Abstract
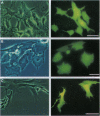
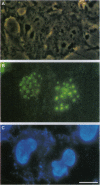
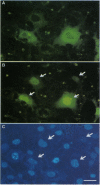
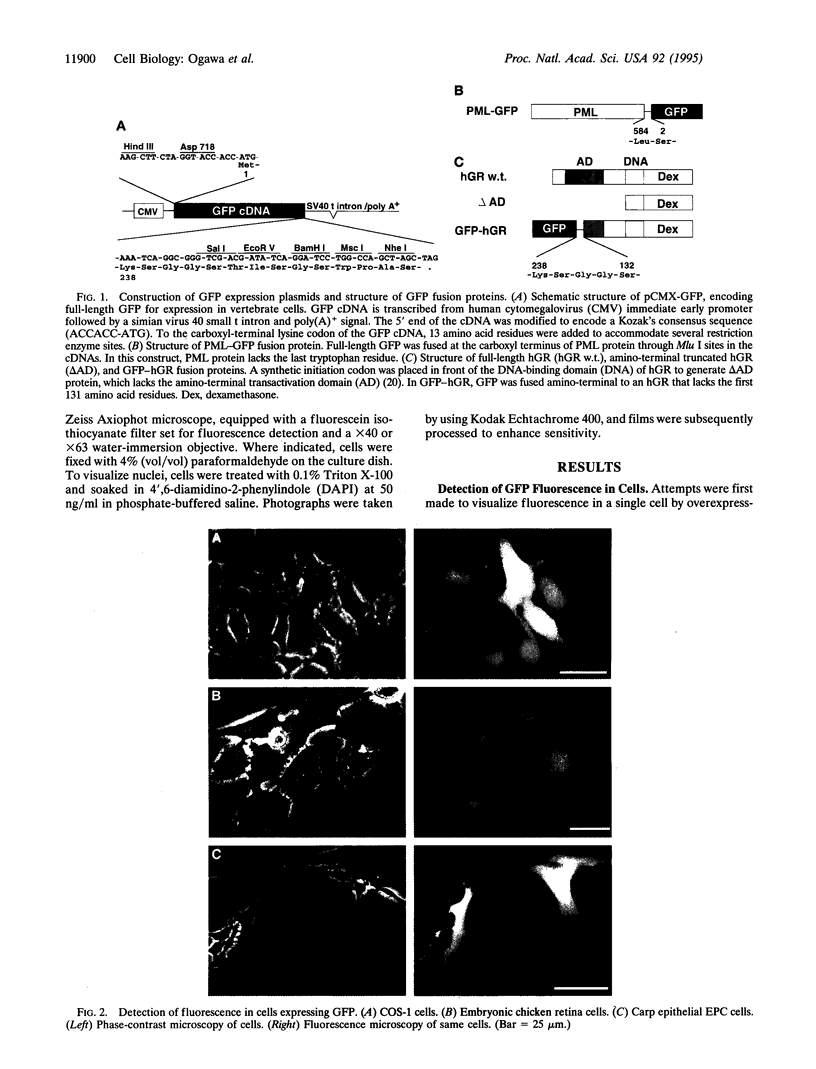
The localization, trafficking, and fluorescence of Aequorea green fluorescent protein (GFP) in cultured vertebrate cells transiently transfected with GFP cDNA were studied. Fluorescence of GFP in UV light was found to be strongest when cells were incubated at 30 degrees C but was barely visible at an incubation temperature of 37 degrees C. COS-1 cells, primary chicken embryonic retina cells, and carp epithelial cells were fluorescently labeled under these conditions. GFP was distributed uniformly throughout the cytoplasm and nucleus independent of cell type examined. When GFP was fused to PML protooncogene product, fluorescence was detected in a unique nuclear organelle pattern indistinguishable from that of PML protein, showing the potential use of GFP as a fluorescent tag. To analyze both function and intracellular trafficking of proteins fused to GFP, a GFP-human glucocorticoid receptor fusion construct was prepared. The GFP-human glucocorticoid receptor efficiently transactivated the mouse mammary tumor virus promoter in response to dexamethasone at 30 degrees C but not at 37 degrees C, indicating that temperature is important, even for function of the GFP fusion protein. The dexamethasone-induced translocation of GFP-human glucocorticoid receptor from cytoplasm to nucleus was complete within 15 min; the translocation could be monitored in a single living cell in real time.
