Abstract
The asymmetric unit of title compound, C12H12N2O4, consists of two independent molecules. In each molecule, the oxadiazine ring has a flattened envelope conformation with the methylene C atom as the flap atom, and the ethoxycarbonyl unit is in a syn-periplanar conformation with respect to the oxadiazine ring as indicated by O—C—C=O torsion angles of 1.9 (4) and 2.5 (4)°. The dihedral angles between the mean plane of the oxadiazine ring and the phenyl ring are 80.07 (13) and 42.98 (14)°. In the crystal, molecules are linked by C—H⋯O hydrogen bonds and stacked in a double-column along the a-axis direction.
Related literature
For the biological activity of oxadiazine derivatives, see: Barbari et al. (2003 ▶); Gsell & Maientisch (1998 ▶). For a related structure, see: Chopra et al. (2004 ▶). For puckering parameters, see: Cremer & Pople (1975 ▶).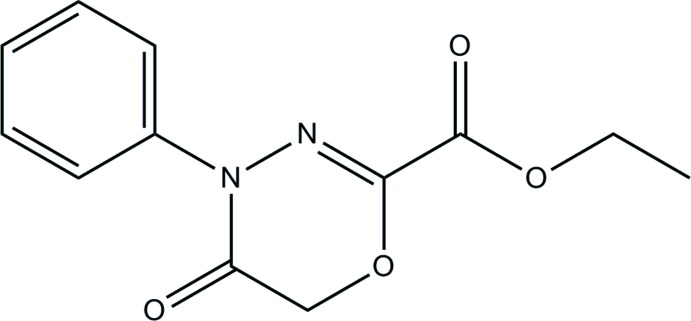
Experimental
Crystal data
C12H12N2O4
M r = 248.24
Triclinic,

a = 9.3499 (7) Å
b = 9.3601 (8) Å
c = 15.2707 (15) Å
α = 104.007 (8)°
β = 99.366 (7)°
γ = 107.862 (7)°
V = 1192.9 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 296 K
0.30 × 0.25 × 0.20 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
9756 measured reflections
4176 independent reflections
2173 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.177
S = 1.03
4176 reflections
327 parameters
H-atom parameters constrained
Δρmax = 0.20 e Å−3
Δρmin = −0.20 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536814011106/is5362sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814011106/is5362Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814011106/is5362Isup3.cml
CCDC reference: 1003020
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2A—H2A2⋯O1A i | 0.97 | 2.55 | 3.150 (3) | 120 |
| C14B—H14A⋯O3A ii | 0.97 | 2.59 | 3.418 (4) | 143 |
| C14B—H14B⋯O5B ii | 0.97 | 2.57 | 3.163 (3) | 120 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
Chandra would like to thank the UGC, New Delhi, for the award of an RFSMS fellowship under the head DV5/Physics/389/RFSMS/2009–2010/10.07.2012.
supplementary crystallographic information
1. Comment
Heterocyclic compounds containing nitrogen and oxygen atoms are of great synthetic interest due to their versatile biological significance. Oxadiazine derivatives are one among those heterocyclic compounds exhibiting various biological activities, for instance 1,2,5-oxadiazine-3,6-diones are potent antiviral agents (Barbari et al., 2003). Also, as an important type of insecticides, oxadiazine derivatives are highly efficient and of low toxicity (Gsell & Maientisch, 1998). With this background on oxadiazine derivatives, we have synthesized the title compound to study its crystal structure.
The two independent molecules (A and B) of the title compound (Fig. 1) in the asymmetric unit exhibit highly planar conformation, with their maximum deviations on ring planes at N1A and N3B are 0.081 (2) Å and 0.055 (2) Å, respectively. The central oxadiazine moiety adopts a flattened envelope conformation with puckering parameters Q(2) = 0.281 (3) Å, Q(3) = 0.118 (3) Å and φ = 325.4 (6)° (Cremer & Pople, 1975). The bond lengths and angles are generally within normal ranges and are comparable to a related structure (Chopra et al., 2004). In the molecules A and B, the oxadiazine moiety makes a dihedral angle of 80.07 (13) and 42.98 (14)°, with the phenyl rings (C4A–C9A and C16B–C21B), respectively. The ethoxycarbonyl unit is in a syn-periplanar conformation with respect to the oxadiazine moiety, as indicated by the torsion angles of 1.9 (4)° (O1A—C1A—C10A—O3A) and 2.5 (4)° (O5B—C13B—C22B—O7B) for A and B, respectively. The crystal structure is stabilized by C—H···O hydrogen bonds and the molecules are stacked in a column along the a axis (Fig. 2).
