Abstract
Myostatin (Mstn), a member of the transforming growth factor β superfamily, plays an inhibiting role in mammalian muscle growth. Mammals like human, cattle, mouse, sheep, and dog carrying null alleles of Mstn display a double-muscle phenotype. Mstn is conserved in fish; however, little is known whether the fish with mutated mstn display a similar phenotype to mammals because of the lack of mutant fish with mstn null alleles. Previously, we knocked out one of the duplicated copies of myostatin gene (mstna) in yellow catfish using zinc-finger nucleases. In this study, we report the identification of the second myostatin gene (mstnb) and knockout of mstnb in yellow catfish. The gene comprises three exons. It is predicted to encode 373 amino acid residues. The predicted protein exhibits 59.3% identity with yellow catfish Mstna and 57.3% identity with human MSTN. Employing TALEN (transcription activator-like effector nucleases) technology, we obtained two founders (from four randomly selected founders) of yellow catfish carrying the mutated mstnb gene in their germ cells. Totally, six mutated alleles of mstnb were obtained from the founders. Among the six alleles, four are nonframeshift and two are frameshift mutation. The frameshift mutated alleles include mstnbnju22, an 8 bp deletion, and mstnbnju24, a complex type of mutation comprising a 7 bp deletion and a 12 bp insertion. They are predicted to encode function null Mstnb. Our results will help to understand the roles of mstn genes in fish growth.
Introduction
Myostatin (Mstn) is a member of the transforming growth factor β superfamily. In mammals, Mstn negatively regulates muscle growth.1 The phenotypes of Mstn knockout mice include a significantly increased myofiber size (hypertrophy) and myofiber number (hyperplasia) compared with their heterozygous and wild-type littermates.2 Spontaneous mutations have been found in mammalian Mstn genes, which lead to a double-muscle phenotype.1 For example, the first reported spontaneous mammalian Mstn mutation is in Belgian Blue cattle. It is an 11 bp deletion in the third exon of Mstn that disrupts the bioactive domain of the protein, resulting in skeletal muscle hyperplasia with more muscle mass than standard breeds.3 In sheep, the mutations in Mstn results in gained body weight and muscle mass.4 In racing dogs, the mutations in Mstn increase muscle mass and enhance racing performance.5 A child carrying a mutated MSTN displays a hypertrophic phenotype.6
Mstn is highly conserved in vertebrates. However, unlike mammals, many fish have more than one mstn copy in their genomes, probably due to genome duplication events during vertebrate evolution.1 For example, there are four mstn paralogs, mstn-1a, -1b, -2a, and -2b in salmonids and two paralogs, mstn and mstn2, in zebrafish.1 Although suppression of mstn and mstn2 in zebrafish yields hyperplasia phenotype,7,8 no spontaneous mstn null mutation has been found in fish.1 Therefore, the function of Mstn in fish remains to be revealed for the lack of direct evidences.
To investigate the roles of mstn gene in fish growth, it is necessary to knock out their mstn. Traditional gene knockout relies on gene targeting in ES cells.9 Zinc-finger nucleases (ZFNs)10–12 and Transcription activator-like effector (TALE) nucleases (TALENs)13–15 have made genome editing possible in animals, of which ES cell strains are not available. TALENs are artificial endonucleases composed of a DNA binding domain derived from TALEs cloned from plant pathogenic bacteria Xanthomonas and an endonuclease domain from a type IIS restriction endonuclease FokI.13–15 A pair of TALENs bind two half-sites of the target DNA fragment, respectively, to generate a double-strand break (DSB) in the spacer between the half-sites. The resulting DSB is then repaired in various ways, including error-prone nonhomologous end joining that gives rise to mutated alleles of the target gene. By introducing TALENs into developing embryos, researchers have created heritable knockout animals, including mutant zebrafish with mutated tnikb, moesina, ppp1cab, cdh5, ponzr1, or crhr2,16,17 IgM knockout rat,18 BmBlos2 knockout silkworm,19,20 and Pibf1, Sepw1, Sry, Uty, Lepr, and C9orf72 knockout mouse.21–23
Previously, we knocked out one of the duplicated copies of myostatin gene (mstna, previously named mstn) in yellow catfish (Tachysurus fulvidraco, previously named Pelteobagrus fulvidraco), one of the most important freshwater aquaculture species in China, using ZFN.24 In this study, we report the identification of a second myostatin gene (mstnb) in yellow catfish. Using TALEN technology, we generated, from two founder fish, 13 heritable yellow catfish carrying either of the two function null alleles of mstnb. Our result will help to understand the roles of mstn genes in fish by obtaining double knockout of mstna and mstnb through crossing mstna+/− and mstnb+/− and witness whether the yellow catfish with null alleles of mstn display the double-muscle phenotype that is beneficial for aquaculture.
Materials and Methods
Animals
Yellow catfish were originally obtained from the Lukou Breeding Base of Freshwater Fisheries Research Institute of Jiangsu Province and cultured in the fish laboratory of Model Animal Research Center, Nanjing University. The research protocol was approved by the Institutional Animal Care and Use Committee of Model Animal Research Center, Nanjing University.
Artificial insemination of yellow catfish
Artificial insemination on yellow catfish was performed as previously described.24 For each mating, eggs were collected by massaging a female yellow catfish in the abdomen repeatedly from anterior to posterior and were mixed with minced testes harvested from a male fish in 5 mL of 0.69% NaCl solution. The fertilized eggs were then dispensed into a dish with 30 mL of fresh water at a density of about 300 eggs per dish.
