimization and sensitivity for high throughput measures of 1,25-(OH)2-D2 and -D3.
2. Materials and Methods
2.1 Patient samples and calibration matrix
Twenty de-identified residual patient serum specimens that were obtained from a reference laboratory (ARUP, USA, http://www.aruplab.com) were analyzed by the University of Wisconsin Hospital and Assay Services core lab for the Institute for Clinical and Translational Research (ICTR) at the University of Wisconsin-Madison using derivatization techniques and LC/MS/MS. The amount of serum used for both LC/MS/MS assays (Amplifex and PTAD) in this study was 0.2 mL. Vitamin D-free Human Serum delipidized for immunoassay and LC/MS application (Golden West Biologicals Inc., Temecula, CA) was used as the calibration matrix.
2.2 RIA methods
Serum samples were assayed for 1,25-(OH)2-D2 and -D3 at the National Reference Laboratory, ARUP, using radioimmunoassay kits (Diasorin, Stillwater, MN, USA). This method did not distinguish between D2 and D3.
2.3 PTAD method of derivatization
All sample volumes, extractions, and methods for the PTAD method were the same as described below for the Amplifex method. A 60 µl aliquot of 0.75 mg/mL PTAD in acetonitrile was added to each dried sample, vortexed for 15 seconds, centrifuged briefly at 14,500 rpm, and allowed to react for one hour at ambient temperature. The derivatized extracts were then transferred to vials for LC/MS/MS analysis.
2.4 Sample procedures, liquid chromatography, and mass spectrometry for the PTAD and Amplifex methods
All procedures were exactly the same for both methods except for the derivatization method. A trial version of Amplifex diene reagent was obtained from AB SCIEX (Chemistry and consumables R&D, Framingham, MA).
2.4.1 Reagents
All solvents were HPLC grade purity or better. Di-isopropyl ether and methanol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hexanes and isopropyl alcohol were obtained from Burdick and Jackson-Honeywell through VWR (West Chester, PA, USA). Acetonitrile was obtained from Fisher Scientific (Farilawn, NJ, USA).
2.4.2 Internal standards and calibration standards
1α, 25-dihydroxy vitamin-D3 (26,26,26,27,27,27-d6) [d6-1,25-(OH)2-D3] and 1α, 25-dihydroxy vitamin-D2 (26,26,26,27,27,27-d6) [d6-1,25-(OH)2-D2]) were obtained from Medical Isotopes (Pelham, NH, USA). An internal standards solution was prepared by dissolving a known amount of d6-1,25-(OH)2-D3 and d6-1,25-(OH)2-D2 in acetonitrile. The concentrations of the internal standards in stock solution were determined by HPLC analysis using a standard concentration vs. absorption curve. Corrections were made for the purity, molar extinction coefficient, and molecular weight of the compounds. The following extinction coefficients were used at 264 nm: ε = 19000 M-1cm-1 for 1,25-(OH)2-D3and ε = 19400 M-1cm-1 for 1,25-(OH)2-D2. The final concentration of internal standards in the working solution was 2.5 pg/µL. Reference standards (1,25-(OH)2-D3 and 1,25-(OH)2-D2 were obtained from Cerilliant (Round Rock, TX). Stock standards and calibration mix solutions were prepared by dissolving a known amount of 1,25-(OH)2-D3 and 1,25-(OH)2-D2 in acetonitrile. Corrections were made for the purity, molar extinction coefficient, and molecular weight of the compounds in the same manner as described above for the internal standards. The final concentrations of the calibration mixes were 0, 15.63, 31.25, 62.5, 125, and 250 pg/mL.
2.4.3 System optimization solution
A mixture of 1,25-(OH)2-D3 and -D2 (analytes & calibrants) and d6-1,25-(OH)2-D3 and -D2 (internal standards) were derivatized with Amplifex reagent in solution and diluted with 1:1 methanol-water to a final concentration of approximately 0.15 µg/mL. This solution was used directly in infusion mode to optimize the MRM parameters and to check the calibration accuracy of the mass spectrometer. After a 1500-fold dilution, this solution may also be used as a standard solution (system suitability solution) to check day-to-day LC and MS performance. This mixture is stable for weeks at 5°C and months at −20°C.