2. Experimental
Ethyl 5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazine-2-carboxylate were obtained from ethyl 2-oxo-2-(2-phenylhydrazinyl) acetate by one pot condensation-cyclization reaction with chloroacetylchloride using potassium carbonate in dry acetone as a solvent. Compounds were purified by column chromatography using petroleum ether and acetone in (2:8) as eluent.
3. Refinement
H atoms were placed at idealized positions and allowed to ride on their parent atoms with C—H distances in the range of 0.93 to 0.97 Å, and with Uiso(H) = 1.2 or 1.5Ueq(C).
Figures
Fig. 1.
Perspective diagram of the title compound with 50% probability displacement ellipsoids.
Fig. 2.
Packing diagram of the title compound viewed down the b axis.
Crystal data
| C12H12N2O4 | Z = 4 |
| Mr = 248.24 | F(000) = 520 |
| Triclinic, P1 | Dx = 1.382 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.3499 (7) Å | Cell parameters from 4176 reflections |
| b = 9.3601 (8) Å | θ = 2.4–25.0° |
| c = 15.2707 (15) Å | µ = 0.11 mm−1 |
| α = 104.007 (8)° | T = 296 K |
| β = 99.366 (7)° | Block, colourless |
| γ = 107.862 (7)° | 0.30 × 0.25 × 0.20 mm |
| V = 1192.9 (2) Å3 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | Rint = 0.034 |
| ω and φ scans | θmax = 25.0°, θmin = 2.4° |
| 9756 measured reflections | h = −11→11 |
| 4176 independent reflections | k = −11→11 |
| 2173 reflections with I > 2σ(I) | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.177 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0687P)2 + 0.0133P] where P = (Fo2 + 2Fc2)/3 |
| 4176 reflections | (Δ/σ)max < 0.001 |
| 327 parameters | Δρmax = 0.20 e Å−3 |
| 0 restraints | Δρmin = −0.20 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.9018 (3) | 0.5053 (2) | 0.41230 (12) | 0.0818 (9) | |
| O2A | 0.9228 (3) | 0.1798 (2) | 0.24645 (13) | 0.0852 (9) | |
| O3A | 0.8395 (2) | 0.7645 (2) | 0.47867 (12) | 0.0751 (8) | |
| O4A | 0.7043 (2) | 0.7358 (2) | 0.33599 (11) | 0.0627 (7) | |
| N1A | 0.8228 (2) | 0.3648 (2) | 0.22576 (13) | 0.0497 (7) | |
| N2A | 0.7753 (2) | 0.4882 (2) | 0.26292 (14) | 0.0496 (8) | |
| C1A | 0.8179 (3) | 0.5490 (3) | 0.35128 (17) | 0.0523 (9) | |
| C2A | 0.8926 (4) | 0.3456 (3) | 0.38006 (18) | 0.0762 (13) | |
| C3A | 0.8834 (3) | 0.2883 (3) | 0.27857 (18) | 0.0590 (10) | |
| C4A | 0.7924 (3) | 0.3134 (3) | 0.12527 (16) | 0.0431 (8) | |
| C5A | 0.6417 (3) | 0.2277 (3) | 0.07184 (16) | 0.0523 (9) | |
