Abstract
Background:
In recent years, adipose tissue, due to the stem cells contained within, has found a new special place in laboratory and clinical applications. These adipose-derived stem cells (ADSCs) have the same characteristics of bone marrow mesenchymal stem cells (BMSCs). Although bone marrow (BM) is not easily accessible and its procurements may be painful, most patients possess excess fat which can be obtained by less invasive methods; this makes adipose tissue ubiquitous, available and an ideal large-scale source for research on clinical applications.
Methods:
BMSCs and ADSCs were harvested from three healthy human and were characterized using flow-cytometry. After they were treated for neurosphere formation using basic fibroblast growth factor, epidermal growth factor, B 27; terminal differentiation was performed. In this study, we used immunocytochemistry, real time-polymerase chain reaction and western blotting techniques for detection and comparison of Nestin, microtubule-associated protein-2 (MAP-2) and glial fibrillary acidic protein (GFAP) markers in human ADSCs and BMSCs.
Results:
Under appropriate conditions ADSCs can differentiate into neuron-like cells and express neural markers the same as BMSCs, also the expression of GFAP marker in differentiated cells derived from ADSCs was significantly lower than the cells derived from BMSCs (P < 0.05). While the expression of MAP-2 marker in both groups was the same.
Conclusions:
However, due to its advantages and according to our results based on the expression levels of GFAP and MAP-2, adipose tissue rather than BM could represent a more appropriate stem cell source for investigating the application of these cells in understanding the pathophysiology and in treatment of neurodegenerative disorders.
Keywords: Glial fibrillary acidic protein, human adipose-derived stem cells, human bone marrow mesenchymal stem cells, microtubule-associated protein-2, Nestin, neural induction
INTRODUCTION
Mesenchymal stem cells (MSCs), which are found in many adult tissues, are morphologically similar to fibroblasts. They are capable of self-renewal and multilineage cell differentiation under appropriate conditions.[1,2] They can differentiate to mature cells of mesodermal origin[3,4] and also transdifferentiate into endodermal origin hepatocytes and ectodermal origin neural cells. According to these special characteristics, including their availability in different tissues, high proliferation rate, long term viability and lack of ethical and legal problems with their usage, they can be promising tools for cell replacement therapy, one of the most important therapeutic approaches for neurological disorders.[5,6]
MSCs were first isolated from BM in 1985 when Owen reported a group of cells other than hematopoietic cells in BM, with self-renewal and multi-differentiation capacity.[7] They have been named as BMSCs which are so far the best characterized adult stem cell.[8]
Isolation of a variety of stem cells from different adult tissues in searching for MSCs suggests the involvement of these cells in tissue repair and regeneration throughout life span.[9,10]
For the first time Zuk in 2001 determined a population of multipotential stem cells in human adipose tissue that opened a new and exciting view in the field of adult stem cells studies.[11] Latter studies showed ADSCs are capable of exhibiting characteristics of neurons and glial cells.[12]
Neural tissue has always been assumed as non-regenerating tissue. Today, we know about postnatal neurogenesis, but its ability is not sufficient to repair damaged areas after injury,[13] therefore cells capable of neuronal differentiation has found important applications in the field of neurodegenerative diseases.[14] Although embryonic stem cells and neural stem cells, due to their ability of undergoing expansion and neuronal differentiation, are the most suggested cell groups for neurogenic cell therapy,[15] there are various limitations to their usage. There are ethical and legal consideration, rejection problems and genetic manipulation issues. By contrast, MSCs of autologous tissue origin are more available, with neither ethical nor immunoreactive considerations.[16]
ADSCs and BMSCs, both MSCs with mesodermal origin, are capable of multilineage differentiation. They both express similar markers and have similar morphologies and doubling times.[3,17] Adipose tissue is ubiquitously available, easily accessible in large quantities under local anesthesia with minimal invasive methods, little patient discomfort and low donor-site morbidity. Hence, it yields a high amount of stem cells which is essential for stem cell based therapies.[18,19] In contrast, BM procurements is painful and frequently needs to be done under anesthesia. Also isolation of BMSCs produces low numbers of MSCs.[20]
Until now, the great majority of studies about neural differentiation potentials of MSCs have been focused on the capacity of BMSCs and there are limited number of studies that have explored the neural differentiation potentials of adult MSCs related to other tissues and the neural differentiation potential of many of the other adult tissues are not clearly known.[3,21]
As mentioned above, between different kinds of MSCs’ sources, adipose tissue has unique characteristics required for an appropriate source of autologous stem cells, on the other hand our induction procedure differ from the previous studies so in this work we decided to compare neurogenic differentiation potential of human ADSCs (hADSCs) and human BMSCs (hBMSCs), using previously published protocols of neural induction,[22] if neurogenic differentiation capacity of hADSCs is similar to BMSCs, adipose tissue can be used as an ideal source of autologous stem cells in degenerative neurological diseases.
