Abstract
Collecting lymphatic ducts contain intraluminal valves that prevent backflow. In mice, lymphatic valve morphogenesis begins at embryonic day 15.5 (E15.5). In the mesentery, Prox1 expression is high in valve-forming lymphatic endothelial cells, whereas cells of the lymphatic ducts express lower levels of Prox1. Integrin α9, fibronectin EIIIA, Foxc2, calcineurin and the gap junction protein Cx37 are required for lymphatic valve formation. We show that Notch1 is expressed throughout the developing mesenteric lymphatic vessels at E16.5, and that, by E18.5, Notch1 expression becomes highly enriched in the lymphatic valve endothelial cells. Using a Notch reporter mouse, Notch activity was detected in lymphatic valves at E17.5 and E18.5. The role of Notch in lymphatic valve morphogenesis was studied using a conditional lymphatic endothelial cell driver either to delete Notch1 or to express a dominant-negative Mastermind-like (DNMAML) transgene. Deletion of Notch1 led to an expansion of Prox1high cells, a defect in Prox1high cell reorientation and a decrease in integrin α9 expression at sites of valve formation. Expression of DNMAML, which blocks all Notch signaling, resulted in a more severe phenotype characterized by a decrease in valves, failure of Prox1high cells to cluster, and rounding of the nuclei and decreased fibronectin-EIIIA expression in the Prox1high cells found at valve sites. In human dermal lymphatic endothelial cells, activation of Notch1 or Notch4 induced integrin α9, fibronectin EIIIA and Cx37 expression. We conclude that Notch signaling is required for proper lymphatic valve formation and regulates integrin α9 and fibronectin EIIIA expression during valve morphogenesis.
Keywords: Notch, Lymphatic valve morphogenesis, Integrin α9, Mouse
INTRODUCTION
The lymphatic system consists of capillaries that uptake interstitial fluid and of collecting ducts that transport the fluid back into the bloodstream. Intraluminal valves in lymphatic collecting ducts ensure unidirectional flow. Mature lymphatic valves are made up of two leaflets that consist of an extracellular matrix (ECM) core surrounded by specialized valve lymphatic endothelial cells (LECs). In mice, lymphatic valve formation begins at embryonic day 15.5 (E15.5). LECs expressing high levels of the transcription factors Prox1 (Prox1high) and Foxc2 cluster at putative valve sites, distinguishable from ductal LECs that express low Prox1 (Prox1low). Connexin 37 (Cx37; Gja4 – Mouse Genome Informatics) is induced in clustered LECs and is required for proper valve morphogenesis (Kanady et al., 2011; Sabine et al., 2012). The Prox1high LECs organize into a ring-like structure that constricts the collecting duct, and then valve LECs invaginate into the lumen (Sabine et al., 2012). A valve leaflet ECM core consisting of laminin α5, collagen IV and fibronectin-EIIIA (FNEIIIA) is formed by the invaginating LECs, enabling leaflet elongation and maturation (Bazigou et al., 2009; Norrmen et al., 2009).
Molecular regulators of valve initiation and maturation are beginning to be understood. Genes encoding the lymphatic proteins Prox1, Foxc2, integrin α9, FNEIIIA and Cx37 are all highly expressed in lymphatic valves (Bazigou et al., 2009; Kanady et al., 2011; Norrmen et al., 2009; Petrova et al., 2004; Sabine et al., 2012). Calcineurin signaling and Cx37 are required to specify the sites of valve formation in the duct wall (Sabine et al., 2012). Interaction of integrin α9 with its ligand FNEIIIA allows for proper valve leaflet ECM core assembly and leaflet elongation. Cx37 is thought to help coordinate the valve-forming LECs into an organized valve structure (Bazigou et al., 2009; Sabine et al., 2012).
Notch signaling requires direct cell-cell contact between Notch ligand-presenting cells and receptor-expressing cells, and regulates cell-fate determination (Andersson et al., 2011). Ligand-induced proteolytic cleavage releases the Notch intracellular domain that translocates to the nucleus where it forms a transcriptional activation complex with CSL, Mastermind-like and co-activators (Borggrefe and Liefeke, 2012). Notch regulates lymphatic sprouting events in physiological and pathological settings (Niessen et al., 2011; Zheng et al., 2011). We have shown that Notch functions in embryonic venous endothelium to restrict the number of venous ECs that adopt the lymphatic fate (Murtomaki et al., 2013).