| C6A | 0.6136 (3) | 0.1792 (3) | −0.02421 (17) | 0.0590 (10) | |
| C7A | 0.7342 (3) | 0.2172 (3) | −0.06527 (17) | 0.0583 (10) | |
| C8A | 0.8838 (3) | 0.3026 (3) | −0.01176 (17) | 0.0586 (10) | |
| C9A | 0.9132 (3) | 0.3525 (3) | 0.08430 (17) | 0.0540 (9) | |
| C10A | 0.7883 (3) | 0.6949 (3) | 0.39674 (18) | 0.0538 (9) | |
| C11A | 0.6807 (3) | 0.8841 (3) | 0.37234 (19) | 0.0649 (11) | |
| C12A | 0.5841 (4) | 0.9068 (3) | 0.2930 (2) | 0.0778 (12) | |
| O5B | 0.4012 (2) | 0.5021 (2) | 0.41180 (12) | 0.0781 (8) | |
| O6B | 0.4292 (2) | 0.1793 (2) | 0.24830 (12) | 0.0734 (8) | |
| O7B | 0.3404 (2) | 0.7627 (2) | 0.47626 (12) | 0.0757 (8) | |
| O8B | 0.2062 (2) | 0.7323 (2) | 0.33296 (11) | 0.0615 (7) | |
| N3B | 0.3194 (2) | 0.3572 (2) | 0.22398 (13) | 0.0472 (7) | |
| N4B | 0.2769 (2) | 0.4843 (2) | 0.26183 (13) | 0.0474 (7) | |
| C13B | 0.3189 (3) | 0.5460 (3) | 0.34960 (16) | 0.0483 (9) | |
| C14B | 0.3933 (4) | 0.3432 (3) | 0.37963 (18) | 0.0704 (11) | |
| C15B | 0.3850 (3) | 0.2857 (3) | 0.27812 (17) | 0.0536 (9) | |
| C16B | 0.2910 (3) | 0.3093 (3) | 0.12345 (15) | 0.0423 (8) | |
| C17B | 0.3345 (3) | 0.4229 (3) | 0.08030 (17) | 0.0526 (9) | |
| C18B | 0.3045 (3) | 0.3784 (4) | −0.01600 (18) | 0.0618 (11) | |
| C19B | 0.2330 (3) | 0.2206 (3) | −0.06714 (17) | 0.0590 (10) | |
| C20B | 0.1896 (3) | 0.1073 (3) | −0.02371 (18) | 0.0611 (10) | |
| C21B | 0.2172 (3) | 0.1515 (3) | 0.07198 (17) | 0.0546 (9) | |
| C22B | 0.2899 (3) | 0.6925 (3) | 0.39472 (17) | 0.0522 (9) | |
| C23B | 0.1793 (3) | 0.8789 (3) | 0.36856 (19) | 0.0650 (11) | |
| C24B | 0.0808 (3) | 0.8990 (3) | 0.2889 (2) | 0.0775 (12) | |
| H2A1 | 0.80160 | 0.27820 | 0.39260 | 0.0920* | |
| H2A2 | 0.98330 | 0.33580 | 0.41530 | 0.0920* | |
| H5A | 0.56050 | 0.20300 | 0.10020 | 0.0630* | |
| H6A | 0.51290 | 0.12080 | −0.06110 | 0.0710* | |
| H7A | 0.71450 | 0.18480 | −0.13000 | 0.0700* | |
| H8A | 0.96490 | 0.32670 | −0.04020 | 0.0700* | |
| H9A | 1.01380 | 0.41210 | 0.12100 | 0.0650* | |
| H11A | 0.77990 | 0.97130 | 0.39730 | 0.0780* | |
| H11B | 0.62810 | 0.87900 | 0.42190 | 0.0780* | |
| H12A | 0.64050 | 0.91920 | 0.24650 | 0.1170* | |
| H12B | 0.55990 | 0.99950 | 0.31520 | 0.1170* | |
| H12C | 0.48950 | 0.81640 | 0.26630 | 0.1170* | |
| H14A | 0.30240 | 0.27520 | 0.39190 | 0.0850* | |
| H14B | 0.48420 | 0.33410 | 0.41520 | 0.0850* | |
| H17B | 0.38380 | 0.52880 | 0.11560 | 0.0630* | |