Molecular cloning of mstnb gene in yellow catfish
Total RNA was isolated from 15-month-old male and female yellow catfish muscle and served as samples for high-throughput SOLEXA sequencing (paired end, 100nt read length) on a Genome Analyzer IIx sequencer (Illumina) by a commercial company (Shanghai Biotechnology Corporation). Briefly, 29.4 μg total RNA was obtained from male yellow catfish muscle and 30.7 μg from female yellow catfish. Total RNA was purified with the RNeasy Micro Kit (Qiagen). The cDNA library was constructed following the TruSeq RNA Sample Preparation Guide (Illumina). cDNA clonal clusters were generated on a flow cell using cBot (Illumina). The flow cell was then placed in a Genome Analyzer IIx (Illumina) for sequencing. Raw reads were preprocessed and trimmed; De novo assembly was performed to produce Contigs using CLC Genomics Workbench (4.8) (CLC Bio). The resulting unigenes were subjected to annotation by blastx alignment with SwissProt database (access date: Oct. 19, 2011). Information of the mstn cDNA sequence was mined from the produced database.
To obtain full-length cDNA of yellow catfish mstnb, 5′-RACE-PCR was performed with a commercial kit (SMARTer RACE cDNA Amplification Kit; Clontech) and KOD FX DNA polymerase (Toyobo) following the manufacturers' instructions. Briefly, total RNA was extracted using the Trizol reagent (Invitrogen) from brains harvested from six 22-month-old female yellow catfish. mRNA was purified from 250 μg total RNA using the Oligotex mRNA Mini Kit (Qiagen) and eluted with 40 μL water. 5′-RACE-Ready cDNA was prepared from 2.75 μL mRNA using the SMARTer RACE cDNA Amplification Kit (Clontech). 5′ RACE PCR was performed using a Universal Primer A Mix (UPM) provided in the kit as a forward primer and a gene-specific primer (GSP) named mstnbRACER3 (Table 1) as a reverse primer, and KOD FX DNA polymerase (Toyobo). The PCR program was 95°C for 2 min, 35 cycles of (98°C for 30 s, 55°C for 30 s, and 68°C for 3 min), and a final extension at 72°C for 10 min. The PCR product was analyzed in a 1% agarose gel and extracted from the gel using the Axyprep DNA Gel Extraction Kit (Axygen), incubated in a 1×Taq PCR prepared with GoTaq DNA polymerase (Promega) at 72°C for 10 min. It was then cloned into the pGEM-T easy vector (Promega). Obtained transformants were sequenced in two directions and resulting sequences were used to assemble the full-length mstnb cDNA sequence.
Table 1.
Primers Used in the Molecular Cloning of Myostain b of Yellow Catfish (Tachysurus fulvidraco)
| Primer name | Sequence |
|---|---|
| mstnbRACER3 | GTACCACGAGGGTTCGCCTTGTTCA |
| mstnbflF12 | TCCGGGATCATGCTTCTC |
| mstnbflR1 | GTGTTAATGCTGCGATCAAAT |
| mstnbflR2 | GCACCTTCATTGGGACTTG |
| mstnbflF3 | GCGAGTCCCGACAGCAGAGT |
| mstnbflR3 | TGGCAGGAATTACGTTATCTTCTCA |
| mstnbflF | GACATGCGACGCGTTTCCTGC |
| mstnbflR | CTTTGTTCATATAGCTAATATGGCAG |
| actbF | GTGCTGTCTTCCCATCCATTG |
| actbR | GACACCTGAACCTCTCATTGC |
To clone full-length mstnb cDNA, RT-PCR was performed. Briefly, total RNA was extracted from yellow catfish muscle using the Trizol reagent (Invitrogen). First-strand cDNA was synthesized using EasyScript First-Strand cDNA Synthesis SuperMix (Transgen). A pair of primers, mstnbflF and mstnbflR (Table 1), were then designed to clone full-length mstnb cDNA using KOD-plus-neo DNA polymerase (Toyobo). The PCR program was 95°C for 2 min, 35 cycles of (98°C for 30 s, 56°C for 30 s, and 68°C for 3 min), and a final extension at 72°C for 5 min.
Genomic DNA of mstnb was amplified from yellow catfish genomic DNA using forward primers (Table 1) of mstnbflF12 and mstnbF3 (complementary to sequences in predicted exon 1), and reverse primers (Table 1) of mstnbflR1, mstnbflR2, and mstnbflR3 (complementary to sequences in predicted exon 3) together with KOD-plus-neo DNA polymerase (Toyobo). The final concentration of each primer was 0.25 μM. The PCR program was 94°C for 2 min, 5 cycles of (98°C for 10s and 68°C for 4 min), 5 cycles of (98°C for 10 s, 66°C for 30 s, and 68°C for 4 min), 5 cycles of (98°C for 10 s, 64°C for 30 s, and 68°C for 4 min), 15 cycles of (98°C for 10 s, 62°C for 30 s, and 68°C for 4 min), and a final extension at 68°C for 7 min. The amplicon with a size of about 2.6 kbp was subcloned into the pGEM-T easy vector and sequenced. The genomic organization of mstnb was obtained by aligning the mstnb cDNA sequence with the mstnb genomic DNA sequence.
Sequence analysis
DNA sequences were analyzed with Vector NTI 11 (www.invitrogen.com). The alignment was generated with Vector NTI 11. The phylogenetic tree of vertebrate Mstns was constructed using the Neighbor-Joining method with MEGA5.25 The bootstrap consensus tree inferred from 1000 replicates was taken to represent the evolutionary history of these analyzed genes.
Detection of mstnb message
The expression pattern of mstnb was examined in adult organs, including the brain, stomach, intestine, liver, skin, muscle, kidney, spleen, gill, heart, testis, and ovary. Total RNA extraction and reverse transcription were performed as described in the “Molecular cloning of mstnb gene in yellow catfish” section. Primers mstnbe2F2 and mstnbe2R2 (Table 1) were used to detect expression of mstnb gene. Gotaq Flexi DNA polymerase (Promega) was used in the PCR according to the manufacturer's instruction. The PCR program was 95°C for 2 min, 35 cycles of (94°C for 30 s, 56°C for 30 s, and 72°C for 60 s), and a final extension at 72°C for 5 min. actinb was amplified as an internal control with primers actbF and actbR (Table 1).