2.4.4 Extraction procedure
The same extraction procedure was used for both derivatization procedures. Serum samples, calibration matrix, and reference standards were equilibrated to ambient temperature, vortexed, and aliquoted (200 µL). Samples were diluted with 700 µL of deionized (MilliQ, 18 MΩ) water in a microcentrifuge tube, vortexed for 30 seconds, and centrifuged at room temperature for 20 seconds at 14,400 rpm (MiniSpin Plus, Eppendorf, Westbury, NY) to re-consolidate the liquid in the tube. A dual column solid phase extraction (SPE) configuration using gravity flow (i.e. - no vacuum) was used for extraction. Chromabond XTR (6 mL, 1 g, Macherey-Nagel, http://www.mn-net.com) and silica (6 cc, 500 mg, Waters, Millford, MA) SPE cartridges were assembled on an extraction manifold using a dual SPE adapter with the Chromabond XTR cartridge on top. The samples were spiked with 20 µL of the internal standard (50 pg, d6-1,25-(OH)2-D3 and -D2) solution, and 20 µL of each calibration mix was added to the top cartridge for use in the calibration curves. Samples, calibration matrix, and reference standards were loaded (900 µL) and allowed to sit for 10 minutes. The top cartridge was eluted with di-isopropyl ether (1 mL × 4, 3 min each elution) under gravity into the silica column. Silica cartridge was washed with 4% (4.5 mL × 2) isopropyl alcohol in hexanes, followed by 6% (6 mL) isopropyl alcohol in hexanes to remove 24,25-(OH)2 D3 from 1,25-(OH)2 D3. Finally analytes were eluted with 25% (4.5 mL) isopropyl alcohol in hexanes. Samples were dried using a water bath and airflow. Methanol (300 µL) was added and the sample vortexed for 1 min, centrifuged at room temperature for 20 seconds at 14,400 rpm to re-consolidate the liquid in the tube, transferred to a smaller vial and dried as above.
2.4.5 Derivatization procedure
Reagent diluent was added to the Amplifex reagent to make a 1 to 1.5 mg/mL solution. A total of 30 µL of the Amplifex reagent was added to the dried sample above and vortexed for 15 seconds, centrifuged at room temperature for 20 seconds at 14,400 rpm to re-consolidate the liquid in the tube, and incubated for 30 min to 1 h at ambient temperature. Next, 30 µL of deionized water was added to each tube, vortexed for 15 seconds, centrifuged at room temperature for 20 seconds at 14,400 rpm to re-consolidate the liquid in the tube, and transferred for LC injection.
2.4.6 LC-MS/MS analysis
Aliquots of the derivatized extracts (10 µL) were separated on a Shimadzu Prominence (Addison, IL, USA) integrated HPLC interfaced with an AB SCIEX (Foster City, CA) QTRAP 5500 Quadrupole - Linear Ion Trap Mass Spectrometer (Framingham, MA, USA) operating in positive TurboIonSpray mode. The analytical column was a Phenomenex (Torrance, CA, USA) Kinetex (2.4 µm, 3.0×150 mm). Samples were eluted at a flow rate of 0.25 mL/min using a binary reversed phase gradient (Channel A = 0.1% formic acid in 18 MΩ/cm water, Channel B = methanol) as follows: 0 min, 2% B; 1 min, 2% B; 17.8 min, 65% B; 18 min, 100% B; 19.8 min, 100% B; 20 min, 2% B; 30 min, 2% B. Relevant MS/MS settings were: CAD gas at 6 psig; CUR at 20 psig; GS1 at 60 psig; GS2 at 30 psig; IS at 5000 V; EP at 10 V; and TEM at 600 °C. The limit of detection was 2.0 pg/L. QA/QC procedures included running calibration blanks, standards, and three replicate spikes of 50 µg/L 1,25-(OH)2-D3. Calibration curves were constructed by plotting the peak area ratio of 1,25-(OH)2-D3/d6-1,25-(OH)2-D2 versus the corresponding concentration ratios and fitting the data using linear regression with no weighting factor.