Notch mediates bi-potential cell fate decisions in multiple biological settings. Valve LECs must be specified from ductal LECs. We hypothesized that Notch may function in this process. Notch1 expression and Notch activation were observed in the valve-forming LECs. We used the Prox1CreERT2-inducible driver (Srinivasan et al., 2007) to delete Notch1 or to inhibit Notch signaling by expressing a dominant-negative Mastermind-like transgene (Tu et al., 2005) in LECs at the initiation of valve formation. Lymphatic endothelial-specific loss of Notch1 or Notch signaling resulted in abnormal Prox1high-Prox1low expression pattern, abnormal valve morphology and reduced integrin α9 and FNEIIIA expression in valve LECs. In human dermal lymphatic endothelial cells (HdLECs), Notch1 or Notch4 activation induced integrin α9, FNEIIIA and Cx37. We report a novel role for Notch in lymphatic valve formation through its regulation of the Prox1 high/low expression pattern, and integrin α9 and FNEIIIA expression.
RESULTS AND DISCUSSION
Notch1 expression and Notch activity became enriched in lymphatic valves
Notch1 expression was analyzed in embryonic mesenteric lymphatics. At E16.5, Notch1 was uniformly expressed in the mesenteric collecting lymphatic ductal endothelium (Fig. 1A). At E17.5, Notch1 expression was highest in the Prox1high LECs at putative valve sites (Fig. 1A). By E18.5, Notch1 expression was enriched in valve-forming LECs and weak Notch1 expression was seen in the collecting duct LECs (Fig. 1A, supplementary material Fig. S1). Notch1 expression was observed in adjacent blood vessels and capillaries.
Fig. 1.
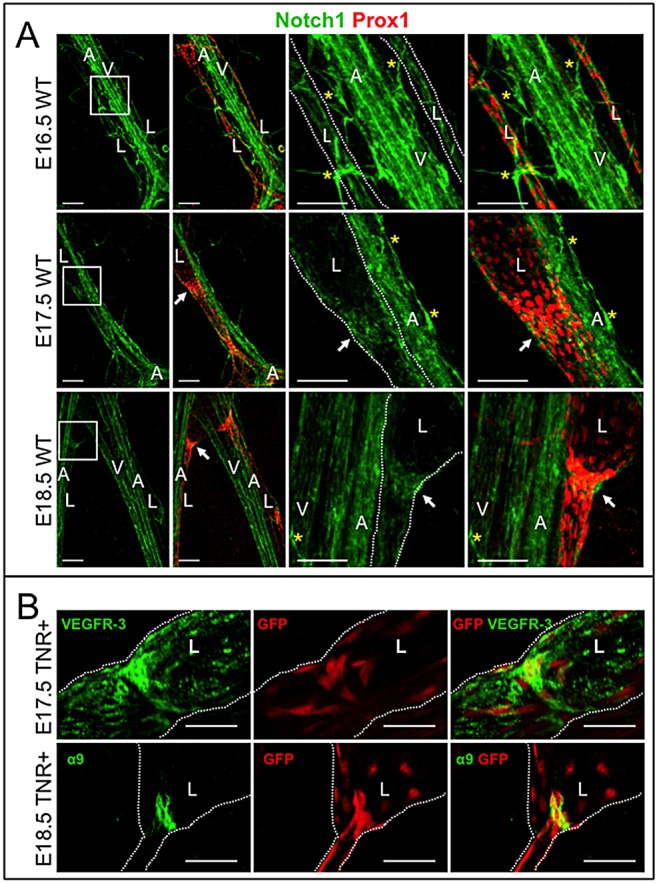
Notch1 expression and Notch activity in embryonic lymphatic duct and valve LECs. (A) E16.5, E17.5 and E18.5 wild-type mesenteries were stained for Notch1 and Prox1. Boxed areas are enlarged on the right. White arrows indicate lymphatic valves. Yellow asterisks mark blood capillaries. Scale bars: 100 µm. (B) TNR mesenteries were stained for GFP to detect Notch activity and VEGFR3 (E17.5) or α9 (E18.5). Scale bars: 50 µm. Lymphatic ducts are marked with white dotted lines. A, artery; L, lymphatic collecting duct; V, vein.