Design of TALENs targeting disruption of mstnb in yellow catfish
The TALENs targeting yellow catfish mstnb were designed with TALENT software (https://tale-nt.cac.cornell.edu/TALENT/).26 Briefly, nucleotide sequence of the 2nd exon of yellow catfish mstnb gene served as an input to search the targeting sites and their corresponding TALENs. TALEN plasmids were assembled and the constructed plasmids were used as templates for in vitro transcription to prepare TALEN mRNA, as previously described,16 using the mMessage mMachine SP6 Kit (Ambion). TALEN mRNAs for microinjection were prepared at a final concentration of 100 ng/μL of each arm.
Examination of TALEN activity in yellow catfish embryos
To test whether the TALEN pair could cut mstnb in yellow catfish genome, we microinjected 5 nL of the mRNAs of TALEN1-mstnb or TALEN2-mstnb pair into the animal poles of yellow catfish embryos at the 1-cell stage. The injected embryos were then grown at 28.5°C in the same conditions as zebrafish embryos. When reaching 72 hpf, 20 embryos were randomly selected to examine mutated mstnb in their genomes using the DNA MiniExtract Kit (Nanjing Runbang Bio-tech Company), as we previously reported.24 Primers mstnbe2F2 and mstnbe2R2 (Table 1) were used for cloning partial exon 2 containing target sites of the TALENs using the same program described in the “Detection of mstnb message” section.
Generation of heritable targeted inactivation of mstnb in yellow catfish
To generate mstnb knockout yellow catfish, we microinjected 5 nL of the mRNAs of TALEN2-mstnb pair into the animal poles of yellow catfish embryos at the 1-cell stage. The injected embryos were then bred at 28.5°C in the same conditions as zebrafish embryos.
After having been raised for 1 year in the laboratory aquarium, the founder yellow catfish were used for artificial insemination by mixing the reproductive cells of a founder yellow catfish with a founder or a wild-type partner. When the offspring of founder yellow catfish (F1) reached 1 month old, tissue samples were taken by clipping a piece of caudal fin from each juvenile. The genomic DNA was then extracted in the way described in the “Examination of TALEN activity in yellow catfish embryos” section. One microliter of the extracted DNA was used as the PCR template to amplify the mstnb fragment containing TALEN2-mstnb target site with the methods as described above. Genotyping was performed by sequencing the PCR products and the sequences were manually read from the chromatogram. The F1 yellow catfish with mutated mstnb alleles were grown in the laboratory aquarium in the way similar to growing zebrafish.
Results
Molecular cloning of myostatin b in yellow catfish
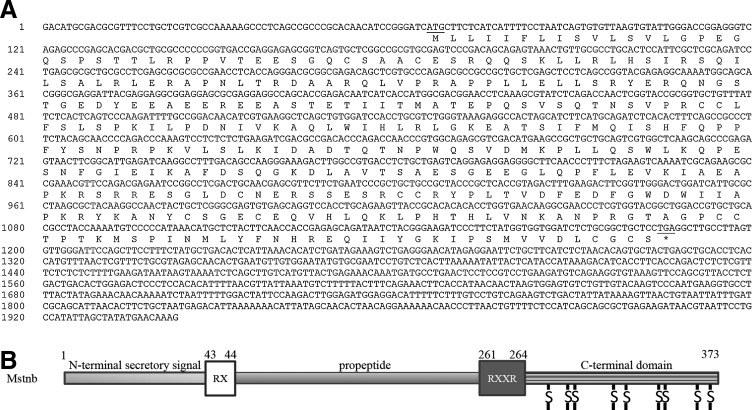
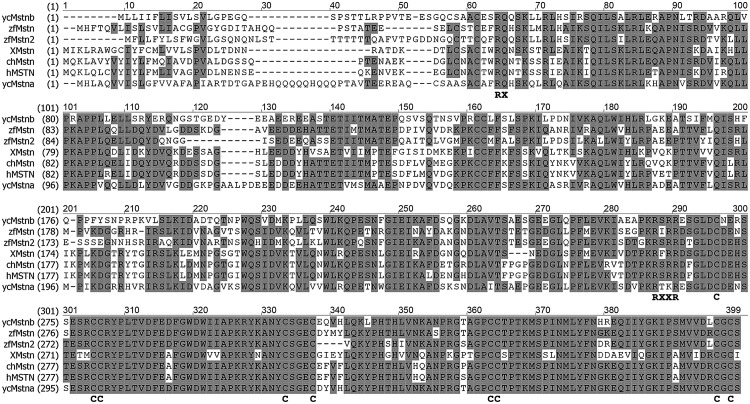
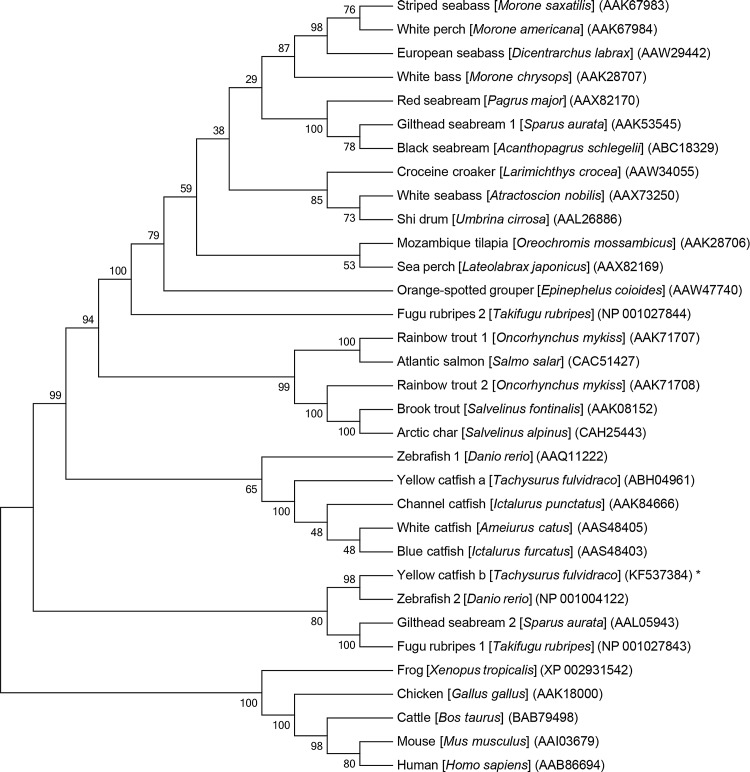
By performing end-to-end PCR, we obtained the full-length mstnb cDNA (GenBank Accession No. KF537384). The mstnb cDNA is 1943 bp in length, consisting of a 65 bp 5′-UTR, a 1122 bp coding sequence (CDS), and a 756 bp 3′ UTR (Fig. 1A). The CDS was predicted to encode a protein with 373 amino acid residues. The predicted protein exhibits 61.1% identity with zebrafish Mstn (AAQ11222), 66.5% identity with zebrafish Mstn2 (NP_001018627), 50.8% identity with Xenopus tropicalis Mstn (XP_002931542), 57.0% identity with chicken Mstn (AAK18000), 57.3% identity with human MSTN (AAB86694), and 60.0% with yellow catfish Mstna (ABH04961) (Fig. 2 and Table 2). Phylogenetic analysis on the vertebrate Mstn family showed that the Mstnb is clustered into the Mstn family (Fig. 3). The results indicate that yellow catfish Mstnb is highly conserved during evolution (Fig. 3).