Validations were performed for linerarity and repeatability data. Standard curve correlation coefficients over four separate runs were greater than 0.9984 for 1,25-(OH)2-D2 and greater than 0.9991 for 1,25-(OH)2-D3. All method blanks were non detect (ND) for 1,25-(OH)2-D2 and 1,25-(OH)2-D3. Back calculated percent recoveries for extracted standards ranged from 89.6 to 115% for 1,25-(OH)2-D2 and from 93.6 to 117% for 1,25-(OH)2-D3. Subsequent re-analysis of the study samples to confirm high repeatability yielded percent recoveries ranging from 87.3 to 109%.
3. Results
The Amplifex LC/MS/MS results were compared to the RIA and PTAD derivatization results for the 20 patient samples. For the comparison to RIA, we combined the derivatization results of D2 and D3 since the RIA does not distinguish the two forms.
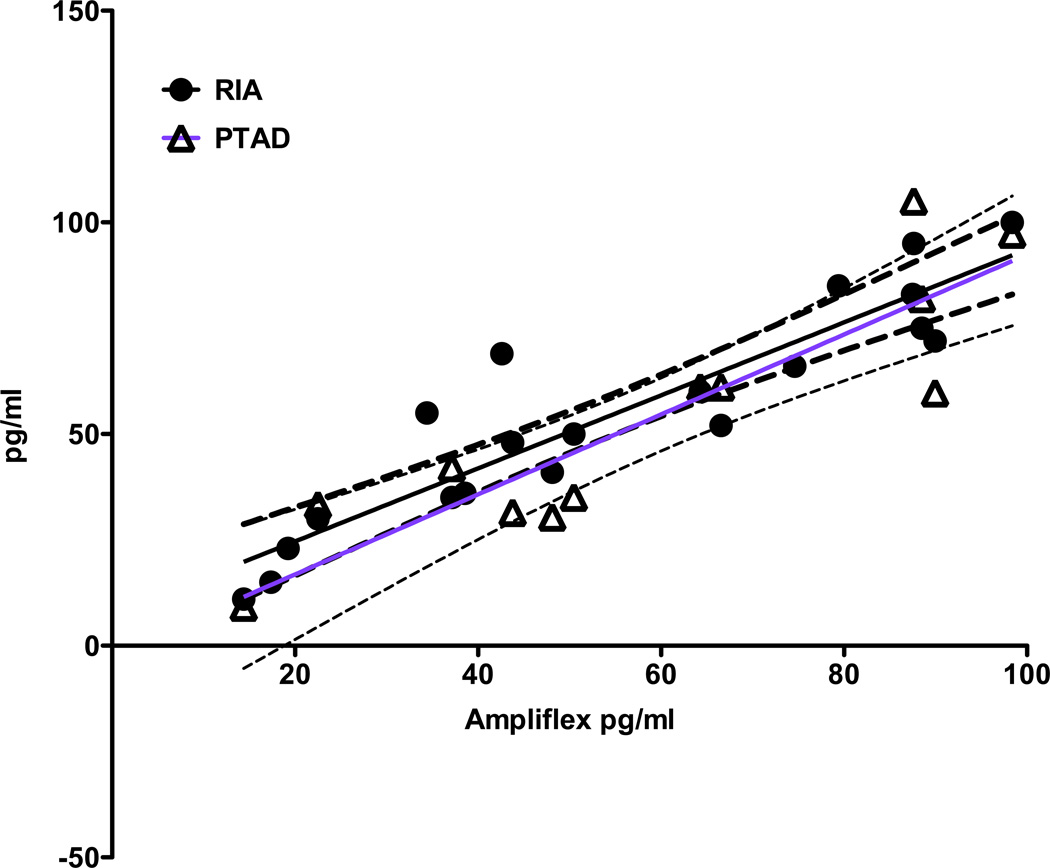
Fig. 2 provides a comparison of the Amplifex derivitization method with the RIA and with the PTAD method for 1,25-(OH)2-D2 and 1,25-(OH)2-D3. A Lin’s Concordance correlation coefficient, (pc) was determined for Amplifex/RIA comparison and the Amplifex/PTAD comparison. Additionally, the upper and lower two-sided 95% correlation were calculated to assess where the least concordance occurred in both comparisons. Amplifex correlated with the RIA (pc) = 0.92, n = 20. The upper two sided 95% pc = 0.97, indicating substantial correlation while the lower two-sided 95% for pc = 0.81, indicating a slightly poorer concordance between the determined concentrations. The Amplifex correlated with the PTAD pc = 0.88, n=12. The upper two sided 95% for pc = 0.96, indicating a substantial correlation while the lower two sided 95% for pc = 0.65, indicating a poorer concordance due to less sensitivity in the PTAD method.