We assessed Notch signaling in mesenteric lymphatic vessels using the transgenic Notch reporter mouse (TNR), which expresses GFP in response to CSL-dependent Notch signaling. TNR mesenteries were stained for GFP and the lymphatic marker, vascular endothelial growth factor receptor 3 (VEGFR3) or integrin α9 (α9). At E17.5, Notch signal activation was seen in clusters of cells at the putative valve sites (Fig. 1B). LECs in the developing collecting ducts and blood vessels adjacent to the ducts also expressed GFP (data not shown). At E18.5, GFP expression was mainly detected in the α9-positive LECs of the lymphatic valves (Fig. 1B). Notch activity was highest in the valve LECs reoriented into lumen. The initial Notch1 expression and Notch activity in collecting duct LECs and subsequent enrichment in valve LECs suggests that Notch1 functions in lymphatic valve development.
Notch1 was required for lymphatic valve morphogenesis and integrin α9 expression
To examine the effect of LEC-specific loss of Notch1 on lymphatic valve development, Prox1CreERT2 drivers were crossed with mice carrying a floxed allele of Notch1 (N1fl/fl) to generate Prox1CreERT2;N1fl/fl (LOF) embryos. Recombination was induced with tamoxifen at E15.5, when murine valve morphogenesis begins, and embryonic mesenteries analyzed at E18.5 (supplementary material Fig. S2). Prox1CreERT2;N1fl/fl embryos were indistinguishable from control littermates (data not shown). Control mesenteric valve-forming LECs that were Prox1high, clustered and reoriented by at least 45° compared with the duct Prox1low LECs (Fig. 2A,B). The regions of Prox1high LECs were expanded in LOF mesenteric lymphatics (Fig. 2A). There was a significant increase in the number of Prox1high LECs normalized to ductal length in LOF mesenteric lymphatics compared with controls (Fig. 2D), indicative of a defect in Prox1high valve LEC clustering or an increase in the Prox1high LEC population. A significant decrease in reoriented Prox1high valve LECs was observed in the Prox1CreERT2;N1fl/fl mutants compared with controls (Fig. 2B,E).
Fig. 2.
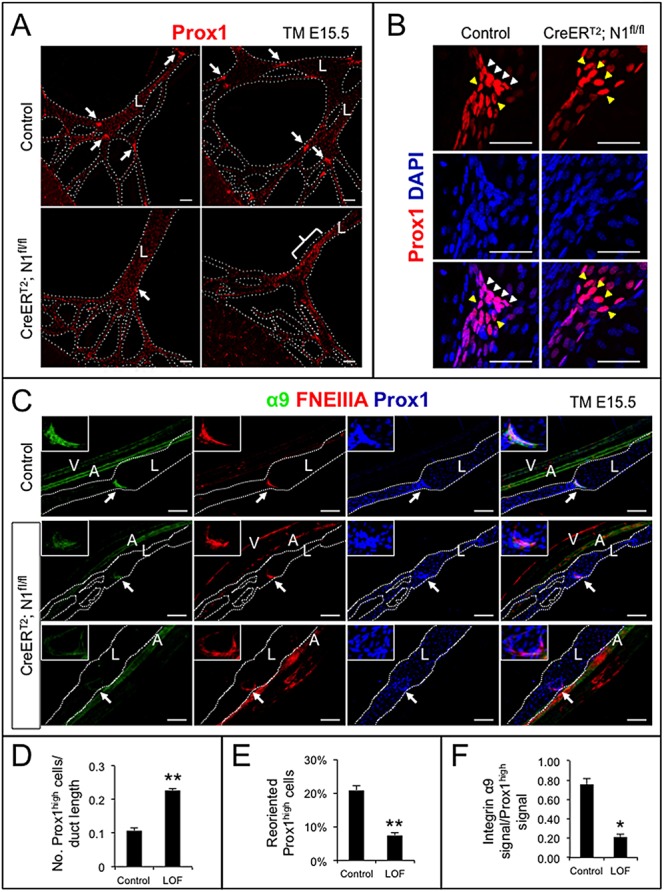
Lymphatic endothelial-specific loss of Notch1 resulted in defective valve morphogenesis and decreased integrin α9 expression. Notch1 was deleted in LECs at E15.5 with tamoxifen and mesenteries isolated at E18.5. (A) N1fl/+ (control) and Prox1CreERT2;N1fl/fl (CreERT2;N1fl/fl) mesenteries stained for Prox1. Arrows indicate Prox1high valves; bracket denotes expansion of Prox1high cells within the duct walls in Prox1CreERT2;N1fl/fl mesenteries. Scale bars: 100 µm. (B) Control and CreERT2;N1fl/fl mesenteries stained for Prox1 and DAPI. White arrowheads indicate Prox1high valve-forming LECs reoriented at least 45° to the duct wall. Yellow arrowheads indicate Prox1high LECs that have failed to reorient. Scale bars: 50 µm. (C) Control and CreERT2;N1fl/fl mesenteries stained for Prox1, α9 and FNEIIIA. White arrows indicate valves magnified in upper left corner. Scale bars: 100 µm. A, artery; L, lymphatic collecting duct; V, vein. (D) Quantification of number of Prox1high cells normalized by duct length. (E) Percentage of reoriented Prox1high cells in Prox1high valve clusters. (F) Quantification of α9-positive signal normalized to Prox1high signal in Prox1high valve LECs. Data are mean±s.e.m. *P<0.05, **P<0.005.