METHODS
HBMSC isolation and culture
All procedures were conducted according to Isfahan University of Medical Sciences, Medical Faculty Ethic Committee approval. Patients with no notable pathologic history were chosen for this study. Human BM of three healthy donors aged 25-40 years was obtained from the patients who were referred to Alzahra Hospital, after they gave informed consent according to the approved procedure. Concurrent to previous study,[23] about 1 mL of marrow was collected from the pelvis of each patient and stored in a heparin containing test tube. BM aspirates were diluted 1:5 in Dulbecco's Modified Eagle's Medium (DMEM), 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and immediately seeded into polystyrene plastic 25 cm2 tissue culture flasks at 37°C in a humidified-atmosphere of 95% air and 5% CO2. After 5 days, flasks were washed with phosphate-buffered saline (PBS) and non-adherent cells were removed by media replacement and adherent cells were expanded in DMEM which contains 10% FBS. Later, half of the medium was replaced every 3-4 days until cells reached 80% confluence (nearly 14 days after the first incubation). For passaging, the cells were detached with trypsin/ethylenediaminetetraacetic acid (EDTA) solution and re-seeded with a density of 4000 cells/cm2. HBMSCs from passages 3-5 were used for the different experiments.
Isolation and culture of hADSCs
Human adipose tissue was obtained from subcutaneous abdominal fat of 3 patients, 27-45 years old, who have been referred to Alzahra Hospital of Isfahan for abdominoplastic surgery after receiving informed consent. Tissue was cultured as described in our previous study.[22] Briefly, samples of fat tissue were washed extensively with sterile PBS in order to remove contaminating debris and red blood cells. Careful surgical removal of the connective tissue surrounding the parenchyma performed and mechanical digested samples were treated with 0.01% collagenase type I in PBS for 30 min at 37°C with gentle agitation. Then the enzyme activity was neutralized by adding DMEM/10% FBS in a 1:1 ratio. After centrifugation for 10 min at 1600 rpm and removal of the supernatant the cellular pellet was washed and then plated in T25 flask which contained DMEM: F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin, in an incubator with a humidified atmosphere containing 5% CO2 at 37°C.
After 24 h, the flasks were washed with PBS and their medium entirely changed. In this way non-adherent cells were removed. Then, adherents ADSCs were expanded by serial passage to improve the purity of the preparation and to generate a homogeneous cell population. In this study cells were separated with 0.25% trypsin - 0.02% EDTA (Gibco, BRL, Paisley, UK) and passaged at the ratio of 1:3 in every passage. We used cells of passages 3-5 in our experiments.
All chemicals, except where specified otherwise, were purchased from Sigma–Aldrich, St. Louis, MO, USA.
Characterization of human MSCs
As there are no universal markers, human MSCs are recognized on the basis of a complex immune phenotype, including the lack of hematopoietic stem cell markers such as CD45, CD34 and CD14 as negative markers and the expression of a number of surface molecules, including CD105, CD44, CD90 as positive markers.[24] Both isolated MSCs were examined for surface markers by flow-cytometry in order to confirm their stemness. The forth passage of MSCs were trypsinized, centrifuged and resuspended to density of 1 × 105 cells for each test. Then the cells were washed twice with 1% bovine serum albumin (BSA)/PBS and incubated with antibodies against positive (CD44, CD90, CD105) and negative (CD14, CD34 and CD45) markers (Chemicon, Temecula, CA, USA) for 30 min. Primary antibodies were directly conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). Negative control staining was performed using a FITC-conjugated mouse IgG isotype and a PE-conjugated mouse IgG isotype antibody. Flow-cytometry was performed with a FACScan flow-cytometry (Becton–Dickinson, San Jose, CA).
Neural induction of human MSCs
We have applied the same conditioning medium and growth factors employed with BMSCs to induce neural differentiation of MSCs obtained from subcutaneous adipose tissue using Ahmadi et al. procedure.[22] The procedure had two steps: Induction of human MSCs into neurosphere-like structures and final differentiation into neural-like cells. For the first step, briefly we dissociated MSCs (80-90% confluence) with 0.25% trypsin - 0.02% EDTA and then plated them on plastic tissue culture plates in a density of 1 × 106 in DMEM: F12 supplemented with 20 ng/ml human epidermal growth factor (EGF), 20 ng/ml basic fibroblast growth factor (b-FGF) and 2% B27 (1:50, Gibco) at 37°C and 5% CO2. Fresh medium was added every 3-4 days. After 7 days neurospheres were dissociated and singled by pipetting in trypsin 0.25%-EDTA 0.02%. Next, the singled cells re-plated in laminin coated chamber slides in differentiation medium consisting of neurobasal medium, 5% FBS, 1% penicillin, 1% L-glutamine, 1% N2, 1% non-essential amino acids, 2% B27, 1% nystatine. The cells were incubated for 1 week under these conditions.