Figure 2.
Correlation of the Amplifex diene method of derivatization with the RIA (circles) and PTAD (triangles) methods of derivatization. The Amplifex diene method correlated well with both methods, but less so at the low concentration standards where the PTAD method was not as sensitive at the Amplifex diene method.
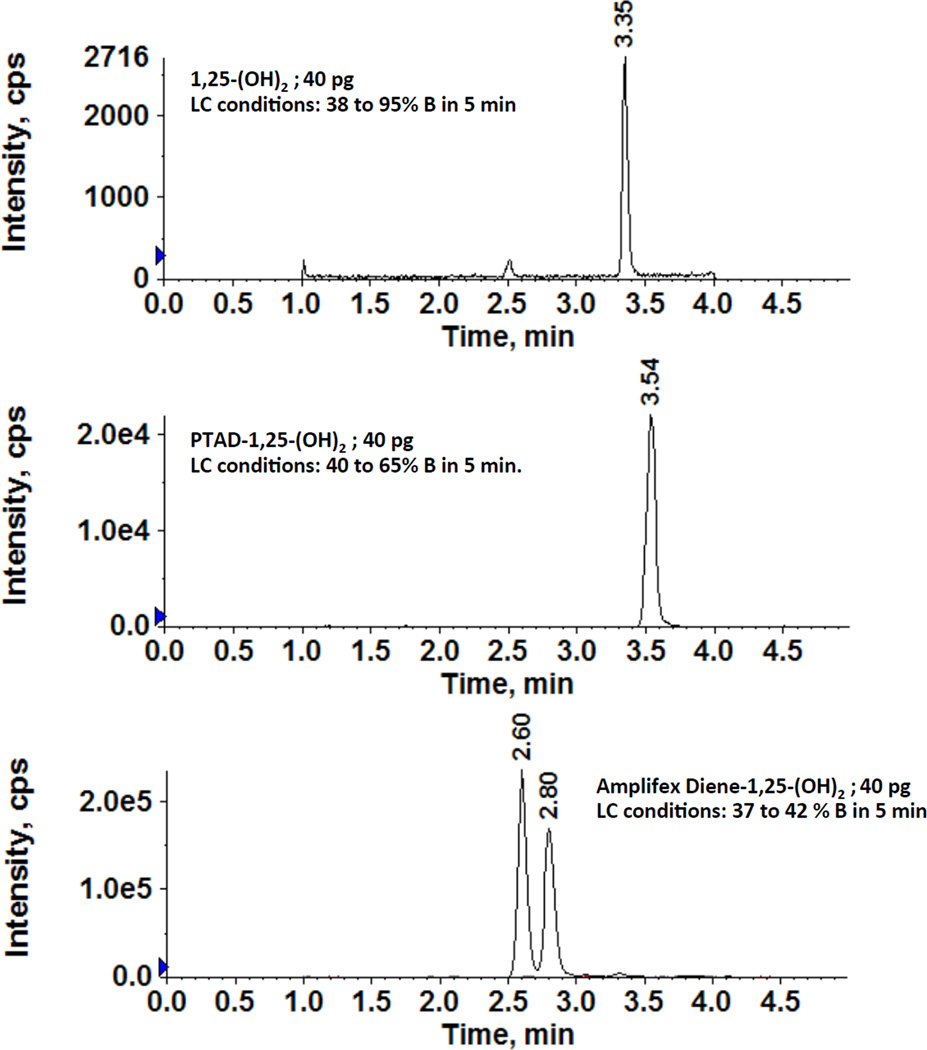
However, the sensitivity of the Amplifex method was greater than that of the PTAD method, as the peak areas were significantly higher for Amplifex and provided a 10 fold higher signal-to-noise ratio than PTAD (Fig. 3). The signal-to-noise ratio was 10 times better with Amplifex, whereas the ratio for PTAD was only 3:1 and could not be distinguished from background at 15 pg/mL and 30 pg/mL. The estimated limit of detection for Amplifex was 2 pg/mL with a 30:1 signal-to-noise ratio (Table 1).