The total number of valves was similar between Prox1CreERT2;N1fl/fl and control mesenteric lymphatics (data not shown). However, the LOF valve leaflets were poorly organized (Fig. 2C). Formation of the lymphatic valve leaflets requires α9 and its ligand FNEIIIA (Bazigou et al., 2009). Prox1high cells of control mesenteric valves expressed α9 and FNEIIIA (Fig. 2C). By contrast, α9 expression was significantly lower in the LOF Prox1high LECs that had migrated into the lumen (Fig. 2F), suggesting that Notch1 regulates α9 expression in valve LECs. Clusters of valve-forming LECs were also reduced in the dermal lymphatics of LOF embryos (supplementary material Fig. S3).
The increase in the number of Prox1high LECs suggests that Notch1 functions to restrict the number of LECs that go on to become Prox1high. Notch1 may also function to cluster the Prox1high cells at sites of valve formation. Even when Prox1high LOF LECs had clustered, the cells failed to reorient and failed to express α9. The abnormal valve leaflets in the LOF mice had a phenotype similar to that described for Cx37 mouse mutants (Kanady et al., 2011; Sabine et al., 2012).
Loss of canonical Notch signaling severely disrupted valve formation and reduced FNEIIIA expression
We speculated that additional Notch proteins compensate for the loss of Notch1, partly based upon incomplete loss of FNEIIIA observed in the LOF valves. We addressed this hypothesis using a transgenic mouse line to express a dominant-negative Mastermind-like1/GFP fusion protein (DNMAML) (Tu et al., 2005) in Prox1-expressing LECs. DNMAML forms an inactive complex with Notch to inhibit canonical Notch/CSL signaling downstream of all Notch proteins without affecting CSL repressor function. DNMAMLfl/fl mice were crossed with the Prox1CreERT2 driver, tamoxifen was administered at E13.5 or E15.5, and mesenteries were collected at E18.5. GFP expression was detected in Prox1CreERT2;DNMAMLfl/+ mesenteric lymphatics (supplementary material Fig. S4), but not in control (DNMAMLfl/+) mesenteries (data not shown). Prox1CreERT2;DNMAMLfl/+ valve morphogenesis phenotype was more severe (Fig. 3) than the Prox1CreERT2;N1fl/fl phenotype (Fig. 2). Control mesenteries displayed the expected Prox1high-Prox1low pattern, with Prox1high LECs clustering at putative valve sites (Fig. 3A,B). Tamoxifen administration at E13.5 or E15.5 disrupted this pattern (Fig. 3A,B), correlating with significantly fewer Prox1high LECs in clusters in Prox1CreERT2;DNMAMLfl/+mesenteric lymphatics compared with control (Fig. 3D). The Prox1high LECs located at Prox1CreERT2;DNMAMLfl/+ valve sites displayed abnormally rounded nuclei, instead of elongated nuclei in control valve LECs (Fig. 3A,B). The overall number of Prox1high, α9 and FNEIIIA triple-positive valves per duct was significantly reduced in Prox1CreERT2;DNMAMLfl/+ mesenteries compared with controls (Fig. 3E). Many of the Prox1CreERT2;DNMAMLfl/+ mesenteric lymphatics had no identifiable lymphatic valves (Fig. 3C). When putative valves were observed in Prox1CreERT2;DNMAMLfl/+ lymphatics, the valves were disorganized and usually expressed only one of two valve markers, α9 or FNEIIIA (Fig. 3C). FNEIIIA expression in Prox1high valve LECs was absent in Prox1CreERT2;DNMAMLfl/+ mutants compared with controls (Fig. 3F). A significant loss of α9 expression was not achieved, largely owing to an insufficient number of identifiable valves that could be analyzed, suggesting that valve initiation was disrupted in the absence of α9 expression. As expected, α9 expression by smooth muscle cells around arteries and was not affected in Prox1CreERT2;DNMAMLfl/+mesenteries (Fig. 3C).