Growth kinetics of the MSCs
To determine whether both kinds of MSCs, used in this work, have the same growth properties we studied their doubling time, cells from passage 3-4 were plated in T25 flasks at 4000 cells/cm2 in DMEM: F12 medium supplemented with 10% FBS and 1% Penicillin/streptomycin, in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. After they reached confluent, cells were trypsynized and counted to calculate the doubling time. Doubling time was obtained using the following formula:
Doubling time = T [log2/log (N2/N1)]
T: Days of expansion; N1: Number of plating cells; N2 : Number of harvested cells at the end of experiment
MTT assay
Growth and viability of neural induced cells were studied by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) biochemical approach, based on the reduction of MTT into formazan crystals by the action of the mitochondrial dehydrogenase enzymes present in viable cells. We used exactly the same protocol for both kinds of adipose and BM differentiated cells. For this purpose, after singling the neurospheres, 3 × 103 cells/well were seeded on 24-well plates and grown in the presence of neural induction medium. MTT powder was dissolved in PBS at 5 mg/ml. 2 weeks after neural induction, differentiated cells were washed with PBS and then treated with a solution at a dilution 1:10 of the MTT stock solution. After 4 h at 37°C the formed formazan crystals were dissolved in a solution consisting of 200 μl of DMSO, giving a spectrophotometrically measurable purple solution. After transferring to a 96-well plate and incubating it for 1 h at room temperature (RT) in the dark, absorbance was detected by a microplate reader (Hiperion MPR 4+, Germany) at a wavelength of 540 nm. Absorbance values correspond to the number of viable cells.
Immunocytochemistry
To evaluate the immune phenotype, MSCs derived from BM and fat tissues after terminal neural differentiation, subjected to immunocytochemistry according to standard protocols.
For this purpose cells were incubated with specific antibodies directed against different phenotypic markers, including: MAP-2, a cytoskeletal protein required for neuronal development, the neural precursor marker Nestin and GFAP which is known as an astrocytic marker. However first cell cultures were fixed for 20 min with 4% paraformaldehyde in PBS and blocked for 45 min at RT with blocking solution (PBS containing 10% goat serum and 2% Triton X-100). Then, the cells were incubated overnight at 4°C with primary antibodies diluted in PBS containing 0.1% Triton X-100 and 1% goat serum in the dark. The following antibodies were used: Mouse anti-GFAP (1:300; Abcam, Cambridge, MA, USA), mouse anti Nestin (1:300, Abcam, Cambridge, MA, USA) and mouse anti MAP-2 (1:300; Abcam, Cambridge, MA, USA). After washing with PBS, slides were exposed to secondary antibody, rabbit anti-mouse FITC (1:500; Abcam, Cambridge, MA, USA) conjugated secondary antibody was incubated at RT for 2 h. For nuclear labeling we stained nuclei using 4’,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma). Negative control included the omission of primary antibodies from the reaction series in each experiment. Experiments were performed in triplicate and samples were observed using fluorescence microscope (Olympus BX51, Japan). To perform quantitative analysis the numbers of immunopositive cells were counted in several non-overlap fields in a minimum total of 100 cells per slide by ImageJ software (NIH, MD, USA). This software was used for merging the pictures of the cells and their nuclei as well.
Real time RT-PCR
PCR is the most powerful technique for detection of small amounts of nucleic acids. Using real-time RT-PCR we can quantify gene expression; hence, in the present study we decided to use real-time RT-PCR technique for measuring the gene expression levels. First RNA samples were prepared after 2 weeks from the beginning of the transdifferentiation procedure. For this purpose, total cellular ribonucleic acid (RNA) was isolated using the RNeasy mini, RNA isolation kit (Qiagen). RT-PCR requires two distinct procedures to be linked together, the first step in this reaction is to selectively convert only the RNA molecules that correspond to protein-encoding genes into complementary deoxyribonucleic acid (cDNA) and next the actual PCR reaction and its subsequent analysis was performed. The RNA was reverse transcribed using RevertAid first strand cDNA synthesis kit (Fermentas) with oligo dT primers. The RT-PCR was performed with gene specific primers and the SYBRGreen PCR Master mix (Qiagen) using a thermal cycler rotor-gene 6000 (Qiagen). The primer sequences (forward, reverse) were as follows: GFAP, 5’-CCGACAGCAGGTCCATGTG-3’, 5’-GTTGCTGGACGCCATTGC-3’; Nestin, 5’-AGCCCTGACCACTCCAGTTTAG-3’, 5’-CCCTCTATGGCTGTTTCTTTCTCT-3’; MAP-2, 5’-TCAGAGGCAATGACCTTACC-3’, 5’-GTGGTAGGCTCTTGGTCTTT-3’; GAPDH, 5’-GAAATCCCATCACCATCTTCCAGG-3’, 5’-GAGCCCCAGCCTTCTCCATG-3’.
The gene of interest was normalized against the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The expression level of each target gene was calculated as 2–ΔΔCt, as previously described.[25]
Western blotting
This technique is used to detect specific proteins in the given sample of tissue extract. In the current study protein extracts were prepared from undifferentiated or differentiated BMSCs and ADSCs. The first step in a western blotting procedure is to separate the macromolecules using gel electrophoresis. But before this stage the cultured cells were tripsinized in order to extract their proteins by lysing cells in cell lysis buffer (1X: 20 mM Tris-Hcl (pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) (Cell Signaling Technology, USA) according to manufacturer's protocols. It should be noted that all procedures related to protein extraction were performed on ice, after that in all of the fallowing steps our samples were shacked very gentle. The protein sample was quantified with Bradford assay and then was added to ×4 sampling buffer. The sample was boiled for 5 min. Total protein was resolved on 12.5% acrylamide gel and electroblotted onto nitrocellulose membrane. Next, the membrane is blocked to prevent any nonspecific binding of antibodies to its surface, with a solution of 5% (W/V) skim milk overnight at RT. The membrane was washed in PBS with 1% BSA and 0.05% tween 20, 3 × 5 min at RT, then was incubated with primary monoclonal antibodies consisting of mouse anti-rat MAP-2 (1:500), anti-GFAP (1:500) and anti-Nestin (1:500). The blots were washed 3 × 5 min in washing buffer and then incubated with a 1:1000 dilution of horse radish peroxidase conjugated secondary antibody (Chemicon) in blocking solution for 2 h at RT. We developed the blots by 3,3’,5,5’ Tetra Methyl Benzidine (TMB) solution (CMG Company, Iran). All of these steps were done separately for each antibody.