Full text
PDF
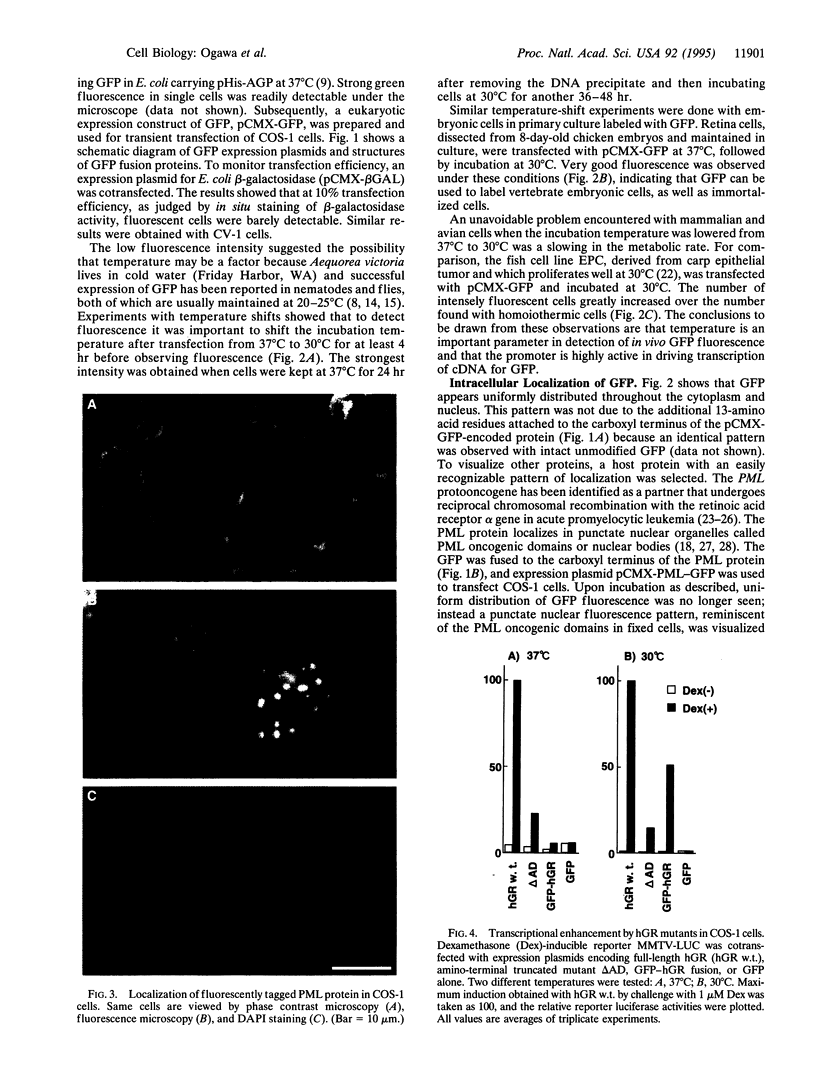
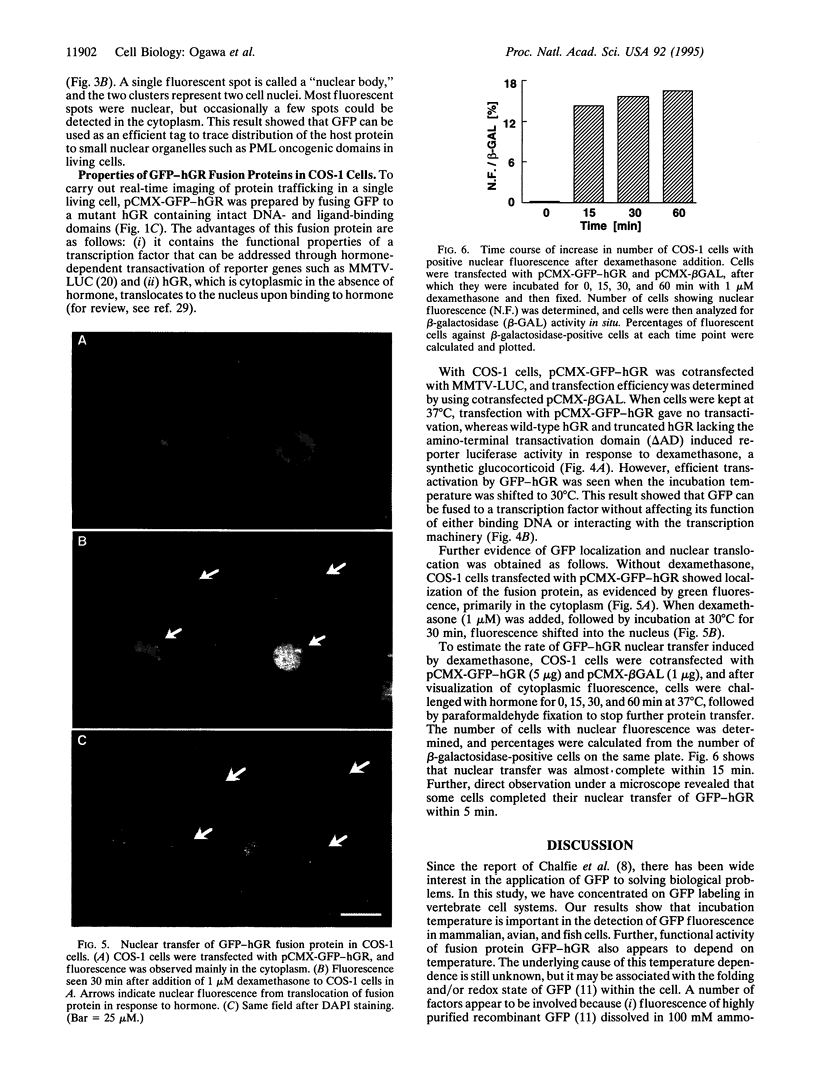

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akner G., Wikström A. C., Gustafsson J. A. Subcellular distribution of the glucocorticoid receptor and evidence for its association with microtubules. J Steroid Biochem Mol Biol. 1995 Jan;52(1):1–16. doi: 10.1016/0960-0760(94)00155-f. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cody C. W., Prasher D. C., Westler W. M., Prendergast F. G., Ward W. W. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry. 1993 Feb 9;32(5):1212–1218. doi: 10.1021/bi00056a003. [DOI] [PubMed] [Google Scholar]
- Delagrave S., Hawtin R. E., Silva C. M., Yang M. M., Youvan D. C. Red-shifted excitation mutants of the green fluorescent protein. Biotechnology (N Y) 1995 Feb;13(2):151–154. doi: 10.1038/nbt0295-151. [DOI] [PubMed] [Google Scholar]
- Dyck J. A., Maul G. G., Miller W. H., Jr, Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994 Jan 28;76(2):333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Goddard A. D., Borrow J., Freemont P. S., Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991 Nov 29;254(5036):1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995 Feb 23;373(6516):663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Heim R., Prasher D. C., Tsien R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Tsuji F. I. Aequorea green fluorescent protein. Expression of the gene and fluorescence characteristics of the recombinant protein. FEBS Lett. 1994 Mar 21;341(2-3):277–280. doi: 10.1016/0014-5793(94)80472-9. [DOI] [PubMed] [Google Scholar]
- Inouye S., Tsuji F. I. Evidence for redox forms of the Aequorea green fluorescent protein. FEBS Lett. 1994 Sep 5;351(2):211–214. doi: 10.1016/0014-5793(94)00859-0. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Kastner P., Perez A., Lutz Y., Rochette-Egly C., Gaub M. P., Durand B., Lanotte M., Berger R., Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992 Feb;11(2):629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M. H., Puvion-Dutilleul F., Guillemin M. C., Viron A., Linares-Cruz G., Stuurman N., de Jong L., Szostecki C., Calvo F., Chomienne C. