Abstract
Over the past several years we have obtained considerable evidence indicating that Ad-IFNα can overcome resistance to the IFNα protein itself. Since cancer cells infected with Ad-IFNα also show high perinuclear cytoplasmic IFNα expression we were interested in whether endoplasmic reticulum (ER) stress and cleavage of caspase 4 could play a major role Ad-IFNα produced cancer cell death.
Indeed procaspase 4 was upregulated and cleaved as early as 12 hours after Ad-IFNα infection of the cancer cells which co-localized with IFNα staining and ER tracker. In contrast immortalized normal human urothelial cells, although exhibiting similar perinuclear IFNα staining showed no cleaved caspase 4. Caspase 4 cleavage was not blocked by the caspase 8 specific inhibitor z-IETD, indicating that caspase 4 activation was independent of caspase 8 activation. Blocking caspase 4 also inhibited activation of caspase 3 in Ad-IFNα containing cells. Finally, the cleaved form of caspase 4 (p10) was detected in Ad-IFNα positive cancer cells from the urine of a patient following intravesical Ad-IFNα/Syn3 treatment. Therefore ER stress and activation of caspase 4 appears to be an important mechanism involved in the direct cancer cell death produced by Ad-IFNα and also occurs in the clinical setting.
Keywords: Adeno-viral mediated interferon α, ER Stress, caspase 4 activation
INTRODUCTION
Bladder cancer is the second most common genitourinary malignancy and the fifth leading cause of cancer deaths in American men. Approximately 70% to 80% of patients with newly diagnosed bladder cancer present with superficial bladder tumors. Although most cases of bladder cancer can be adequately managed with periodic transurethral resection (TUR), intravesical Bacillus Calmette-Guerin (BCG) therapy and surveillance, this treatment is far from optimal since approximately 50% of superficial tumors recur after TUR and BCG therapy and up to 30% evolve into more aggressive, potentially lethal cancers. Clearly, more effective therapies are necessary to improve overall survival and provide an alternative to radical cystectomy.
We, in collaboration with others, have well documented that prolonged, high concentrations of IFNα occur after Ad-IFNα exposure both in cell culture and in vivo after intravesical instillation of Ad-IFNα/Syn3 (1–2). Preclinical studies also showed considerable potential for intravesical treatment with Ad-IFNα in the presence of Syn3 as an excipient for superficial bladder cancer (1–2). In addition it has been documented that Ad-IFNα has unique cytotoxic properties distinct from those of the IFNα protein and that Ad-IFNα can overcome resistance to IFNα itself (1–4). This latter finding could be quite important because many human bladder and other cancer cell lines have been shown to be resistant to the IFNα protein (5) and therefore one might assume there will be different levels of sensitivity the IFNα-produced cell death in various tumors. In addition, Ad-IFNα has little or no cytotoxicity to normal cells.
These studies have lead to a Phase 1 study with intravesical Ad-IFNα/Syn3 for BCG refractory superficial bladder cancer which has recently been completed (6). However, the molecular mechanisms by which Ad-IFNα kills cancer cells resistant to the IFNα protein itself needs further study and is a high priority for our ongoing research. We have shown there are two major cytotoxic effects in cancer cells that are resistant to the IFN protein (7). The first involves a strong bystander effect and the second is associated with Ad-IFNα infection, subsequent high levels of intracellular IFNα produced and the resultant accumulation within the ER-Golgi network. The second cause of cell death is the focus of our present study.
We initially noted that various cancer cell types infected with Ad-IFNα produced high perinuclear cytoplasmic IFNα expression (1, 3). Since the ER is also localized in this region and caspase 4 is an ER-resident caspase known to be implicated in ER stress-induced apoptosis it became of interest to determine if endoplasmic reticulum (ER) stress and resultant caspase 4 activation occurred after Ad-IFNα treatment. This in turn could play a major role in the direct effect of Ad-IFNα produced cancer cell death in Ad-IFNα resistant cells. ER stress leads to caspase activation through various mechanisms triggering an evolutionarily conserved series of signal-transduction events, which constitutes the unfolded protein response. Although these signaling events aim to ameliorate the accumulation of unfolded proteins in the ER when these events are severe or protracted they can induce cell death.