FIG. 1.
Complete nucleotide and deduced amino acid sequences of yellow catfish (Tachysurus fulvidraco) mstnb cDNA and schematic diagram showing the function domains of the protein. (A) The complete nucleotide and deduced amino acid sequences of yellow catfish mstnb cDNA. The start codon (ATG) and stop codon (TGA) are underlined. (B) Schematic diagram showing the domains of yellow catfish Mstnb. The protein with 373 amino acid residues comprises an N-terminal secretory signal, a proteolytic site (RX), a propeptide, a tetrabasic proteolytic processing site (RXXR), and a bioactive C-terminal domain with a conserved pattern of nine cysteine (S) residues.
FIG. 2.
Amino acid sequence alignment of yellow catfish (T. fulvidraco) Mstn with other representative vertebrates' Mstn. The amino acid identities of Mstns from different animals were determined using MEGA5. Identical amino acid residues are cross hatched and dashes represent gaps for alignment purposes. The predicted proteolytic site (RX) to remove the signal sequence, the proteolytic site (RSSR) to generate bioactive form of Mstn, and the conserved nine cysteine residues in the bioactive domain are boxed. zf: zebrafish; X: Xenopus tropicalis; yc: yellow catfish; ch: chicken; h: human.
Table 2.
Protein Sequence Identities of Yellow Catfish (T. fulvidraco) Myostatin b with Other Vertebrates' Myostatins
| Identity (%) | ||||
|---|---|---|---|---|
| Common name of animals | Scientific name of animals | GenBank accession number | Overall | Bioactive domain |
| Yellow catfish | Tachysurus fulvidraco | KF537384 | / | / |
| Yellow catfish | T. fulvidraco | ABH04961 | 59.3 | 92.7 |
| White catfish | Ameiurus catus | AAS48405 | 59.8 | 92.7 |
| Channel catfish | Ictalurus punctatus | AAK84666 | 60.0 | 92.7 |
| Blue catfish | Ictalurus furcatus | AAS48403 | 60.3 | 92.7 |
| Brook trout | Salvelinus fontinalis | AAK08152 | 61.0 | 89.9 |
| Rainbow trout | Oncorhynchus mykiss | AAK71707 | 62.4 | 90.8 |
| Rainbow trout | O. mykiss | AAK71708 | 61.8 | 90.8 |
| Gilthead seabream | Sparus aurata | AAK53545 | 60.8 | 89.9 |
| Gilthead seabream | S. aurata | AAL05943 | 63.9 | 85.3 |
| White seabass | Atractoscion nobilis | AAX73250 | 60.5 | 90.8 |
| Striped seabass | Morone saxatilis | AAK67983 | 61.0 | 90.8 |
| European seabass | Dicentrarchus labrax | AAW29442 | 61.0 | 90.8 |
| White bass | Morone chrysops | AAK28707 | 60.2 | 89.9 |
| Red seabream | Pagrus major | AAX82170 | 61.5 | 89.9 |
| Black seabream | Acanthopagrus schlegelii | ABC18329 | 60.3 | 88.1 |
| Atlantic salmon | Salmo salar | CAC51427 | 62.6 | 89.9 |
| Arctic char | Salvelinus alpinus | CAH25443 | 61.0 | 88.1 |
| White perch | Morone americana | AAK67984 | 61.0 | 90.8 |
| Croceine croaker | Larimichthys crocea | AAW34055 | 60.8 | 89.9 |
| Shi drum | Umbrina cirrosa | AAL26886 | 60.5 | 89.9 |
| Mozambique tilapia | Oreochromis mossambicus | AAK28706 | 59.4 | 90.8 |
| Fugu rubripes | Takifugu rubripes | NP_001027843 | 62.2 | 83.5 |
| Fugu rubripes | T. rubripes | NP_001027844 | 59.7 | 88.1 |
| Sea perch | Lateolabrax japonicus | AAX82169 | 57.5 | 79.8 |
| Orange-spotted grouper | Epinephelus coioides | AAW47740 | 61.6 | 90.8 |
| Zebrafish | Danio rerio | AAQ11222 | 61.1 | 89.0 |
| Zebrafish | D. rerio | NP_001004122 | 66.5 | 89.5 |
| Frog | Xenopus tropicalis | XP_002931542 | 50.8 | 68.8 |
| Chicken | Gallus gallus | AAK18000 | 57.0 | 85.3 |
| Cattle | Bos taurus | BAB79498 | 57.3 | 84.4 |
| Mouse | Mus musculus | AAI03679 | 57.6 | 85.3 |
| Human | Homo sapiens | AAB86694 | 57.3 | 85.3 |
FIG. 3.