Figure 3.
Amplifex diene derivatization of 1,25-(OH)2-D3 provided a higher signal-to-noise ratio than the PTAD derivatization method, increasing sensitivity. The analyte was 40 pg for both methods (offsetting of chromatograms was used for comparison purpose).
Table 1.
The signal-to-noise (S/N) ratio and the Lowest Level of Detection(LLOD) are presented for 1,25-DHVD3-Amplifex diene derivative and 1,25-DHVD2-PTAD derivative.
| 15.6 pg/mL calibration standard ID | Observed S/N ratio |
Estimated LLOD* pg/mL |
|---|---|---|
| 1,25-DHVD2-Amplifex diene dvt. | 30:1 | 2 |
| 1,25-DHVD3-Amplifex diene dvt. | 30:1 | 2 |
| 1,25-DHVD2-PTAD dvt. | no signal | 30 |
| 1,25-DHVD3-PTAD dvt. | 3:1 | 15 |
Based upon 3:1 signal-to-noise ratio.
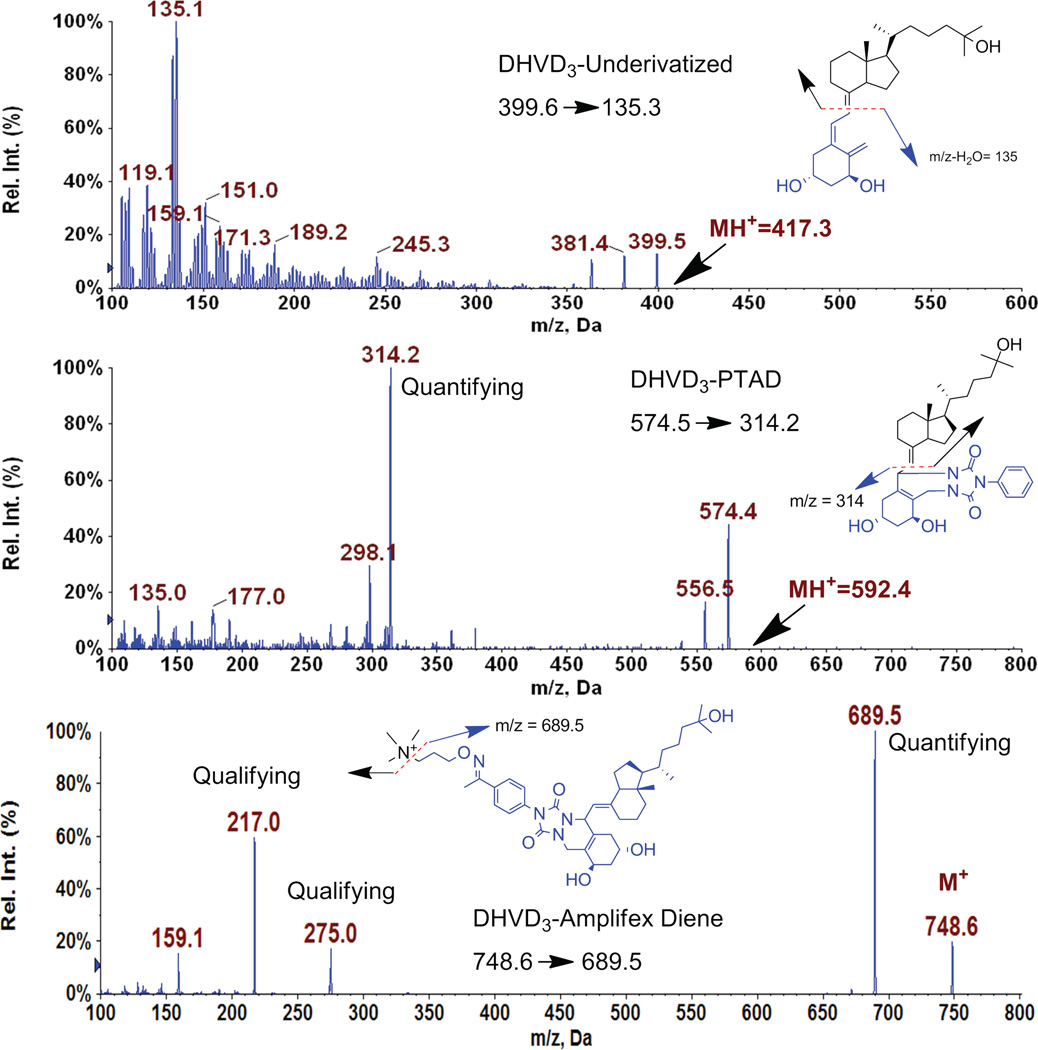
Fig. 4 demonstrates the difference in the fragmentation patterns observed for 1,25-(OH)2-D3 when derivatized with Amplifex or PTAD. Amplifex resulted in a defined fragmentation pattern in which most of the ion current was funneled to the quantifying transition. 1,25-(OH)2-D3 PTAD and its fragment ion underwent dehydration. The quantifying ion carried only a small portion of the analyte.