We report that caspase 4 cleavage is a very early marker of Ad-IFNα induced ER stress and when inhibited by caspase 4 targeted RNAi downstream activation of caspase 3 is blocked. In contrast no caspase 4 cleavage occured after Ad-IFN α infection of normal urothelial cells. Moreover, cleaved caspase 4 and perinuclear IFN α were seen in tumor cells from the urine following intravesical Ad-IFNα/Syn3 treatment (6).
MATERIALS AND METHODS
Cell lines
The bladder cancer cell line, UM-UC9, was provided by Dr Barton Grossman. The KU7 cell line was also obtained from Dr. Grossman who originally received the cells from Dr. M. Tachibana, Keio University, Japan (8) and were used for some of our experiments. The KU7 cell line was initially thought to be a bladder cancer cell line but recent mapping results from cells obtained from Japan and stocks we had available have shown the cells to be HeLa. Therefore they will be referred to as HeLa cells hereafter. This recent finding, however, did not effect any results or conclusions reached but rather extend them to other cell types. The UM-UC-9 and HeLa cell lines were grown in MEM and RPMI 1640, respectively, in 10% fetal bovine serum and incubated at 37°C in 5% CO2 and 95% air. The telomerase immortalized normal urothelial cell line (hTERT-NHUC) was provided by Dr Margaret Knowles and grown in K-SFM medium with cholera toxin (9).
Reagents and adenovirus Infection
Ad-IFNα2b (Ad-IFNα) was obtained from Merck (formerly Canji/Schering-Plough). Cells were exposed to the adenoviral vector at a 50 or 100 multiplicity of infection (50–100 MOI) for 3 hr in medium without serum. The virus was then removed and complete medium added. Transfection frequency was checked by Ad-IFNα staining in order to insure the transfection rates were comparable in different experiments. Intron A was obtained from Schering-Plough Corporation, Kenilworth, N.J.
Western blotting
The cancer cells were transfected with a 50–100 MOI of Ad-IFNα. Occasionally the cells were treated with 25 µM of the caspase 8 specific inhibitor zIETDfmk purchased from Enzyme Systems Products (Livermore, CA). Cells which were still attached at each time point were lysed in SDS buffer [150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris (pH 8.0)], and protease inhibitors (Roche Diagnostics)], subjected to 15% SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were blotted with the following antibodies: cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 (Cell Signaling Technology, Beverly, MA and caspase 4 (Abcam, Cambridge, MA). Equal loading was confirmed by blotting with anti-β-Actin antibody (Sigma, St. Louis, MO). The blots were incubated with chemiluminescent substrate (Amersham, Piscataway, NJ) and then developed by exposure to x-ray film.
Immunochemical analysis
For interferon studies immunochemistry was done according to previously described methods (1). Briefly, cancer cells were seeded on cover-slips in 6-well plate and treated with Ad-IFNα. 48 hrs following infection the cells were fixed with 4% formalin and treated with 0.3% hydrogen peroxide in absolute methanol for 30 min to block endogenous peroxidase activity, followed by antigen retrieval. Cells were incubated overnight with rabbit polyclonal antibody against human IFNα at a 1:500 dilution (PBL, Piscataway, NJ, USA) and then stained using the Vector ABC Elite Kit protocol.
Confocal microscopy analysis for active caspase 4, caspase 3, IFNα and ER localization
At various times post-infection, the cells were fixed in ice-cold methanol, blocked in PBS with 10% normal donkey serum and incubated overnight with a polyclonal sheep antibody against IFNα (PBL, Piscataway, NJ) diluted 1:1000 in PBS with 1% donkey serum, with polyclonal goat antibody against cleaved caspase-4 (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:500, and monoclonal antibody against KDEL/Grp78 (Stressgen, Ann Arbor, MI) for ER tracking, diluted 1:200. In some experiments, a polyclonal rabbit antibody against cleaved caspase 3 was used for caspase 3 staining (Cell Signaling Technology, Beverly, MA).
Cells were washed three times in PBS, then incubated for 1 h with an Alexa-546-labeled donkey anti-mouse antibody, Alexa-488-labeled donkey anti-rabbit antibody and Alexa-647-labeled donkey anti-goat antibody (Molecular Probes, Eugene, OR), as well as the DNA dye DAPI (Molecular Probes, Eugene, OR) or Topro3 (Invitrogen, San Diego, CA) Digital micrographs were acquired using serial laser excitation on a Leica TCS SP5 laser scanning confocal microscope, and then were imported into a computer for processing using the Leica Application Suite (LAS) Microscope Software. In order to get rid of the background noise, signal thresholds were set up according to corresponding control slides. Selected images are representative of at least 10 microscopic fields analyzed for each condition.