Statistical analysis
Statistical comparison of data of surface markers, immunocytochemistry and real time RT-PCR obtained with the different kinds of MSCs treated with neural differentiation medium was carried out according to the one-way ANOVA followed by Tukey's post hoc test. Differences between the mean of parameters were considered statistically significant when P < 0.05. The experiments were replicated at least three times. Data were presented as mean ± standard error of mean.
RESULTS
Phenotypic characterization of MSCs
To assess the changes of both kinds of MSCs’ morphology (MSCs derived from BM and adipose tissue) before and after neural induction, we analyzed morphology of neurogenic induced cells within 2 weeks of induction using bright field and phase contrast microscopy (Nikon Eclipse TS100). A homogeneous and proliferating adherent cell population was obtained from human BM and adipose tissue after 3-4 weeks of isolated cell's culturing.
MSCs derived from adipose tissue were similar to BMSCs morphologically, forming a monolayer of spindle-shaped morphology at confluence. BMSCs and ADSCs proliferated rapidly in vitro and within 3-4 passages after initial plating of the primary culture, they produced a homogenous population and grew in a spindle-shaped, typical fibroblast-like morphology [Figure 1a and b].
Figure 1.
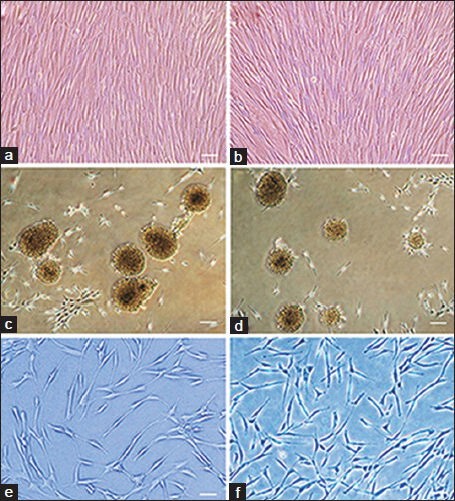
Phase contrast image of (a) hBMSCs and (b) hADSCs Both kinds of cultures were filled with elongated fibroblast-like cells. (c and d) Neurospheres dissociated from the tissue culture dish plastic substrate after 7 days culturing, surrounded by some fibroblast-like cells, almost all of the cells participate in neurosphere formation. (c) Spheres from BM samples culturing; (d) spheres derived from adipose tissue culturing. Differentiated cells derived from (e) hBMSCs and (f) hADSCs 2 weeks after neural induction. We can see bi- and multipolar cells with elongated processes. Scale bars in a and b = 150 μm, c and d = 200 μm and in e and f = 100 μm
Morphological changes during neuronal differentiation
Here we used exactly the same protocol to induce both kinds of MSCs toward the neurogenic lineage. This protocol involved two steps: Conversion of MSCs into neurosphere-like structures and final differentiation into neuron-like cells. During neurosphere formation, we did not observe any significant differences between these two kinds of cells [Figure 1c and d]. As differentiation progressed during the second step, the cells changed their characteristics but changes in both cell groups were exactly the same.
The cell processes became thinner and longer, similar to neural cells and other morphologic changes, such as small growing of perinuclear cytoplasm were observed [Figure 1e and f]. When the grown and proliferated cells were seen under the microscope the bipolar, spheroid cell mass began to adhere and spread across the growth surface. Moreover, proliferation rate in differentiated ADSCs was similar to the proliferation rate of differentiated BMSCs.
Characterization of MSCs
MSCs immunophenotype has been analyzed by flow-cytometry in order to confirm BM and adipose tissue-derived MSCs, as shown in Table 1. Isolated cells were collected and tested for CD44, CD90 and CD105 expressions, which are markers specific to MSCs and the test results in cell culture were positive aforementioned. The test was negative for antibodies CD14, CD45 and CD34, which are specific markers to hematopoietic stem cells.
Table 1.
Immunophenotype of BM and adipose-derived MSCs
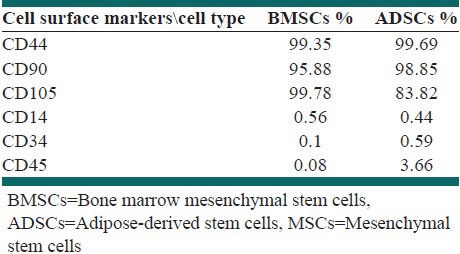
According to the results above, both kinds of MSCs showed the same phenotype [Table 1], with expression of CD105, CD44 and CD90, absence of hematopoietic markers. Related to these results both kinds of cell populations derived from BM and adipose tissue were MSCs and one-way ANOVA analysis indicated that the difference mean of above markers was no significant between two kinds of MSCs.