Figure 4.
The fragmentation pattern for the underivatized 1,25-(OH)2-D3, PTAD-derivatized 1,25-(OH)2-D3 and Amplifex diene reagent-derivatized 1,25-(OH)2-D3. The Amplifex Diene:1,25-(OH)2-D3 quantifying fragment ion is a high molecular weight ion that provides cleaner MRM profile.
4. Discussion
The results indicate that the Amplifex compound provides a means for increasing the sensitivity of LC/MS/MS over the PTAD method and that it compares well with RIA. The method also uses a small sample volume, achieving reliable measures with 200 µL of human serum. This method provides a precise measurement of vitamin D and obviates the need for RIA. As a mass spectrometry method, both D2 and D3 can be measured simultaneously.
The PTAD method requires a larger sample volume than the Amplifex method of derivatization to obtain reliable sensitivity. The use of TAD-based reagents, which react selectively with diene-containing compounds, for organic synthesis began with the Cookson Reagent, PTAD [7]. This dienophillic reactive property of TAD was quickly adapted for use as an analytical derivatization agent with the development of several fluorophoric varieties of TAD-containing molecules [8]. Although these legacy derivatization reagents have been applied successfully to vitamin D metabolite LC/MS/MS assays [3, 9], the reagents are more non-polar, non-ionic and require larger sample aliquots for the sub-ng/ml detection required for 1,25-(OH)2-D3 and -D2.
The mass spectrometer used for detection in an LC/MS/MS system relies on ionized and desolvated analyte molecules being transferred from the ionization source to the high vacuum mass analyzer by voltage gradient. Therefore, more efficient ionization of target compounds results in a stronger signal for these compounds. The goal of the addition of a derivatization reagent moiety to a target compound should be to maximize the selectivity and sensitivity of the detector response. Although derivatization chemistry has been used for decades, most of the existing derivatization reagents for HPLC analysis were developed to optimize other desirable analytical characteristics, such as the addition of a chromophore for UV detection or a fluorophore for fluorescence detection. To date, a limited number of derivatizing agents have been developed specifically for LC/MS.
The Amplifex diene derivatization reagent was developed by combining a quaternary amine containing an organic compound with TAD, creating multiple benefits for the LC/MS analysis of cis-diene-containing compounds, such as 1,25-(OH)2-D. First, the stereochemistry of this compound allows for selective reaction with cis-diene compounds, which prevents endogenous trans-diene compounds in the sample matrix from being derivatized. This cis-diene specificity allows for more selective detection of dihydroxyvitamin D metabolites, such as 1,25-(OH)2-D3 and -D2. Second, the fragmentation of the Amplifex diene:1,25-(OH)2-D3 product is limited to several defined peaks, whereas the PTAD derivatized 1,25-(OH)2-D3 and underivatized 1,25-(OH)2-D3 fragment into several peaks. This difference allows more of the fragmentation collision energy of the mass spectrometer to be utilized and transferred to quantifying and qualifying MRM products rather than extraneous products. Third, the quantifying MRM product contains the entire 1,25-(OH)2-D structure, which results in different m/z values for the 1,25-(OH)2-D3 and 1,25-(OH)2-D3 product ions. This is beneficial in preventing isobaric cross talk between the two analytes. This result is unlike that of the reaction with PTAD, which creates a common quantifying m/z for the 1,25-(OH)2-D3 and 1,25-(OH)2-D2 product ions. Fourth, the Amplifex diene: 1,25-(OH)2-D metabolite products are more polar but remain soluble in organic solvent. This hydrophilic property of the derivatization reaction products allows for the use of more rapid HPLC separation techniques. Finally, and most importantly, the quaternary amine functionality of the Amplifex diene derivatization reagent results in much more efficient ionization in the MS ionization source due to its quaternary amine functional group. The sum of these benefits is the highly selective and sensitive detection of 1,25-(OH)2-D metabolites.