Silencing of caspase 4 with an RNA interference (RNAi)
A human caspase 4 RNA interference (RNAi) (5'ac aaccgcaact gcctcagtct gaa3')and non-targeting universal control RNAi were purchased from Invitrogen, San Diego, CA. Transfections were performed using Lipofectamine RNAiMax (Invitrogen, San Diego ,CA) according to the manufacturer's instructions. Silencing of caspase 4 was evaluated by realtime RT-PCR and Western blotting comparing samples transfected with a non-targeting universal control and caspase 4 targeting RNAi.
Collection of cells from urine
Briefly, 20 ml of urine from a patient treated seven days previously with intravesical Ad-IFN/Syn3 (6) was pelleted by centrifugation at 400 × g for 10 minutes and the supernatant discarded. The cell pellets was re-suspended in 1 ml of normal saline and cells were deposited on poly-L-lysine-coated microscope slides using a cytospin centrifuge. Slides were air-dried for one hour, fixed with acetone briefly and then stored at 4°C. Hematoxylin-eosin staining was performed to identify bladder cancer cells in the patient’s urine samples. In addition, immunofluorescent staining was carried out as described as above to examine the expression of IFNα and whether evidence of cleaved caspase 4 was present using confocal microscopy.
RESULTS
Caspase 4 is cleaved in IFNα protein resistant cancer cells following Ad-INFα treatment but not normal urothelial cells and is an early molecular event of apoptosis mediated by Ad-IFN α
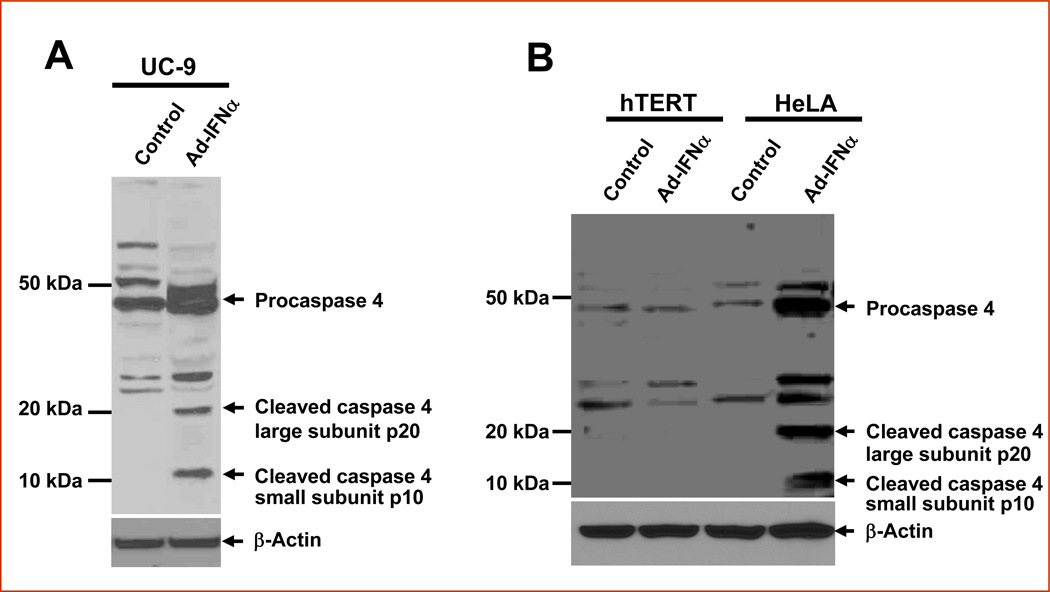
Cancer cells infected with Ad-IFNα show high perinuclear cytoplasmic IFNα expression and led to our examination of whether endoplasmic reticulum (ER) stress could play a major role in Ad-IFNα produced cell death in such cancer cells. Because caspase 4 is an ER-resident caspase known to be implicated in ER stress-induced apoptosis, we decided to investigate whether or not caspase 4 is activated following the Ad-INFα treatment. By Western analysis the activation of caspase 4 was clearly detected in the IFNα resistant UC9 bladder cancer cells (Fig.1a) and cervical cancer HeLa cells but not normal urothelial cells (Fig.1b) 48 hrs after Ad-INFα treatment using a specific antibody which recognizes both the large unit (p20) and small unit (p10) of cleaved caspase 4 as well as the procaspase 4.