Fig. 3.

Lymphatic endothelial-specific loss of Notch signaling resulted in loss and defective valve formation and decreased FNEIIIA expression. DNMAML expression was induced in LECs at (A) E13.5 or (B,C) E15.5 with tamoxifen and mesenteries isolated at E18.5. (A,B) DNMAMLfl/+ (control) and Prox1CreERT2;DNMAMLfl/+ (CreERT2;DNMAMLfl/+) mesenteries stained for Prox1. White arrows indicate Prox1high regions magnified on the right. White arrowheads mark reoriented Prox1high LECs with typical elongated nuclei. Yellow arrowheads indicate Prox1high LECs that have not reoriented and/or display abnormal rounded nuclei. Scale bars: 100 µm. (C) Control and CreERT2;DNMAMLfl/+ mesenteries stained for Prox1, α9 and FNEIIIA. Middle row shows mutant with mild valve phenotype. Bottom row shows severely affected mesenteric lymphatic ducts with no discernible valves. White arrows indicate valves magnified in upper left-hand corner. Scale bars: 100 µm. A, artery; L, lymphatic collecting duct; V, vein. (D) Quantification of Prox1high cells located in clusters. (E) Quantification of Prox1high/α9/FNEIIIA triple-positive valves per duct. (F) Quantification of FNEIIIA-positive signal normalized to Prox1high signal in Prox1high valve LECs. (D-F) Prox1CreERT2;DNMAMLfl/+ (mutant) and DNMAMLfl/+ (control). Data are mean±s.e.m. *P<0.05, **P<0.005.
α9 and its ligand FNEIIIA are indispensable for valve leaflet formation (Bazigou et al., 2009). As α9 and FNEIIIA expression is lost in Notch signaling-deficient mesenteries with defective valves, we propose that Notch functions in valve morphogenesis by inducing expression of these proteins in valve LECs. Furthermore, the disruption of Prox1 expression pattern in Prox1CreERT2;DNMAMLfl/+mesenteries and failure of Prox1high cells to cluster at valve sites suggest that Notch regulates the establishment of a valve LEC identity and subsequent clustering and/or valve leaflet formation.
Notch induced integrin α9, FNEIIIA and Cx37 in human dermal lymphatic endothelial cells
Notch functions by eliciting transcriptional responses and it has been previously demonstrated that Notch suppresses Prox1 and induces VEGFR3 in HdLECs (Kang et al., 2010; Murtomaki et al., 2013). We sought to determine whether Notch regulates the expression of valve genes, α9, FNEIIIA, Cx37, calcineurin and Foxc2.
HdLECs were infected with an adenovirus encoding a constitutively active Notch1 intracellular domain (N1IC), a constitutively active allele of Notch4 (N4int3), lacZ or GFP (Shawber et al., 2007; Tung et al., 2009). N1IC or N4int3 induced α9 transcripts (Fig. 4A), whereas only N1IC increased α9/β1 surface expression (Fig. 4A). The absence of increased α9 surface in N4int3-expressing HdLECs may be due to its reduced activity relative to N1IC (supplementary material Fig. S5). The transcriptional repressors Hey1 and Hey2 did not affect α9 levels, suggesting that α9 is a direct target of Notch transcriptional activation or regulated indirectly by other Notch-induced proteins.
Fig. 4.