The phylogenetic tree of vertebrate Mstns was constructed using the Neighbor-Joining method with MEGA5. The percentage of replicate trees in which the associated genes clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The Mstn protein sequences of different animals (scientific name shown in italic) were retrieved from the GenBank database (www.ncbi.nlm.nih.gov/) and the GenBank accession numbers are shown in brackets.
Similar to Mstna, Mstnb has an N-terminal secretory signal (1st–42nd aa), a proteolytic site to remove the signal sequence (RX) (43rd–44th aa), a propeptide (45th–260th aa), a tetrabasic proteolytic processing site (RXXR) (261st–264th aa), and a bioactive C-terminal domain (265th–373rd aa) with a conserved pattern of nine cysteine residues (Figs. 1B and 2). The mature form of Mstnb (bioactive C-terminal peptide), predicted to be generated by cleavage of the precursor protein at the tetrapeptide site, exhibits more than 79% identity to that of other vertebrate Mstns (Table 2).
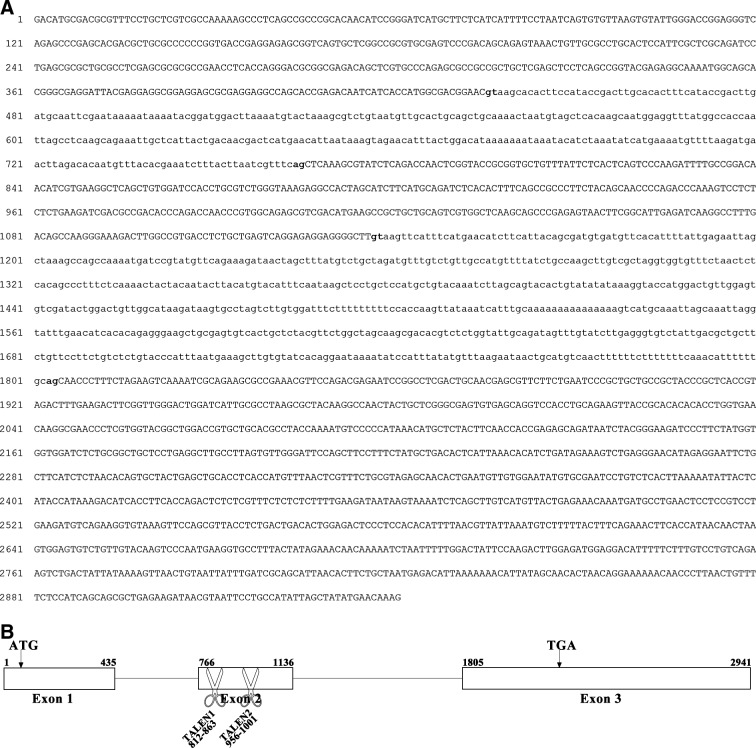
The genomic sequence of mstnb gene in yellow catfish is 2941 bp in length (Genbank Accession No. KF537385). It comprises three exons, namely 435 bp of exon 1, 371 bp of exon 2, and 1137 bp of exon 3 in length, respectively. It is interspaced by two introns, namely 330 bp of intron 1 and 668 bp of intron 2 in length, respectively. The start codon ATG is in the 1st exon and the stop codon TGA is in the 3rd exon (Fig. 4). It shares the same genomic organization with that of mstna.27
FIG. 4.
Complete nucleotide sequences of yellow catfish (T. fulvidraco) mstnb genomic DNA and schematic diagram showing the genomic organization of yellow catfish mstnb gene. (A) Complete nucleotide sequences of yellow catfish mstnb genomic DNA. Exons are shown in uppercase and introns are shown in lowercase. The splicing signals of introns (gt…ag) are shown in bold. (B) A schematic diagram showing the genomic organization of yellow catfish mstnb gene. Exons are shown as boxes and introns are shown as solid lines. Start codon (ATG) and stop codon (TGA) are marked with arrows in exon 1 and exon 3, respectively. Scissors show the recognition loci of designed TALENs.
Expression of mstnb in different adult organs
The RT-PCR results suggested that a high level of mstnb message was present in the brain, liver, muscle, spleen, testis, and ovary, a low level in skin, and no detectable expression in the stomach, intestine, kidney, gill, and heart (Fig. 5).
FIG. 5.
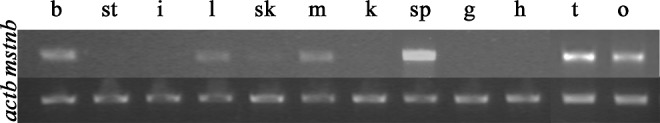
Expression of mstnb in different adult tissues of yellow catfish (T. fulvidraco). b, brain; st, stomach; i, intestine; l, liver; sk, skin; m, muscle; k, kidney; sp, spleen; g, gill; h, heart; t, testis; and o, ovary. mstnb expression was detected in the brain, liver, skin, muscle, spleen, testis, and ovary. The expression actinb was served as an internal control.