Fig. 1.
Activation of caspase 4 mediated in the IFNα-resistant cancer cell lines 48 hrs after Ad-INFα treatment. The cells were harvested for Western analyses using antibodies against caspase-4 active cleaved fragment (Mr ~ 20 000 and Mr ~ 10 000) and the procaspase 4 (Mr ~ 46 000) which were shown as indicated. β-Actin was used as a control for equal loading. A, UC-9 bladder cancer cells show typical caspase 4 cleavage. B, HeLa cells also show similar caspase 4 cleavage whereas none is apparent in normal human urothelial cells (hTERT-NHUC).
There are several mechanisms involved in Ad-IFNα -induced bladder cancer cell death (6). First, both normal and bladder cancer cells produce high and prolonged levels of IFNα after Ad-IFNα transfection and expression. If the cancer cells are sensitive to IFNα, they will undergo an apoptotic cell death, largely through a TRAIL-related, caspase-8-dependent mechanism. If the cancer cells are resistant to IFNα, they could be killed by either bystander factors or by direct cytotoxicity caused by the high levels of Ad-IFNα produced. In order to define whether caspase 4 activation was involved only in cells showing high perinuclear IFNα levels or bystander produced cytotoxicity as well, we examined the effects of Ad-IFNα on caspase-4 processing by immunofluorescence staining and confocal microscopy using both UC-9 and HeLa cells. The cleaved caspase 4 small subunit p10 was only found in Ad-IFNα-expressing cells (Fig.2a) indicated that ER stress induced apoptosis and activation of caspase 4 might be the mechanism involved only in the direct cytotoxicity caused by Ad-INFα and that the bystander effect of Ad-INFα could be triggered by other unknown pathway(s), which is currently under investigation in our laboratory. In addition both Ad-INFα and activated caspase 4 were found to be co-localized with the enhanced ER signals shown by using ER tracker, KDEL (Grp78, Grp94), a retrieval motif essential for the precise sorting of KDEL proteins along the secretory pathway which perform critical functions in the ER related to protein folding as well as assembly (Fig. 2a).
Fig. 2.
The cleaved caspase-4 small subunit p10 occurs early in IFNα expressing cells which co-localizes with the ER. A, Cleaved caspase-4 cells (red) are seen in only IFNα expressing cells (green) 48 h after Ad-IFNα treatment and both co-localize with the enhanced ER signals (blue).
B, The small subunit p10 of caspase 4 (green) was detected in the Ad-IFNα transfected cells (red) 12 hr after Ad-IFNα infection whereas caspases 3 was not activated at the same time point. At 24hr after Ad-IFNα treatment, both cleaved caspase 4 (green) and caspase 3 (blue) forms were stained. The active form of caspase 3 was detected in both Ad-IFNα-expressing (solid arrow) and non-expressing cells (dashed arrow) whereas caspase 4 was only seen in IFNα expressing cells. This indicates that caspase 4 was activated only in cells which were infected with Ad-IFNα whereas caspase 3 was activated by both Ad-IFNα infection and bystander factors. Nuclei were counterstained with DAPI (grey-white). HeLa cells are shown in this experiment but similar results were seen in UC-9 cells. Original Magnification × 400.
Our previous study showed the proteolytic processing of caspases 3, 8 and 9 occurred after Ad-IFNα treatment in cancer cells (3). These observations were extended by directly assessing the proteolytic processing of caspase 4 over time following Ad-IFNα treatment. As shown in Figure 2b, p10 initially appeared in the Ad-IFNα transfected cells at 12 hr after Ad-IFNα treatment whereas caspase 3 was not proteolytically processed at the same time point, indicating that caspase 4 activation could be an early molecular event of apoptosis mediated by Ad-IFNα. Caspase 3 was activated subsequently 28 hr after Ad-IFNα treatment and this active form of caspase 3 was detected in both Ad-IFNα expressing and non-expressing cells by immunofluorescent confocal microscopy (Fig.2b), consistent with our previous studies on caspase 3 activation which showed that both direct infection of Ad-IFNα and the bystander factors produced caused caspase 3 activation (1, 3, 4).
