Abstract
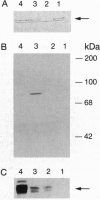
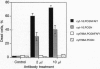
Fas, a member of the tumor necrosis factor receptor family, can induce apoptosis when activated by Fas ligand binding or anti-Fas antibody crosslinking. Genetic studies have shown that a defect in Fas-mediated apoptosis resulted in abnormal development and function of the immune system in mice. A point mutation in the cytoplasmic domain of Fas (a single base change from T to A at base 786), replacing isoleucine with asparagine, abolishes the signal transducing property of Fas. Mice homozygous for this mutant allele (lprcg/lprcg mice) develop lymphadenopathy and a lupus-like autoimmune disease. Little is known about the mechanism of signal transduction in Fas-mediated apoptosis. In this study, we used the two-hybrid screen in yeast to isolate a Fas-associated protein factor, FAF1, which specifically interacts with the cytoplasmic domain of wild-type Fas but not the lprcg-mutated Fas protein. This interaction occurs not only in yeast but also in mammalian cells. When transiently expressed in L cells, FAF1 potentiated Fas-induced apoptosis. A search of available DNA and protein sequence data banks did not reveal significant homology between FAF1 and known proteins. Therefore, FAF1 is an unusual protein that binds to the wild type but not the inactive point mutant of Fas. FAF1 potentiates Fas-induced cell killing and is a candidate signal transducing molecule in the regulation of apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Armitage R. J., Maraskovsky E., Tough T. W., Roux E., Schooley K., Ramsdell F., Lynch D. H. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993 Dec 1;178(6):2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M. P., Mett I. L., Varfolomeev E. E., Chumakov I., Shemer-Avni Y., Camonis J. H., Wallach D. Self-association of the "death domains" of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995 Jan 6;270(1):387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- Boldin M. P., Varfolomeev E. E., Pancer Z., Mett I. L., Camonis J. H., Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995 Apr 7;270(14):7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Brodsky M. H., Warton M., Myers R. M., Littman D. R. Analysis of the site in CD4 that binds to the HIV envelope glycoprotein. J Immunol. 1990 Apr 15;144(8):3078–3086. [PubMed] [Google Scholar]
- Chinnaiyan A. M., O'Rourke K., Tewari M., Dixit V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995 May 19;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Heller R. A., Krönke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol. 1994 Jul;126(1):5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Mutations in the Fas antigen gene in lpr mice. Semin Immunol. 1994 Feb;6(1):3–8. doi: 10.1006/smim.1994.1002. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Meterissian S., Ford R. J. Fas/APO-1 expression and function on malignant cells of hematologic and nonhematologic origin. J Immunother Emphasis Tumor Immunol. 1993 Oct;14(3):234–241. doi: 10.1097/00002371-199310000-00011. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Stanger B. Z., Leder P., Lee T. H., Kim E., Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995 May 19;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Two TNF receptors. Immunol Today. 1992 May;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]