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994 Mar 1;13(5):1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Molloy R., Moss G. W., Howe J. R., Hughes T. E. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995 Feb;14(2):211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Morin J. G., Hastings J. W. Energy transfer in a bioluminescent system. J Cell Physiol. 1971 Jun;77(3):313–318. doi: 10.1002/jcp.1040770305. [DOI] [PubMed] [Google Scholar]
- Morise H., Shimomura O., Johnson F. H., Winant J. Intermolecular energy transfer in the bioluminescent system of Aequorea. Biochemistry. 1974 Jun 4;13(12):2656–2662. doi: 10.1021/bi00709a028. [DOI] [PubMed] [Google Scholar]
- Okada T. S., Yasuda K., Araki M., Eguchi G. Possible demonstration of multipotential nature of embryonic neural retina by clonal cell culture. Dev Biol. 1979 Feb;68(2):600–617. doi: 10.1016/0012-1606(79)90230-6. [DOI] [PubMed] [Google Scholar]
- Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992 Feb 15;111(2):229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Mann K. G. Chemical and physical properties of aequorin and the green fluorescent protein isolated from Aequorea forskålea. Biochemistry. 1978 Aug 22;17(17):3448–3453. doi: 10.1021/bi00610a004. [DOI] [PubMed] [Google Scholar]
- Sengupta P., Colbert H. A., Bargmann C. I. The C. elegans gene odr-7 encodes an olfactory-specific member of the nuclear receptor superfamily. Cell. 1994 Dec 16;79(6):971–980. doi: 10.1016/0092-8674(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994 Jun 2;369(6479):400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- Weis K., Rambaud S., Lavau C., Jansen J., Carvalho T., Carmo-Fonseca M., Lamond A., Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994 Jan 28;76(2):345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]