Western analysis also indicated that proteolytic processing of caspases 4 appeared as early as 12 hr after Ad-IFNα treatment with activation of caspase 3 and 9 occurring at approximately 28 hr after Ad-IFNα infection (Fig.3). The caspase 4 and caspase 3 results were consistent with the results obtained by confocal analysis (Fig. 2b).
Fig. 3.
Upregulation and activation of caspase 4 mediated by Ad-IFNα measured at different time points in UC-9 and compared with activation of caspases 3 and 9. The cells were harvested for Western analyses using antibodies against caspase-4 active cleaved fragment (Mr ~ 20 000 and Mr ~ 10 000) and the procaspase 4 (Mr ~ 46 000) which were shown as indicated. The same blot was stripped and reprobed with antibody against the active caspase 3 (Mr ~ 17,000) and caspase 9 (Mr ~ 37,000)forms. β-Actin was used as a control for equal loading. Caspase 4 activation was seen as early as 12–18 hrs after Ad-IFN infection whereas caspase 3 and caspase 9 occured only 28hrs after Ad-IFN infection. Procaspase 4 also increased over time.
Confocal analysis shows caspase 4 activation is independent of caspase 8 activation in cancer cells and is not present in normal urothelial cells after Ad-IFNα infection
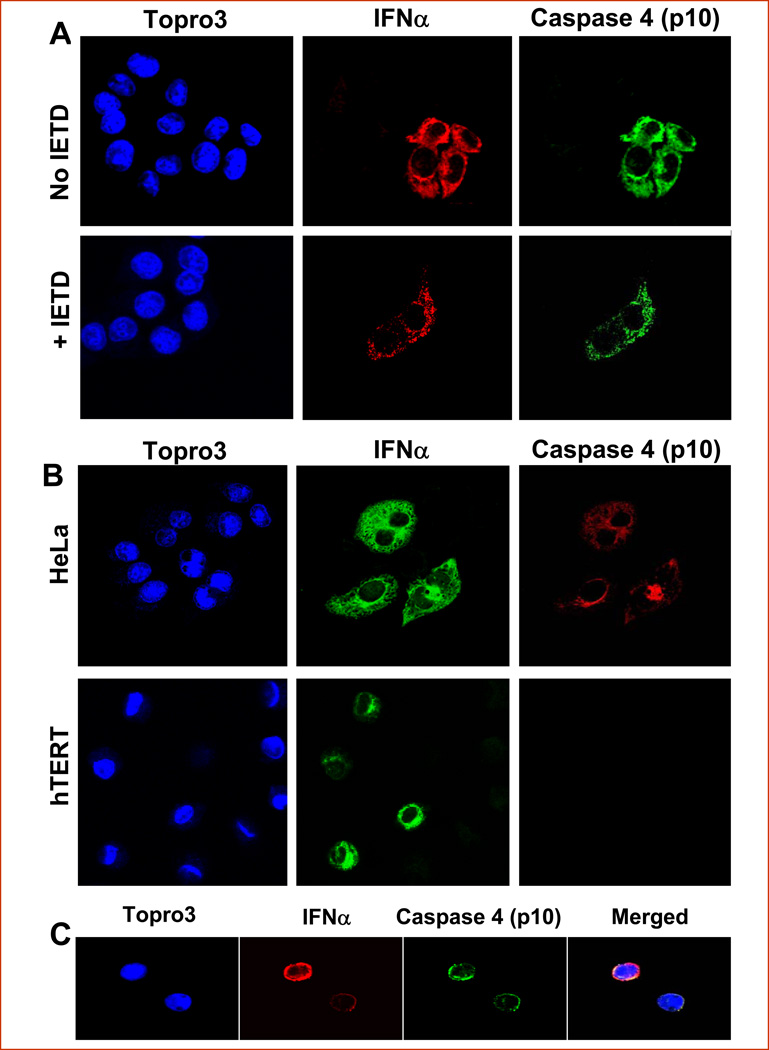
To define the role of caspase 8 in the proteolytic processing of caspases 4, we added the specific inhibitor zIETDfmk to completely block caspase 8 at the concentration and conditions used in our laboratory. As shown in Figure 4a, p10 was still detected at 48 hrs after Ad-INFα treatment in the presence of zIETDfmk, indicating that the inhibitor had little or no effect on activation of caspase-4 and that Ad-IFNα mediated apoptosis occurs via a mechanism that was independent of caspase-8 activation. Parallel Western immunoblotting experiments confirmed that the inhibitor completely blocked TRAIL-induced caspase 8 activation at the concentrations employed (data not shown).