Activities of the TALENs to induce mstnb indel mutations in yellow catfish embryos
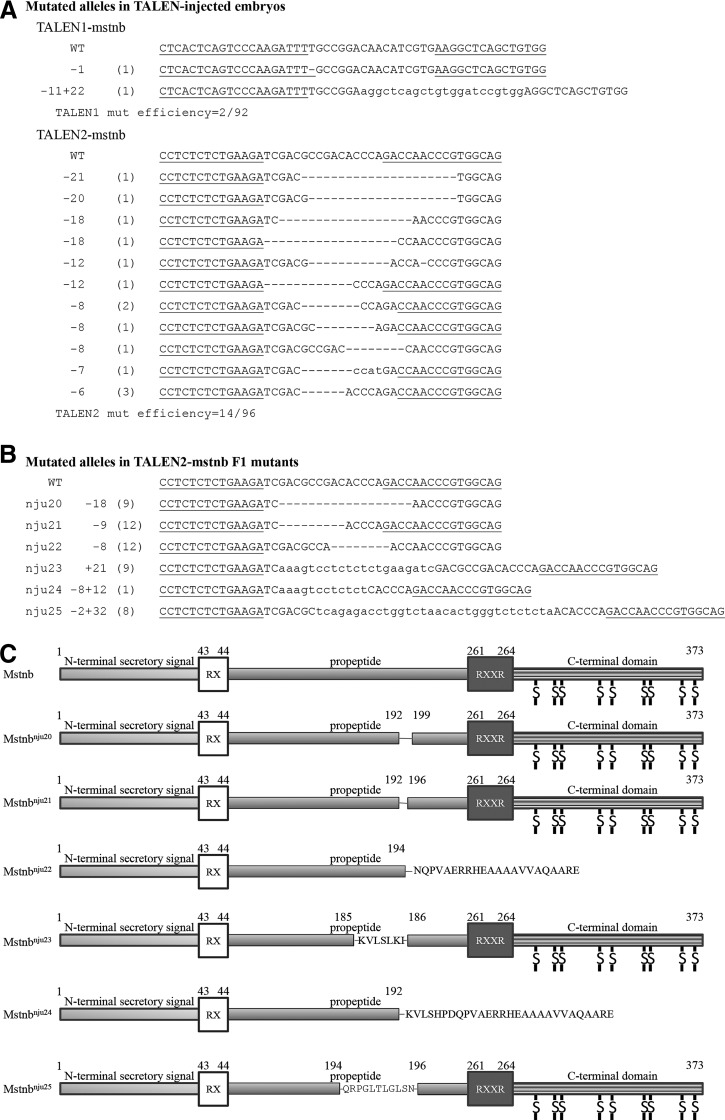
The activities of two pairs of TALENs recognizing the 2nd exon of yellow catfish mstnb were tested in yellow catfish embryos (Table 3, Fig. 4B). Sequencing analyses revealed that 2 of 92 molecules amplified from the TALEN1-mstnb-microinjected yellow catfish embryos at 24 hpf were mutated, and that 14 of 96 molecules amplified from TALEN2-mstnb-microinjected embryos were mutated. Of the two mutated molecules induced by TALEN1-mstnb, one was a deletion and the other was a complex containing both deletion and insertion (Fig. 6A). Of the 14 mutated molecules induced by TALEN2-mstnb, all were deletions (Fig. 6A). The results demonstrated that both TALEN1-mstnb and TALEN2-mstnb had the ability to cut genomic mstnb in yellow catfish genome, with TALEN2-mstnb having a higher activity.
Table 3.
Sequences of TALEN Pairs Targeted to Yellow Catfish (T. fulvidraco) mstnb
| TALEN | Left half-site (sense strain) | Right half-site (anti-sense strain) | Spacer (sense strain) |
|---|---|---|---|
| TALEN1-mstnb | tCTCACTCAGTCCCAAGATTT | tCCACAGCTGAGCCTT | TGCCGGACAACATCGTG |
| TALEN2-mstnb | tCCTCTCTCTGAAGAT | tCTGCCACGGGTTGGTCT | CGACGCCGACACCC |
FIG. 6.
Generation of mstnb knockout yellow catfish (T. fulvidraco) using engineered TALENs. (A) TALEN1-mstnb and TALEN2-mstnb induced various mutations in injected embryos. Number in the leftmost of the panels shows the number of nucleotides deleted (−) or inserted (+) in the mutated mstnb gene. Number in the bracket shows the frequency of the mutated molecules. (B) Six mutated alleles were found in mutants of F1 yellow catfish, including mstnbnju20, mstnbnju21, mstnbnju22, mstnbnju23, mstnbnju24, and mstnbnju25. Partial sequence of each allele is shown. Numbers following the allele names show the number of nucleotides deleted (−) or inserted (+) in the mutated mstnb. Number in the bracket shows the number of mutants carrying the mutated alleles. (C) Schematic diagram shows that six mutated proteins would be produced from the six different strains of yellow catfish carrying different mutated mstnb alleles. mstnbnju22 and mstnbnju24 are frame shift mutations and encode truncated proteins lacking the bioactive C-terminal domain. Single lines in the propeptide domain show loss of amino acids. Amino acid sequences in the propeptide domain show inserted amino acid fragments. Amino acid sequences following incomplete propeptide domain are due to frame shift reading.