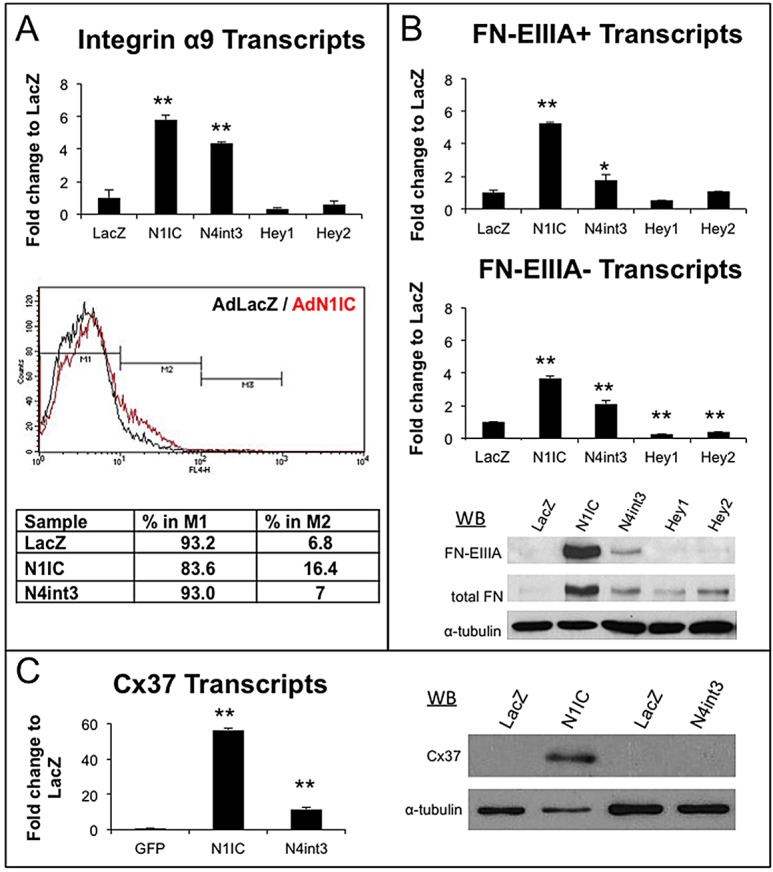
Notch activation induced integrin α9, FNEIIIA and Cx37 in HdLECs. HdLECs were adenovirally infected with N1IC, N4int3, Hey1, Hey2 or lacZ-coding viruses. RNA and protein isolated 48 h post-infection. (A) qRT-PCR for α9 and FACs for integrin α9/β1. (B) qRT-PCR for fibronectin transcripts with EIIIA (FN-EIIIA+) and without EIIIA (FN-EIIIA−). Western blots for FN-EIIIA, total fibronectin or α-tubulin. (C) qRT-PCR and western blot for Cx37. Data are mean±s.d. *P≤0.05, **P≤0.005.
Mammalian fibronectin consists of various isoforms, created through alternative splicing (Muro et al., 2003). FNEIIIA splice variant is expressed in the developing lymphatic valve ECM core (Bazigou et al., 2009). We evaluated transcript and protein levels for fibronectin with (FN-EIIIA+) or without (FN-EIIIA−) the EIIIA domain. Expression of N1IC or N4int3 increased levels of FN-EIIIA+ and FN-EIIIA− transcripts, and of total fibronectin and FN-EIIIA+ proteins (Fig. 4B). The antibody used to detect total fibronectin recognizes FN-EIIIA+ and FN-EIIIA− proteins, thus the increase in total fibronectin levels may be due to increased FN-EIIIA+ levels or to a combination of increased FN-EIIIA+ and FN-EIIIA− levels.
N1IC or N4int3 strongly induced Cx37 transcripts and protein in HdLECs (Fig. 4C). As Cx37 is required for reorientation of Prox1high LECs and lymphatic valve formation (Kanady et al., 2011; Sabine et al., 2012), the reorientation phenotype in the LOF models may be due to a reduction in Cx37 expression. Expression of N1IC or N4int3 modestly induced Foxc2 transcripts 1.5-fold (supplementary material Fig. S6A), whereas the calcineurin subunit Cnb1 was unaffected by Notch activation (supplementary material Fig. S6B).
Conclusions
We demonstrate that lymphatic specific loss of either Notch1 or inhibition of canonical Notch signaling with DNMAML disrupted lymphatic valve morphogenesis. Loss of Notch1 expanded the region of Prox1high cells in ducts, disrupted reorientation of Prox1high cells at valve sites, and reduced α9 expression by valve-forming LECs leading to poor valve leaflet formation. Inhibition of canonical Notch signaling resulted in a more severe valve phenotype than that observed for Notch1 deletion. Prox1CreERT2;DNMAML ductal lymphatics had significantly fewer valves, and a loss of Prox1high LEC clustering. Prox1high cells that did cluster at valve sites in Prox1CreERT2;DNMAML mutants had rounded nuclei and loss of FNEIIIA expression. In vitro studies with Notch1 or Notch4 activation suggest that Notch functions in part to induce α9, FNEIIIA and Cx37 expression in valve LECs.