Fig. 4.
A specific inhibitor for caspase 8, IETD, does not effect activation of caspase-4 nor is caspase 4 activated in normal urothelial cells after Ad-IFNα infection but is seen in tumor cells from a patient following intravesical Ad-IFNα treatment. A. A, Activated caspase 4 (p10) (green) was detectable in both presence and absence of the specific caspase 8 inhibitor zIETDfmk in the Ad-IFN (red) transfected HeLa cells 48 hrs after Ad-INFα treatment. B, HeLa cells 48 hrs after transfection with Ad-IFNα were used as positive control (Top). Activated caspase 4 (p10) (red) was seen in Ad-IFNα (green) transfected HeLa cells whereas it was not detectable in the Ad-IFNα transfected hTERT-NHUC cells at the same time point (Bottom). The nuclei were counterstained with Topro 3 (blue). C, The active form of caspase 4 (p10) was detected in exfoliated bladder cancer cells in voided urine from a patient undergoing intravesical Ad-IFNα treatment (6). A urine sample was collected from a patient 7 days after receiving Ad-IFNα Syn3 treatment and centrifuged briefly. The exfoliated cells were smeared on slides and fixed instantly with acetone. Cleaved caspase 4 (p10) (green) was clearly detected in IFNα (red) containing bladder cancer cells. The nuclei were counterstained with Topro 3 (blue). Original magnification × 400.
We previously reported that Ad-IFNα was cytotoxic to cancer cell lines, whereas it was not cytotoxic to normal urothelium (4). As shown in Figure 4b, Ad-IFNα infection did not induce the activation of caspase 4 in normal urothelial cells, consistent with the Western blot results (Fig. 1b). No detectable cleaved caspase 4 was found even though the urothelial cell line hTERT-NHUC had similar adenoviral infection efficiency and Ad-IFNα infected normal urothelial cells showed similar high amounts of perinuclear interferon α expression.
Caspase 4 activation was confirmed in exfoliated bladder cancer cells in voided urine from a patient undergoing intravesical Ad-IFNα/Syn3 treatment
An intravesical Phase l study using Ad-IFNα has recently been completed for superficial bladder cancer (6). Based on the results obtained we thought it could be informative to examine the status of caspase 4 in exfoliated bladder cancer cells in voided urine from a patient in this clinical trial. Urine was collected from this patient 7 days after receiving intravesical Ad-IFNα treatment and the exfoliated bladder cancer cells were examined by immunofluorescent confocal microscopy. Although the number of cancer cells were few, those that were found in the urine displayed coincident caspase 4 activation and Ad-IFNα expression (Fig.4c). These results supported our cell culture studies and extended them into the in vivo situation.
Inhibition of caspase 4 by caspase 4 targeting RNAi blocks early caspase 3 activation
Finding early activation of caspase 4 followed by the activation of caspase 3 suggested there may be a direct relationship between the two. In order to examine this more closely caspase 4 activation was blocked by caspase 4 targeted RNAi addition prior to Ad-IFNα infection. The conditions to block caspase 4 was initially optimized and confirmed by Western blotting and realtime RT-PCR (data not shown). We examined the 48 hr time point after Ad-IFNα infection since no caspase 3 was activated at the 24 hour time point as shown by both Western blotting and confocal analysis. In addition, because considerable cytotoxicity also occurs at the 48 hr time point as a result of the bystander effect, which in turn produces caspase 3 activation (3), we were only interested in examining the cells which contained INFα. The basis for this approach is the fact that cells which are killed by the bystander effect do not show any perinuclear IFN staining and the cells which have perinuclear IFN staining as a reflection of direct Ad-IFN transfer also show co-localization of caspase 4 activation (see Fig. 4). Therefore we could use confocal analysis to determine if blockage of caspase 4 activation in cells which showed perinuclear IFN staining also blocked caspase 3 activation. Indeed we found that in cells showing perinuclear IFN staining in which caspase 4 activation had been blocked by the addition of the caspase 4 targeted RNAi that caspase 3 activation was also inhibited. An example is shown in Fig. 5. However, this was the result seen in all cases where caspase 4 had been inhibited by the addition of caspase 4 targeted RNAi. These findings strongly suggest that the rapid activation of caspase 4 is not only an early signal of ER stress but is also a marker of subsequent cell death produced by Ad-IFNα infection.