Growth kinetics of the MSCs
Both kinds of studied MSCs were similar in duplication rate. Average doubling times for BM and adipose tissue derived MSCs were 1.46 ± 0.03 and 1.5 ± 0.02 days respectively with no significant difference between the mean of their doubling time (P > 0.05).
Cell viability and proliferation rate of differentiated MSCs
We compared the survival and proliferation rate of differentiating BMSCs and ADSCs using MTT assay after 2 weeks of differentiation. MTT assay demonstrated no significant difference in the mean of the optical density between neural induced stem cells derived from BM compared with neural induced stem cells derived from adipose tissue after 2 weeks post induction (0.252 ± 0.041% vs. 0.256 ± 0.048%) (P > 0.05). In contrast we observed a significant differences between the mean optical density (OD) of differentiated cells of both sources and their related MSCs (P < 0.01). The mean OD for BM derived stem cells and differentiated cells, adipose tissue derived stem cells and differentiated cells were 0.141 ± 0.014, 0.252% ± 0.041% and 0.153 ± 0.018, 0.256% ± 0.048%, respectively.
According to our results, it has been found that using this differentiation protocol after 2 weeks of induction not only our cells were still alive and we did not see much dead cells in the differentiation medium, but also compare with control groups proliferation rate and cell viability were significantly increased.
Immunocytochemistry after neural induction
To investigate the expression of Nestin, MAP-2 and GFAP markers in neural differentiated cells derived from adipose and BM tissues, immunocytochemistry staining was performed [Figures 2 and 3].
Figure 2.
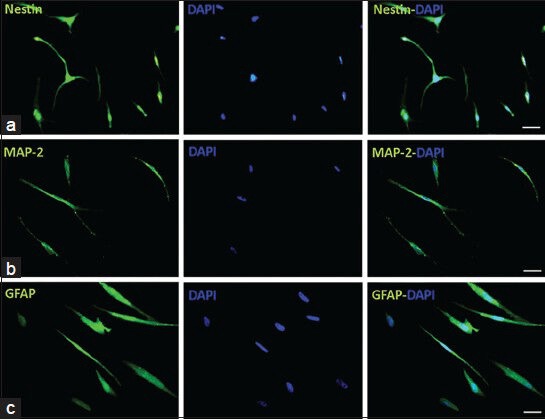
Immunocytochemistry of neural-like cells derived from human bone marrow mesenchymal stem cells. Cells were positive for Nestin, MAP-2 and GFAP, while they were more positive for MAP-2 compare with GFAP, cell nuclei were counterstained with DAPI (blue), scale bars: Nestin, MAP-2 and GFAP = 50 μm
Figure 3.
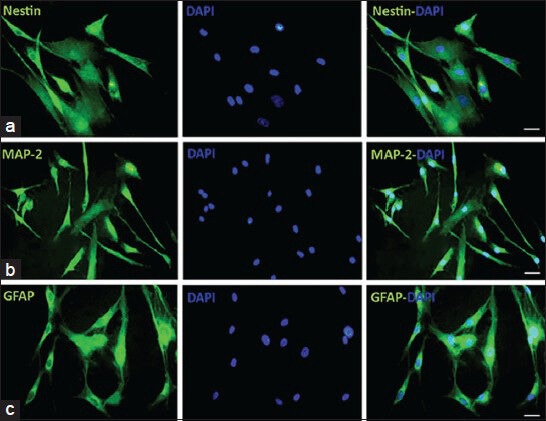
Immunocytochemistry of neural-like cells derived from human adipose-derived stem cells. Cells were positive for Nestin, Map-2 and GFAP in this cell population, the same as bone marrow mesenchymal stem cells derived cells, the numbers of GFAP positive cells were lower than Map-2 positive cells, cell nuclei are labeled with DAPI (blue). Scale bars: Nestin, MAP-2 and GFAP = 50 μm
As it has shown in Figure 4, the mean percentage of Nestin positive cells was 34.61% ± 3.72% in neural induced cells derived from BM, whereas the mean percentage of these cells was 36.11% ± 4.41% in neural induced cells derived from adipose tissue and there was no significant difference between these 2 groups (P > 0.05).
Figure 4.
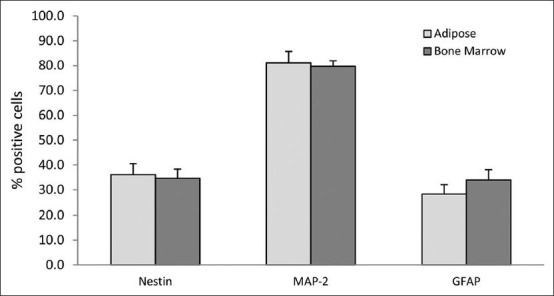
Comparative analysis between the mean percentages of immunoreactive positive cells for some neural cell's markers in differentiated cells derived from hBMSCs and hADSCs. As we can see there were no significant differences between these two kinds of cultures (P > 0.05)
Immunostaining analysis showed that the mean percentage of MAP-2 positive cells in the neural induced cells derived from adipose tissue was 81.11% ± 4.57%, which did not significantly differ from 79.72% ± 2.2% in neural induced cells derived from BM (P > 0.05).