We propose that Notch signaling functions in multiple steps of valve formation. Notch regulates Prox1high/low cell ratios and/or clustering to promote valve initiation or early steps in valve morphogenesis. Notch also promotes reorientation of Prox1high valve-forming LEC and induction of FNEIIIA and α9 in valve LECs, consistent with a role in valve morphogenesis. Thus, Notch signaling is essential for LECs to properly adopt the valve LEC fate.
MATERIALS AND METHODS
Mouse lines
Prox1CreERT2 (Srinivasan et al., 2007), Notch1fl/fl (Yang et al., 2004) and DNMAML (Tu et al., 2005) mice have been described previously. Notch1fl/fl, ROSA:LacZfl/fl and TNR mice were from Jax Labs. Tamoxifen in corn oil was administered via oral gavage or intraperitoneal injection (10 mg/40 g) to females at E13.5 or E15.5. Two to six litters were analyzed for each time-point.
Immunohistochemistry
Mesenteries collected at E16.5, E17.5 and E18.5 were fixed in 4% paraformaldehyde, blocked in 5% donkey serum, 0.2% BSA, 0.5% Triton-X100 and PBS, and incubated overnight with primary antibodies (supplementary material Table S1). Mesenteries were washed, incubated with secondary Alexa Fluor antibodies (Invitrogen) diluted in 0.2% BSA, 0.3% Triton-X100 and PBS, and washed and mounted with Vectashield with DAPI (Vector Labs). MOM kit (Vector Labs) was used with mouse monoclonal antibodies. Images were acquired using a laser scanning confocal Zeiss LSM 510 Meta microscope and LSM software, or a Nikon A1 confocal microscope and NIS Elements software.
Constructs and cell culture
HdLECs isolated from human neonatal foreskins (Columbia IRBAAAB-1700) were maintained in EGM-2MV BulletKit (Lonza) with VEGFC (10 ng/ml, RnD) (Murtomaki et al., 2013). HdLECs were infected with adenovirus expressing constitutively active Notch1 (N1IC) or Notch4 (N4int3), or Hey1, Hey2, lacZ or GFP (Shawber et al., 2007; Tung et al., 2009). Expression verified by qRT-PCR and/or western blot analyses.
RT-PCR, western blots and flow cytometry
HdLEC were assessed 48 h after adenoviral infections. RNA was isolated (RNeasy Mini Kit, Qiagen) and cDNA synthesized (Verso cDNA synthesis kit, Fisher). qPCR performed with Sybr Green Master Mix (ABI) or Absolute Blue qPCR SYBR Green ROX Mix (Fisher) using 7300 Real-Time PCR System (ABI). Gene-specific PCR products cloned into pDrive (Stratagene) served as standards. β-Actin was used to normalize qRT-PCRs. Protein was isolated in TENT lysis buffer (Shawber et al., 2007) and westerns were performed. HdLECs were incubated with anti-integrin α9β1 antibody (abcam), followed by anti-rabbit-APC (Jackson Immunoresearch). Ten-thousand cells per group were counted using FACSCalibur and CellQuestPro acquisition software (BD Biosciences). Antibody and primer details can be found in supplementary material Tables S1 and S2.
Statistical analysis
Image J used to quantitate phenotypes. Number of embryos and images analyzed summarized in supplementary material Table S3. Number of Prox1high cells was normalized to μm duct length. Prox1high cell threshold was determined for control ducts and applied to all images analyzed. Percentage of Prox1high cells located in clusters was determined. A valve cluster was defined as a group of Prox1high cells in which the nuclei appear to be in close contact with each other. Percentage of Prox1high cells in Prox1high clusters reorienting at least 45° to the duct wall was determined. Number of Prox1/α9/FNEIIIA triple-positive valves determined per duct. α9- and FNEIIIA-positive signal was normalized to Prox1high signal at site of valve formation. Significance determined using two-tailed Student's t-test. In vitro data represents three or more independent experiments.
Supplementary Material
Acknowledgements
Images were collected in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
J.K. and C.J.S. provided guidance and funded the studies. A.M. wrote the manuscript with contributions from C.J.S. J.K. and M.K.U. Experiments were performed by A.M., M.K.U., C.K., J.Z. and T.N.