Fig. 5.
The inhibition of caspase 4 by caspase 4 targeted RNAi blocks downstream caspase 3 activation. Top panels show that 48 hrs after Ad-IFNα treatment of HeLa cells both cleaved caspase 4 (green) and cleaved caspase 3 (blue) forms are present in the same cells containing IFNα (red). The bottom panel shows that caspase 4 targeted RNAi blocked caspase 4 being cleaved as well as cleavage of caspase 3 in cells similarly treated with Ad-IFN which contained IFNα expressing nuclei were counterstained with DAPI (grey-white). Original magnification × 400.
Discussion
Adenoviruses expressing interferon α (Ad-IFNα) show a significant potential for the treatment of superficial bladder cancer (1–2, 6). Although bladder cancer cells sensitive to recombinant human interferon α (IFNα) are often killed through a tumor necrosis factor, apoptosis-inducing ligand (TRAIL)-related pathway, our previous studies also showed that intravesical administration using Ad-IFNα in the presence of the excipient, Syn3, also produced a marked regression of superficial human bladder tumors derived from the IFNα-resistant cancer cells (1–2). In addition we have previously documented that Ad-IFNα produces a strong bystander effects in cancer cells (3–4). These results implied that Ad-IFNα can have highly important additional mechanisms of cell kill in addition to the high levels of IFNα produced. We are presently attempting to obtain the exact identification of these bystander effect factors.
In our present study we have centered on the direct cytotoxicity mediated by Ad-IFNα. Although we have already shown that Ad-IFNα induced proteolytic processing of caspases 3, 8 and 9 (3), the molecular background of the antitumor effects of Ad-IFNα and the mechanisms of overcoming resistance to the IFNα protein in cancer cells remained undefined. The strong perinuclear IFNα staining which was seen uniformly following Ad-IFNα treatment to various types of cancer cells (3) and was never observed after exposure to the IFNα protein led us to examine the possible role endoplasmic ER stress in the cancer cell death. The accumulation of unfolded proteins in the ER mediates a cellular stress induced by multiple stimuli and pathological conditions. The capacity of the ER to process proteins is limited and the accumulation of unfolded and misfolded proteins , in this case IFNα, can lead to ER stress associated apoptosis.
We have recently reported that ER stress is observed as a direct effect of Ad-IFNα exposure but not by bystander factors. (10). In the present study we found that caspase 4 activation is a particularly early molecular event following Ad-IFNα transfection and expression of cancer cells. To date we do not have a complete explanation for the reason that cleaved caspase 4 is detected only in cancer cell lines but not in the normal urothelial cells. A clue to this difference may come from a recent observation. We have found that autophagy is produced after Ad-IFNα treatment of either interferon resistant cancer cells or the normal urothelial cell line (TERT-NHUC) (11). However, after Ad-IFNα infection autophagosomes, which are an early stage of autophagy, were seen in the cancer cells whereas autophagolysosomes, a later stage of autophagy, were observed mostly in normal cells by electron microscopy (11). Conditioned medium from either normal or bladder cancer cells, however, produced no autophagy when placed on the bladder cancer cells, although again marked cytotoxicity was observed under these conditions, indicative of bystander factors being produced (11). This in turn suggested that the autophagy seen after Ad-IFNα infection was related to the direct effect of Ad-IFNα infection and expression rather than to the bystander factors produced.
It was also documented that Ad-IFNα treatment produces the LC3-II autophagic protein form in cancer cells but not normal cells, which in turn was inhibited by the autophagic inhibitor, 3-methyladenine (3-MA), resulting in a significant increase in apoptotic cell death. We concluded from these findings that the autophagy seen in normal urothelial cells is a protective response and is allowed to be completed, providing a survival mechanism following Ad-IFN treatment, whereas the autophagy produced in interferon resistant cancer cells is not allowed to be completed and is insufficient to significantly inhibit cytotoxicity. These differences could at least in part be related to the fact that no caspase 4 activation was seen in the normal cells, since the complete autophagy found in normal urothelial cells could protect the cells resulting in a lack of caspase 4 activation. In addition the fact that inhibition of caspase 4 by caspase 4 siRNA also inhibited the activation of caspase 3 in cells containing high perinuclear IFNα (Fig.5) suggests that the activation of caspase 4 not only is an early marker of ER stress but also signals downstream activation of caspase 3 which in turn is an effector of subsequent cell death.