Generation of heritable targeted inactivation of mstnb in yellow catfish
About 2000 embryos were microinjected with TALEN2-mstnb mRNA at the 1-cell stage to generate mstnb mutant founders. When the founders reached 12 months old, all the males and some of the females were sexually mature. Male founder P1 and P4 were then mated with a wild-type (wt) female to generate F1 offspring named P1wt and P4wt, respectively. Male founder P2 and female founder M3 were mated to generate an F1 offspring named P2M3. One hundred thirty-four fish in group P1wt, 34 in group P2M3, and 256 in P4wt were genotyped and six mutated alleles were found, namely mstnbnju20–25, respectively (Table 4). The mutant rates were 5.9% in P1wt, 0.0% in P2M3, and 16.4% in P4wt, respectively. Eight juveniles in P1wt carried mstnbnju20, a deletion of nt972-nt989 in mstnb genomic DNA (nt642-nt659 in mstnb cDNA), predicted to encode a mutated Mstnb lacking the 193rd–198th amino acid residues (part of the propeptide) (Fig. 6B, C). Twelve fish in P4wt carried mstnbnju21, a deletion of nt972-nt980 in mstnb genomic DNA (nt642-nt650 in mstnb cDNA), predicted to encode a mutated Mstnb protein lacking the 193rd–195th amino acid residues (part of the propeptide) (Fig. 6B, C). Twelve in P4wt carried mstnbnju22, a deletion of nt978-nt986 (nt648-nt656 in cDNA) and an insertion of a single A in mstnb genomic DNA, predicted to encode a truncated Mstnb protein of only the 1st–192nd amino acid residues of the wild-type Mstnb protein and an additional fragment (NQPVAERRHEAAAAVVAQAARE) due to reading frame shift (Fig. 6B, C). Nine fish in P4wt carried mstnbnju23, an insertion of a 21 bp fragment (AAAGTCCTCTCTCTGAAGATC) between nt971 and nt972 in mstnb genomic DNA (nt641 and nt642 in cDNA), predicted to encode a mutated Mstnb protein with an insertion of a fragment (KVLSLKI) between the 185th and 186th amino acid residues (Fig. 6B, C). One fish in P4wt carried mstnbnju24, a complex of a deletion of nt972-nt979 (nt642-nt649 in cDNA) and an insertion of a 12 bp fragment (AAAGTCCTCTCT) in mstnb genomic DNA, predicted to encode a truncated protein of only the 1st–192nd amino acid residues of wild-type Mstnb and an additional fragment (KVLSHPDQPVAERRHEAAAAVVAQAARE) due to the inserted 12 bp DNA fragment and reading frame shift (Fig. 6B, C). Eight fish in P4wt carried mstnbnju25, a complex of a deletion of nt977-nt978 (nt647-nt648 in cDNA) and an insertion of a 32 bp fragment (TCAGAGACCTGGTCTAACACTGGGTCTCTCTA), predicted to encode a mutated Mstnb protein in which the original 195th amino acid residue D was replaced with a fragment (QRPGLTLGLSN) (Fig. 6B, C). In summary, we have obtained 50 F1 mutants, of which 13 carry reading frame shift mutations that are predicted to encode function null proteins (Table 4).
Table 4.
Summary of the Identification of the Yellow Catfish (T. fulvidraco) Carrying Disrupted mstnb
| Founder no. | Number of juveniles carrying mutated mstn/total examined | Genotype of juveniles carrying mutated mstn (number of juveniles) |
|---|---|---|
| P1 | 8/134 | mstnbnju20/+ (8) |
| P2M3 | 0/34 | N/A |
| P4 | 42/256 | mstnbnju21/+ (12), mstnbnju22/+ (12), mstnbnju23/+ (9), mstnbnju24/+ (1) and mstnbnju25/+ (8) |
Discussion
Unlike mammals, fish have duplicated copies of myostatin gene. Previously, we reported molecular cloning of yellow catfish mstna using PCR with degenerate primers.27 Performing deep sequencing of the transcriptome of yellow catfish muscle, we successfully obtained mstnb cDNA. Analyses of the genomic sequence of mstnb revealed that its exon 3 is more conserved than exon 1 or 2 during evolution. Consistently, the bioactive C-terminal domain, the mature form of Mstn that is encoded by exon 3 of mstnb, displays high identity (69%–93%), whereas Mstnb overall shares 51%–67% amino acid identity to Mstns of other vertebrates, including human, cattle, chicken, frog, and zebrafish (Table 2). Moreover, phylogenic analysis revealed that yellow catfish mstnb is clustered in the fish mstnb cluster and mstna in the fish mstna cluster. The results suggest that the two genes have a common origin and separated in ancient genome duplication in fish.
Unlike mammalian Mstns that are predominately expressed in developing somite and skeletal muscles during development and adulthood,2,28–30 fish mstn mRNA was found to be highly expressed in other organs, including the brain, eyes, intestine, skin, gill filaments, gonad, heart, kidney, and spleen, besides muscle.31,32 Previously, we reported that yellow catfish mstna is widely expressed in different adult tissues similar to other fishes.27 Compared with mstna, yellow catfish mstnb is not expressed in the stomach, intestine, kidney, gill, or heart, and is more weakly expressed in other tissues, including muscle, except for spleen. The partially overlapped expression of these two mstn genes suggests they may share redundant functions in some tissues, but play different functions in some other tissues.
Because no spontaneous mutants carrying null mstn alleles have been found in fish, it is necessary to knock out the genes in fish genome to understand the roles of mstn in fish growth. Previously, we knocked out mstna using the ZFN technology with a pair of ZFNs that cleaved mstna in exon 1.24 However, the difficulty in obtaining ZFN pairs with high activity limits its application. For example, the mutation rates of mstna induced by the two ZFN pairs that we selected to knock out yellow catfish mstna were from 0% to 2%.24 Recently, the TALEN technology has proved to be robust and efficient in gene modification of any organism. Employing the TALEN technology, researchers have produced knockout animals, including rat, silkworm, zebrafish, and mouse.16–23,33–35 In this study, we report knocking out mstnb in yellow catfish using TALEN technology. This is the first successful heritable application of TALEN in farmed fish. Although the mutation rates of mstnb alleles induced by the TALEN2-mstnb pair were 14% in yellow catfish embryos, we obtained 50 F1 yellow catfish carrying mutated mstnb alleles from 2 of 4 randomly selected founders. Of the 50 F1 mutants, 13 are predicted to carry mstnb null alleles. Our results support that TALEN is a powerful genetic tool to do genome editing in farmed fish.
Besides its powerfulness, TALEN is considered to yield less off-target mutation than ZFN.36 Because of the lack of whole genome information, we are unable to predict potential off-target sites of the TALEN pairs we used to knock out mstnb in yellow catfish genome. However, the off-target mutation could be separated from the targeted mutation by backcrossing the mutants to the wild-type background even if any off-target mutation exists in our F1 mutants.
Because it takes at least 1 year for yellow catfish to be sexually mature, we only have F1 mstnb heterozygous mutant currently. Of the various types of heterozygous mutants, they all display normal growth like their wild-type siblings and we have not observed any muscle phenotype. This phenomenon is consistent with the phenotype of heterozygous mutants of Mstn knockout mice.2 Upon obtaining homozygous mutants of mstnb (mstnb−/−) and mstna (mstna−/−), and double homozygous mutants of mstna and mstnb (mstna−/−; mstnb−/−), we will know the roles of mstna and mstnb in the growth of yellow catfish.