Funding
The Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University is supported by a NIH grant [P30 CA013696]. The confocal microscope was purchased with a National Institutes of Health (NIH) grant [S10 RR025686]. Work was supported by the NIH [R01CA136673 (J.K. and C.J.S.), R01HL112626 (J.K.) and R21 EB016515-01 (C.J.S.)], by a DOD pre-doctoral fellowship [W81XWH-10-1-0304 (M.K.U.)] and by the Research to Prevent Blindness and Eye Surgery Fund (T.N. and J.Z.). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.101188/-/DC1
References
- Andersson E. R., Sandberg R., Lendahl U. (2011). Notch signaling: simplicity in design, versatility in function. Development 138, 3593-3612 10.1242/dev.063610 [DOI] [PubMed] [Google Scholar]
- Bazigou E., Xie S., Chen C., Weston A., Miura N., Sorokin L., Adams R., Muro A. F., Sheppard D., Makinen T. (2009). Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 17, 175-186 10.1016/j.devcel.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe T., Liefeke R. (2012). Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 11, 264-276 10.4161/cc.11.2.18995 [DOI] [PubMed] [Google Scholar]
- Kanady J. D., Dellinger M. T., Munger S. J., Witte M. H., Simon A. M. (2011). Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 354, 253-266 10.1016/j.ydbio.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Yoo J., Lee S., Tang W., Aguilar B., Ramu S., Choi I., Out H. H., Shin J. W., Dotto G. P., Koh C. J., Detmar M., Hong Y. K. (2010). An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood 116, 140-150 10.2353/ajpath.2008.080378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro A. F., Chauhan A. K., Gajovic S., Iaconcig A., Porro F., Stanta G., Baralle F. E. (2003). Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 162, 149-160 10.1083/jcb.200212079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A., Uh M. K., Choi Y. K., Kitajewski C., Borisenko V., Kitajewski J., Shawber C. J. (2013). Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 140, 2365-2376 10.1242/dev.083865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen K., Zhang G., Ridgway J. B., Chen H., Kolumam G., Siebel C. W., Yan M. (2011). The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood 118, 1989-1997 10.1182/blood-2010-11-319129 [DOI] [PubMed] [Google Scholar]
- Norrmen C., Ivanov K. I., Cheng J., Zangger N., Delorenzi M., Jaquet M., Miura N., Puolakkainen P., Horsley V., Hu J., et al. (2009). FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 185, 439-457 10.1083/jcb.200901104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova T. V., Karpanen T., Norrmén C., Mellor R., Tamakoshi T., Finegold D., Ferrell R., Kerjaschki D., Mortimer P., Ylä-Herttuala S., et al. (2004). Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 10, 974-981 10.1038/nm1094 [DOI] [PubMed] [Google Scholar]
- Sabine A., Agalarov Y., Maby-El Hajjami H., Jaquet M., Hägerling R., Pollmann C., Bebber D., Pfenniger A., Miura N., Dormond O., Calmes J. M., et al. (2012). Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 22, 430-445 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- Shawber C. J., Funahashi Y., Francisco E., Vorontchikhina M., Kitamura Y., Stowell S. A., Borisenko V., Feirt N., Podgrabinska S., Shiraishi K., et al. (2007). Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J. Clin. Invest. 117, 3369-3382 10.1172/JCI24311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R. S., Dillard M. E., Lagutin O. V., Lin F.-J., Tsai S., Tsai M.-J., Samokhvalov I. M., Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422-2432 10.1101/gad.1588407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L., Fang T. C., Artis D., Shestova O., Pross S. E., Maillard I., Pear W. S. (2005). Notch signaling is an important regulator of type 2 immunity. J. Exp. Med. 202, 1037-1042 10.1084/jem.20050923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. J., Hobert O., Berryman M., Kitajewski J. (2009). Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis 12, 209-220 10.1007/s10456-009-9139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Klein R., Tian X., Cheng H.-T., Kopan R., Shen J. (2004). Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev. Biol. 269, 81-94 10.1016/j.ydbio.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Zheng W., Tammela T., Yamamoto M., Anisimov A., Holopainen T., Kaijalainen S., Karpanen T., Lehti K., Ylä-Herttuala S., Alitalo K. (2011). Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118, 1154-1162 10.1182/blood-2010-11-317800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