Finally, we have been able to examine cancer cells in the urine from a patient 7 days after intravesical Ad-INFα/Syn3 treatment. Although few tumor cells were found, all of the cancer cells having IFNα also showed activated caspase 4 by confocal analysis (Fig.4c). Numerous tumor cells with high IFNα were seen in the superficial bladder tumors produced in our human tumor mouse model after Ad-IFNα infection (1). We believe that such high perinulear IFNα results in ER stress, activating caspase 4 and other ER stress signals which ultimately leads to tumor cell kill. Identifying tumor cells in the patient’s urine after intravesical Ad-INFα/Syn3 treatment which had both high IFNα and caspase 4 activation strongly suggests that the results we have reported here from studies done in cell culture may also be quite relevant in the human setting where approximately 50% of the patients with BCG refractory superficial bladder cancer have obtained a complete remission after a single intravesical instillation of Ad-INFα/Syn3 in our Phase I trial(6).
Acknowledgments
Supported by Bladder SPORE Grant P50 CA91846 to all authors and a Bladder SPORE Career Development Award to ZY.
REFERENCES
- 1.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, McConkey DJ, Papageorgiou A, Munsell M, Philopena J, Engler H, Demers W, Maneval DC, Dinney CP, Connor RJ. Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcome resistance to IFN-alpha protein. Mol Ther. 2004;10(3):525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, Benedict WF. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: implications for clinical investigation. Cancer Gene Therapy. 2006;13(2):125–130. doi: 10.1038/sj.cgt.7700865. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X-Q, Yang Z, Dong L, Papageorgiou A, McConkey D, Benedict WF. Adenoviral mediated interferon a overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Therapy. 2007;14:241–250. doi: 10.1038/sj.cgt.7701011. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Dong L, Chapman E, Benedict WF. Conditioned medium from Ad-IFN-alpha-infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells: further evidence for a strong bystander effect. Cancer Gene Therapy. 2008;12:817–822. doi: 10.1038/cgt.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papageorgiou A, Lashinger L, Millikan R, Benedict WF, Dinney CP, McConkey DJ. Autocrine TRAIL production mediates interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64:8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 6.Benedict WF, Fisher MB, Cutler DL, et al. Results of a Phase 1 trial with intravesical Ad-IFN-a/Syn3 for superficial bladder cancer including putative marker studies (abstract) Proceedings of the 100th Annual Meeting of the American Association for Cancer Research, Abstract # 1449. 2009 [Google Scholar]
- 7.Fisher MB, Zhang XQ, McConkey DJ, Benedict WF. Measuring soluble forms of extracellular cytokeratin18 identifies both apoptotic and necrotic mechanisms of cell death produced by adenoviral-mediated interferon alpha: possible use as a surrogate marker. Cancer Gene Therapy. 2009;16(7):567–572. doi: 10.1038/cgt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T, Shinohara N, Sazawa A, et al. An Improved Intravesical Model Using Human Bladder Cancer Cell Lines to Optimize Gene and Other Therapies. Cancer Gene Therapy. 2000;7(12):1575–1580. doi: 10.1038/sj.cgt.7700261. [DOI] [PubMed] [Google Scholar]
- 9.Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA. Expression of hTERT immortalizes normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037–5045. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XQ, Yang Z, Benedict WF. Direct Gene Transfer of Adenoviral-mediated Interferon α into Human Bladder Cancer Cells but Not the Bystander Factors Produced Induces Endoplasmic Reticulum Stress-Related Cytotoxicity. Cancer Gene Therapy. doi: 10.1038/cgt.2010.76. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang XQ, Dunner K, Benedict WF. Autophagy is induced by adenoviral-mediated interferon α treatment in interferon resistant bladder cancer and normal urothelial cells as a cell death protective mechanism but not by the bystander factors produced. Cancer Gene Therapy. 2010;8:579–584. doi: 10.1038/cgt.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]