Results of the mean percentage of GFAP positive cells in neural induced cells derived from BM and adipose tissue was exactly similar to the previous results, there was no significant difference between cells derived from these two kinds of tissues (P > 0.05), the mean percentage of GFAP positive cells in neural induced cells derived from BM was 33.94% ± 4.18% and the mean percentage of GFAP positive cells in neural induced cells derived from adipose tissue was 28.27% ± 3.85%.
As it has shown in Figure 4, there was no significant difference between the mean percentage of Nestin, MAP-2 and GFAP positive cells in differentiated cells derived from BMSCs and ADSCs.
Western blot
We used western blot analysis for monitoring the differentiation potential and neurogenic ability of BMSCs and ADSCs in protein expression levels of the neural differentiating cells.
Both differentiated groups showed expression of the protein MAP-2. There was no significant difference between differentiated BMSCs and ADSCs. For Nestin, both groups showed the expression of this protein and it seems there was no significant difference between both neural induced groups. In the GFAP test, the differentiated groups’ results were positive for GFAP expression [Figure 5a].
Figure 5.
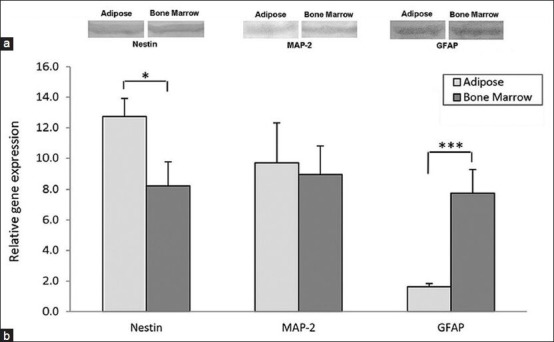
(a) Western blot analysis in order to detect neural cell proteins Nestin, MAP-2 and GFAP during neural differentiation of hBMSCs and hADSCs, after 2 weeks both bone marrow and adipose tissue derived cultures expressed these proteins and it seems with no significant differences, (b) comparative analysis of neural cells markers in differentiated cells derived from hBMSCs and hADSCs examined by real time RT-PCR. The expression of GFAP (***P < 0.001) and Nestin (*P < 0.05) genes had a significant differences but the expression of MAP-2 in these two kinds of induced cells was nearly the same (P < 0.05)
Based on these results, according to particular differentiation condition, specific markers of some neal-like cells showed a difference in the level of expression between the original stem cells and neural induced cells. Our data showed we succeeded in promoting the neurogenic potential of BMSCs and ADSCs and there was no significant difference between them.
Real time RT-PCR analysis
The increase in neural lineage related messenger RNA (mRNA) levels on neural induction was confirmed using real time RT-PCR technique. Real time RT-PCR analysis demonstrated positive expression of all three markers in differentiated cells from these two kinds of MSCs after 2 weeks, but there were some differences in the expression of these genes between them.
The results of one-way ANOVA analysis from real time RT-PCR technique showed that the average gene expression levels of Nestin in the adipose tissue derived samples 12.74 ± 1.18 was higher than the average of this gene expression in the samples derived from BM 8.24 ± 1.52 with a significant difference (P < 0.05) [Figure 5b].
The average gene expression levels of MAP-2 down-regulated in BM derived samples, this gene expression decreased from 9.7 ± 2.63 in adipose-derived samples to 8.94 ± 1.87 in the samples derived from BM, but this reduction was not significant (P > 0.05).
Unlike MAP-2 gene, expression of GFAP showed significant upregulation in BM derived samples (P < 0.001), the average of this gene expression was 1.62 ± 0.22 in adipose tissue derived samples while this was 7.76 ± 1.5 in the samples derived from BM.
According to these results it is obvious that although the expression levels of Nestin and GFAP was significantly different between neural induced cells from BMSCs and ADSCs, MAP-2 expression level was similar and we did not see any significant differences between them.
DISCUSSION
Our results demonstrated both kinds of MSCs produced a homogenous population and grew in a spindle-shaped, fibroblast-like morphology. Likewise, they behaved in the same manner in terms of expressing MSCs’ surface markers and they had the same doubling time. Furthermore, we found that hADSC cells can be induced to differentiate into cells with selected characteristics of neuronal and glial markers, exactly the same as cell derived from BM.
Until now scientists performed some comparative studies in order to learn about different characteristics of various MSCs and their advantages to each other, though there are few studies which have compared neural potential of ADSCs and BMSCs in human. The first one was done by De Ugarte et al., in which both kinds of cells isolated from the same patient. Their selected protocol for inducing neuronal changes was chemical one which is not suggested these days, despite this they did not observe any significant differences for yield of MSCs, growth kinetics, cell senescence, differentiation capacity and gene transduction efficiency, between these two cell populations.[26]
Furthermore, Krampera et al. described neural-like differentiation of human MSCs obtained from BM, fat, spleen and thymus, induced either with chemical factors or with co-culture with human Schwann cells. The process was transient and reversible, as MSCs recovered basal morphology and phenotype, as well as their multilineage differentiation potential. Their results show that a MSC reservoir is present in tissues other than BM and that MSCs of different origin have similar neural differentiation potential,[9] though they used completely different induction protocol, their results were consistent with ours, while according to our previous study[22] using a two-step neural differentiation procedure causes more stable expression of neural markers compare with chemical method of induction.