Like the other TAD derivatization agents, the Amplifex diene derivatization reagent reacts at room temperature in less than 30 min (t1/2 = 4 min, data not shown), stabilizes 1,25-(OH)2-D3 and -D2 from thermal and photochemical transformations once reacted, and creates a product that is stable for several months when stored at −20°C or lower. With the improved sensitivity, the smaller sample volume (200 µL vs. 400 µL serum), and the high selectivity, this assay can provide the most efficient and reliable method for measuring 1,25-(OH)2-D3 and -D2 in biomedical and clinical research.
Table 2.
Concentration of 1,25-(OH)2-D3,D2 in pg/ml by different LC/MS methods and by RIA.
| Amplifex | RIA | PTAD |
|---|---|---|
| 14 | 11 | 9 |
| 23 | 30 | 33 |
| 39 | 36 | |
| 48 | 41 | 30 |
| 51 | 50 | 35 |
| 67 | 52 | 61 |
| 64 | 60 | 61 |
| 90 | 72 | 60 |
| 79 | 85 | |
| 88 | 95 | 105 |
| 98 | 100 | 97 |
| 17 | 15 | |
| 19 | 23 | |
| 37 | 35 | 42 |
| 44 | 48 | 32 |
| 34 | 55 | |
| 75 | 66 | |
| 43 | 69 | |
| 89 | 75 | 82 |
| 87 | 83 |
Highlights.
1,25 dihydroxyvitamin D2, D3 is the active form of vitamin D and requires sensitive methods for measuring in human serum.
A new ionizing agent, Amplifex, was tested for its ability to enhance liquid chromatography/mass spectrometry measurement.
The Amplifex method significantly correlated with values measured by standard RIA and mass spectrometry.
Sensitivity of 10 fold resulted over other derivatizing methods.
This new method resulted in increased sensitivity and required half the sample volume of other methods.
Acknowledgements
We would like to thank Marc K. Drezner, the Director of the Institute for Clinical and Translational Research for his encouragement to develop the vitamin D methods and to acknowledge the support of this study by the Institute for Clinical and Translational Research at the University of Wisconsin-Madison as the Clinical and Translational Science Award (CTSA) program, the National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. Additionally we thank the Wisconsin National Primate Research Center for their support of the LC/MS/MS and the vitamin D project through the base funding: NIH P51RR000167/P51OD011106
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holt LE. The American Journal of Clinical Nutrition. 1968;21:1136–1137. doi: 10.1093/ajcn/21.10.1136. [DOI] [PubMed] [Google Scholar]
- 2.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Anal Bioanal Chemistry. 2008;391:1917–1930. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netzel BC, Cradic KW, Bro ET, Girtman AB, Cyr RC, Singh RJ, Grebe SKG. Increasing Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Throughput by Mass Tagging: A Sample-Multiplexed High-Throughput Assay for 25-Hydroxyvitamin D2 and D3. 2011 doi: 10.1373/clinchem.2010.157115. [DOI] [PubMed] [Google Scholar]
- 4.Yuan C, Kosewick J, He X, Kozak M, Wan S. Communications in Mass Spectrometry. 2011;25:1241–1249. doi: 10.1002/rcm.4988. [DOI] [PubMed] [Google Scholar]
- 5.Strathmann FG, Laha TJ, Hoofnagle AN. Clin Chem. 2011;57:1279–1285. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashi T, Simada K, Toyo’oka T. Journal of Chromatography B. 2009;878:1654–1661. doi: 10.1016/j.jchromb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Cookson RC. Organic Syntheses. 1988 [Google Scholar]
- 8.Knapp DR. Handbook of anlalytical derivatization reactions. John Wiley & Sons, Inc.; 1979. [Google Scholar]
- 9.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Anal Bioanal Chem. 1971;391:1930. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]