| H18B | 0.33210 | 0.45420 | −0.04610 | 0.0740* | |
| H19B | 0.21390 | 0.19040 | −0.13190 | 0.0710* | |
| H20B | 0.14180 | 0.00130 | −0.05890 | 0.0730* | |
| H21B | 0.18650 | 0.07580 | 0.10180 | 0.0660* | |
| H23A | 0.27740 | 0.96760 | 0.39330 | 0.0780* | |
| H23B | 0.12680 | 0.87300 | 0.41810 | 0.0780* | |
| H24A | 0.13650 | 0.91110 | 0.24200 | 0.1160* | |
| H24B | 0.05550 | 0.99110 | 0.31050 | 0.1160* | |
| H24C | −0.01310 | 0.80770 | 0.26270 | 0.1160* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.1398 (19) | 0.0665 (13) | 0.0441 (12) | 0.0626 (14) | −0.0008 (11) | 0.0092 (10) |
| O2A | 0.149 (2) | 0.0725 (14) | 0.0581 (13) | 0.0775 (15) | 0.0200 (12) | 0.0185 (11) |
| O3A | 0.1091 (16) | 0.0733 (14) | 0.0425 (12) | 0.0502 (13) | 0.0084 (10) | 0.0023 (10) |
| O4A | 0.0855 (13) | 0.0551 (12) | 0.0523 (11) | 0.0435 (11) | 0.0094 (9) | 0.0070 (9) |
| N1A | 0.0726 (14) | 0.0467 (12) | 0.0389 (12) | 0.0355 (11) | 0.0130 (10) | 0.0119 (10) |
| N2A | 0.0628 (13) | 0.0466 (13) | 0.0433 (13) | 0.0308 (11) | 0.0118 (10) | 0.0078 (10) |
| C1A | 0.0688 (17) | 0.0472 (16) | 0.0420 (16) | 0.0269 (14) | 0.0091 (12) | 0.0114 (13) |
| C2A | 0.130 (3) | 0.0616 (19) | 0.0494 (18) | 0.0566 (19) | 0.0152 (16) | 0.0158 (15) |
| C3A | 0.089 (2) | 0.0500 (17) | 0.0475 (17) | 0.0387 (16) | 0.0135 (14) | 0.0171 (14) |
| C4A | 0.0540 (15) | 0.0418 (14) | 0.0389 (14) | 0.0270 (12) | 0.0097 (11) | 0.0109 (11) |
| C5A | 0.0491 (15) | 0.0557 (17) | 0.0525 (16) | 0.0221 (13) | 0.0155 (12) | 0.0116 (13) |
| C6A | 0.0495 (16) | 0.0674 (18) | 0.0533 (17) | 0.0251 (14) | 0.0037 (13) | 0.0080 (14) |
| C7A | 0.077 (2) | 0.0672 (19) | 0.0397 (15) | 0.0435 (16) | 0.0124 (14) | 0.0117 (14) |
| C8A | 0.0625 (17) | 0.0724 (19) | 0.0577 (18) | 0.0366 (15) | 0.0271 (14) | 0.0264 (15) |
| C9A | 0.0483 (15) | 0.0568 (17) | 0.0581 (17) | 0.0235 (13) | 0.0114 (12) | 0.0156 (14) |
| C10A | 0.0669 (17) | 0.0499 (16) | 0.0464 (16) | 0.0282 (14) | 0.0140 (13) | 0.0096 (13) |
| C11A | 0.0750 (19) | 0.0528 (17) | 0.0652 (19) | 0.0331 (15) | 0.0164 (15) | 0.0033 (14) |
| C12A | 0.098 (2) | 0.078 (2) | 0.077 (2) | 0.0574 (19) | 0.0208 (17) | 0.0264 (18) |
| O5B | 0.1242 (17) | 0.0720 (14) | 0.0437 (11) | 0.0611 (13) | −0.0019 (10) | 0.0102 (10) |
| O6B | 0.1116 (16) | 0.0736 (14) | 0.0603 (13) | 0.0652 (13) | 0.0217 (11) | 0.0245 (11) |
| O7B | 0.1060 (16) | 0.0771 (14) | 0.0410 (11) | 0.0505 (13) | 0.0045 (10) | 0.0002 (10) |