Zemelko et al. studied on neurogenic potential of human MSCs isolated from BM, adipose tissue and endometrium. It was shown that all three types of MSC cultures demonstrate multipotent plasticity and predisposition to neurogenesis. Their induction procedures were different and their emphasis was on the endometrial derived MSCs.[27]
Zavan et al. investigated this issue with regard to skin- and ADSCs. Though they induced neural differentiation through neurosphere formation using EGF and FGF-2, our final neural induction protocol had some more steps. Their results represented the spheres have been able to proliferate and differentiate to Schwann and glial-like cells.[28]
Most of the recent comparative studies are about other differentiation potentials of stem cells in animal models, though their results confirm ours in terms of similarity between stem cells. For example Radtke et al. performed an experiment in order to characterize equine muscle tissue- and periosteal tissue-derived cells as MSCs and evaluate their proliferation capacity and osteogenic potential in comparison with BM-and adipose tissue-derived MSCs. They found that equine muscle and periosteum which are sources of MSCs, have osteogenic potential comparable to that of equine adipose- and BM-derived MSCs, which could make them useful for tissue engineering applications in equine medicine.[29] In another study done by Jo et al., in 2013, in vivo osteogenic potential of adult MSCs from adipose tissue and BM was compared with fetal MSCs from umbilical cord and umbilical cord blood using a rat critical-sized femoral defect model. Based on quantitative assessment no significant difference was seen between adult MSCs and fetal MSCs.[30]
Likewise Ning et al. decided to induce neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells of the rats. They found that similar to ADSCs, vascular smooth muscle cells could also be induced to differentiate into neuron-like cells.[31]
Furthermore Yu et al. have assessed the myogenic potential of MSCs isolated from three different sources: BM, fetal tissue and adipose tissue. All kinds of stem cells, regardless of source, predominantly commit to myogenic lineage, as shown by the significant upregulation of myogenic gene markers and positive myosin heavy chain staining.[32]
A study by Karpov et al. performed a study which aimed to investigate the effect of BM-and adipose tissue-derived MSC transplantation on left ventricular (LV) function and infarct area in the rat model of ischemic heart failure. They showed treatment with both BMSCs and ADSCs ameliorates LV function and reduces histological scar size.[33]
Using a series of immunohistochemistry, Real time RT-PCR and western blotting experiments, we have found that hADSC and hBMSC cells can be induced to undergo morphologic and phenotypic changes consistent with developing neuronal and glial cells. These cells expressed several phenotypic properties of neuronal tissue, including the expression of Nestin, MAP-2 and GFAP. Expression of the markers in ADSC cells was very similar to the pattern of their expression seen in BMSC cells. Both undifferentiated hADSCs and BMSCs expressed neuronal progenitor marker Nestin at the levels of mRNA and proteins, similar to several other stem and progenitor cell populations,[17] demonstrating it cannot be used as a marker for assumed neurogenic potential. Furthermore, the low levels of MAP-2 and GFAP expression in undifferentiated ADSCs are consistent with the results reported by Jang et al.[34] which was exactly the same as the expression of these markers in BMSCs. According to these results it could be suggest that ADSCs may retain a native potential for neural differentiation.
Our experiments demonstrate that differentiated cells expressed increased immunoreactivities for neuronal marker MAP-2 and glial marker GFAP as well as the increased mRNA expression of these markers compared to undifferentiated stem cells; indicating that both ADSCs and BMSCs differentiate into neural cells through B27, b-FGF and EGF-mediated differentiation. The result by western blot analysis was coincident with immunocytochemistry and Real time RT-PCR.
As we know Nestin is expressed in neurons at an early stage of development and MAP-2 is expressed during the neuronal differentiation of neural precursor cells and the astrocyte marker GFAP is detectable during fetal glial development.[17,35] Treatment with the materials advised in our differentiation protocol resulted in the selective expression by MSCs of the neuronal-specific proteins Nestin, MAP-2 and GFAP, but we did not examined weather they can have any effect on the expression of Schwann or oligodendrocyte markers which needs to be considered in the future studies.
As we can see in the Figure 5, after differentiation process, despite the increase in GFAP marker expression fallowing neural induction in both cell groups which were used, expression of this marker in the cells derived from ADSCs was significantly lower than differentiated cells derived from BMSCs. Since the expression of MAP-2 (mature neurons marker) in differentiated cells from both groups was exactly the same, decreased expression of GFAP (glial cells marker) could be an important and very helpful point in the field of neural differentiation. According to the current results adipose tissue might be a better choice in order to isolate stem cells to use in the experiments need neural-like cells.
A normal mature neuron is defined by its polarity in cytology and specific protein expression in defined locations and also by its excitability, or its ability to fire action potentials and to communicate with other cells through the formation of functional synaptic structures.[36] Most studies rely upon morphological changes and neural markers gene expression at the mRNA or protein level. However, some previous studies performed electrophysiological analysis on induced MSCs.[37,38] In the present study, we did not examined electrophysiological analysis, therefore we cannot determine whether our protocol is efficient enough to do so and this subject still needs to be clarified.