Acknowledgment
This work was supported by the Fund for Independent Innovation on Agriculture Science and Technology of Jiangsu Province [CX(11)1036], Graduate Student Research and Innovation Program of Jiangsu Province (CXZZ12_0048), the Scientific Research Foundation of Graduate School of Nanjing University (2013CW10), and the National Natural Science Foundation of China (31171434).
Disclosure Statement
No competing financial interests exist.
References
- 1.Stinckens A, Georges M, Buys N. Mutations in the myostatin gene leading to hypermuscularity in mammals: indications for a similar mechanism in fish? Anim Genet 2011;42:229–234 [DOI] [PubMed] [Google Scholar]
- 2.McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997;387:83–90 [DOI] [PubMed] [Google Scholar]
- 3.Grobet L, Martin LJR, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. . A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 1997;17:71–74 [DOI] [PubMed] [Google Scholar]
- 4.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, et al. . A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 2006;38:813–818 [DOI] [PubMed] [Google Scholar]
- 5.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. . A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet 2007;3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. . Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. New Engl J Med 2004;351:1030–1031 [DOI] [PubMed] [Google Scholar]
- 7.Acosta J, Carpio Y, Borroto I, González O, Estrada MP. Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J Biotechnol 2005;119:324–331 [DOI] [PubMed] [Google Scholar]
- 8.Lee C-Y, Hu S-Y, Gong H-Y, Chen MH-C, Lu J-K, Wu J-L. Suppression of myostatin with vector-based RNA interference causes a double-muscle effect in transgenic zebrafish. Biochem Biophys Res Commun 2009;387:766–771 [DOI] [PubMed] [Google Scholar]
- 9.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet 2005;6:507–512 [DOI] [PubMed] [Google Scholar]
- 10.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 1996;93:1156–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith J, Berg JM, Chandrasegaran S. A detailed study of the substrate specificity of a chimeric restriction enzyme. Nucleic Acids Res 1999;27:674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, et al. . Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol 2001;21:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, et al. . Breaking the Code of DNA Binding Specificity of TAL-Type III Effectors. Science 2009;326:1509–1512 [DOI] [PubMed] [Google Scholar]
- 14.Moscou MJ, Bogdanove AJ. A Simple Cipher Governs DNA Recognition by TAL Effectors. Science 2009;326:1501. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, et al. . Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res 2011;39:6315–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 2011;29:699–700 [DOI] [PubMed] [Google Scholar]
- 17.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, et al. . In vivo genome editing using a high-efficiency TALEN system. Nature 2012;491:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesson L, Usal C, Menoret S, Leung E, Niles BJ, Remy S, et al. . Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol 2011;29:695–696 [DOI] [PubMed] [Google Scholar]
- 19.Ma S, Zhang S, Wang F, Liu Y, Liu Y, Xu H, et al. . Highly Efficient and Specific Genome Editing in Silkworm Using Custom TALENs. PLoS One 2012;7:e45035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajwan S, Takasu Y, Tamura T, Uchino K, Sezutsu H, Zurovec M. Efficient disruption of endogenous Bombyx gene by TAL effector nucleases. Insect Biochem Mol Biol 2013;43:17–23 [DOI] [PubMed] [Google Scholar]
- 21.Qiu Z, Liu M, Chen Z, Shao Y, Pan H, Wei G, et al. . High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic Acids Res 2013;41:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hu YC, Markoulaki S, Welstead GG, Cheng AW, Shivalila CS, et al. . TALEN-mediated editing of the mouse Y chromosome. Nat Biotechnol 2013;31:530–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, et al. . Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol 2013;31:23–24 [DOI] [PubMed] [Google Scholar]
- 24.Dong Z, Ge J, Li K, Xu Z, Liang D, Li J, et al. . Heritable Targeted Inactivation of Myostatin Gene in Yellow Catfish (Pelteobagrus fulvidraco) Using Engineered Zinc Finger Nucleases. PLoS One 2011;6:e28897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, VanDyk JK, et al. . TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res 2012;40:W117–W122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan J, Wang X, Song W, Chen J, Li C, Zhao Q. Molecular cloning and expression pattern of myostatin gene in yellow catfish (Pelteobagrus fulvidraco). DNA Seq 2007;18:279–287 [DOI] [PubMed] [Google Scholar]
- 28.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 1997;7:910–916 [DOI] [PubMed] [Google Scholar]
- 29.Ji S, Losinski RL, Cornelius SG, Frank GR, Willis GM, Gerrard DE, et al. . Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol 1998;275:R1265–R1273 [DOI] [PubMed] [Google Scholar]
- 30.Kocamis H, Kirkpatrick-Keller DC, Richter J, Killefer J. The ontogeny of myostatin, follistatin and activin-B mRNA expression during chicken embryonic development. Growth Dev Aging 1999;63:143–150 [PubMed] [Google Scholar]
- 31.Rodgers BD, Weber GM, Sullivan CV, Levine MA. Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: oreochromis mossambicus and Morone chrysops. Endocrinology 2001;142:1412–1418 [DOI] [PubMed] [Google Scholar]
- 32.Maccatrozzo L, Bargelloni L, Radaelli G, Mascarello F, Patarnello T. Characterization of the myostatin gene in the gilthead seabream (Sparus aurata): sequence, genomic structure, and expression pattern. Mar Biotechnol (NY) 2001;3:224–230 [DOI] [PubMed] [Google Scholar]
- 33.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. . A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 2011;29:143–148 [DOI] [PubMed] [Google Scholar]
- 34.Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 2011;39:9283–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotech 2012;30:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackburn PR, Campbell JM, Clark KJ, Ekker SC. The CRISPR system-keeping zebrafish gene targeting fresh. Zebrafish 2013;10:116–118 [DOI] [PMC free article] [PubMed] [Google Scholar]