CONCLUSIONS
Finally, we found these two kinds of stem cells in the presence of b-FGF, EGF and B27 differentiate into neural-like cells without any significant differences. Our results provide an acceptable assurance for our attempts to use ADSCs as a proper autologous adult stem cell population for cell replacement therapy. In spite of widespread advances in usage of MSCs, to increase the duration of cell differentiation to investigate differentiated cells functional applications in vivo, to determine the molecular mechanisms underlying the differentiation process and for evaluation of the easiest ways in order to achieve success in treating patients, there is a long way to go and further studies are recommended.
ACKNOWLEDGMENTS
The authors are grateful to Iranian Council of Stem Cell Technology, Isfahan University of Medical Sciences for financial support (Grant no. 189068). We declare no conflict of interest.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair – Current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–97. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 6.Pachón-Peña G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–51. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen M. Lineage of osteogenic cells and their relationship to the stromal system. Bone Miner Res. 1985;3:1–25. [Google Scholar]
- 8.Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–30. [PubMed] [Google Scholar]
- 9.Krampera M, Marconi S, Pasini A, Galiè M, Rigotti G, Mosna F, et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382–90. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Nadig RR. Stem cell therapy-Hype or hope? A review. J Conserv Dent. 2009;12:131–8. doi: 10.4103/0972-0707.58329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA. The adipose-derived stem cell: Looking back and looking ahead. Mol Biol Cell. 2010;21:1783–7. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safford KM, Rice HE. Stem cell therapy for neurologic disorders: Therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005;6:57–62. doi: 10.2174/1389450053345028. [DOI] [PubMed] [Google Scholar]
- 13.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 14.Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg. 2005;116:1453–60. doi: 10.1097/01.prs.0000182570.62814.e3. [DOI] [PubMed] [Google Scholar]
- 15.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 16.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: An overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 17.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int 2012. 2012 doi: 10.1155/2012/812693. 812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 21.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–23. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi N, Razavi S, Kazemi M, Oryan S. Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue Cell. 2012;44:87–94. doi: 10.1016/j.tice.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, et al. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: Misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeili A, Zaker SR. Differential expression of glycine receptor subunit messenger RNA in the rat following spinal cord injury. Spinal Cord. 2011;49:280–4. doi: 10.1038/sc.2010.109. [DOI] [PubMed] [Google Scholar]
- 26.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 27.Zemelko V, Kozhukharova I, Alekseenko L, Domnina A, Reshetnikova G, Puzanov M, et al. Neurogenic potential of human mesenchymal stem cells isolated from bone marrow, adipose tissue and endometrium: A comparative study. Cell Tissue Biol. 2013;7:235–44. [PubMed] [Google Scholar]
- 28.Zavan B, Michelotto L, Lancerotto L, Della Puppa A, D’Avella D, Abatangelo G, et al. Neural potential of a stem cell population in the adipose and cutaneous tissues. Neurol Res. 2010;32:47–54. doi: 10.1179/174313209X385743. [DOI] [PubMed] [Google Scholar]
- 29.Radtke CL, Nino-Fong R, Esparza Gonzalez BP, Stryhn H, McDuffee LA. Characterization and osteogenic potential of equine muscle tissue- and periosteal tissue-derived mesenchymal stem cells in comparison with bone marrow- and adipose tissue-derived mesenchymal stem cells. Am J Vet Res. 2013;74:790–800. doi: 10.2460/ajvr.74.5.790. [DOI] [PubMed] [Google Scholar]
- 30.Jo CH, Yoon PW, Kim H, Kang KS, Yoon KS. Comparative evaluation of in vivo osteogenic differentiation of fetal and adult mesenchymal stem cell in rat critical-sized femoral defect model. Cell Tissue Res. 2013;353:41–52. doi: 10.1007/s00441-013-1619-5. [DOI] [PubMed] [Google Scholar]
- 31.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–8. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu T, Chua CK, Tay CY, Wen F, Yu H, Chan JK, et al. A generic micropatterning platform to direct human mesenchymal stem cells from different origins towards myogenic differentiation. Macromol Biosci. 2013;13:799–807. doi: 10.1002/mabi.201200481. [DOI] [PubMed] [Google Scholar]
- 33.Karpov AA, Uspenskaya YK, Minasian SM, Puzanov MV, Dmitrieva RI, Bilibina AA, et al. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int J Exp Pathol. 2013;94:169–77. doi: 10.1111/iep.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A, et al. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004;117:4289–99. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- 36.Lu P, Tuszynski MH. Can bone marrow-derived stem cells differentiate into functional neurons? Exp Neurol. 2005;193:273–8. doi: 10.1016/j.expneurol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Padovan CS, Jahn K, Birnbaum T, Reich P, Sostak P, Strupp M, et al. Expression of neuronal markers in differentiated marrow stromal cells and CD133+stem-like cells. Cell Transplant. 2003;12:839–48. doi: 10.3727/000000003771000183. [DOI] [PubMed] [Google Scholar]
- 38.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]