| O8B | 0.0810 (13) | 0.0600 (12) | 0.0489 (11) | 0.0438 (11) | 0.0083 (9) | 0.0084 (9) |
| N3B | 0.0641 (13) | 0.0467 (12) | 0.0399 (12) | 0.0330 (11) | 0.0126 (10) | 0.0134 (10) |
| N4B | 0.0577 (13) | 0.0451 (12) | 0.0418 (12) | 0.0283 (11) | 0.0086 (9) | 0.0077 (10) |
| C13B | 0.0578 (16) | 0.0470 (15) | 0.0394 (15) | 0.0233 (13) | 0.0056 (12) | 0.0110 (12) |
| C14B | 0.111 (2) | 0.0634 (19) | 0.0514 (17) | 0.0511 (18) | 0.0157 (15) | 0.0216 (15) |
| C15B | 0.0681 (17) | 0.0512 (16) | 0.0485 (16) | 0.0303 (14) | 0.0134 (13) | 0.0176 (14) |
| C16B | 0.0453 (14) | 0.0443 (15) | 0.0402 (14) | 0.0239 (12) | 0.0109 (11) | 0.0083 (12) |
| C17B | 0.0541 (15) | 0.0503 (16) | 0.0542 (17) | 0.0209 (13) | 0.0102 (12) | 0.0174 (14) |
| C18B | 0.0666 (18) | 0.081 (2) | 0.0532 (17) | 0.0378 (17) | 0.0191 (14) | 0.0312 (16) |
| C19B | 0.0553 (17) | 0.083 (2) | 0.0397 (15) | 0.0378 (16) | 0.0083 (12) | 0.0067 (16) |
| C20B | 0.0577 (17) | 0.0594 (18) | 0.0550 (18) | 0.0253 (15) | 0.0054 (13) | −0.0013 (15) |
| C21B | 0.0563 (16) | 0.0498 (16) | 0.0551 (17) | 0.0231 (14) | 0.0108 (12) | 0.0089 (14) |
| C22B | 0.0593 (16) | 0.0550 (17) | 0.0440 (16) | 0.0280 (14) | 0.0106 (12) | 0.0105 (13) |
| C23B | 0.078 (2) | 0.0525 (17) | 0.0672 (19) | 0.0377 (15) | 0.0175 (15) | 0.0060 (14) |
| C24B | 0.098 (2) | 0.078 (2) | 0.084 (2) | 0.0613 (19) | 0.0261 (18) | 0.0335 (18) |
Geometric parameters (Å, º)
| O1A—C1A | 1.340 (3) | C5A—H5A | 0.9300 |
| O1A—C2A | 1.427 (4) | C6A—H6A | 0.9300 |
| O2A—C3A | 1.206 (4) | C7A—H7A | 0.9300 |
| O3A—C10A | 1.199 (3) | C8A—H8A | 0.9300 |
| O4A—C10A | 1.320 (3) | C9A—H9A | 0.9300 |
| O4A—C11A | 1.462 (3) | C11A—H11B | 0.9700 |
| O5B—C13B | 1.345 (3) | C11A—H11A | 0.9700 |
| O5B—C14B | 1.423 (3) | C12A—H12A | 0.9600 |
| O6B—C15B | 1.210 (3) | C12A—H12B | 0.9600 |
| O7B—C22B | 1.195 (3) | C12A—H12C | 0.9600 |
| O8B—C22B | 1.323 (3) | C13B—C22B | 1.503 (4) |
| O8B—C23B | 1.462 (3) | C14B—C15B | 1.493 (4) |
| N1A—C4A | 1.444 (3) | C16B—C17B | 1.375 (4) |
| N1A—C3A | 1.365 (3) | C16B—C21B | 1.383 (4) |
| N1A—N2A | 1.390 (3) | C17B—C18B | 1.383 (4) |
| N2A—C1A | 1.272 (3) | C18B—C19B | 1.380 (4) |
| N3B—N4B | 1.393 (3) | C19B—C20B | 1.376 (4) |
| N3B—C16B | 1.446 (3) | C20B—C21B | 1.376 (4) |
| N3B—C15B | 1.365 (3) | C23B—C24B | 1.489 (4) |
| N4B—C13B | 1.265 (3) | C14B—H14A | 0.9700 |
| C1A—C10A | 1.503 (4) | C14B—H14B | 0.9700 |
| C2A—C3A | 1.491 (4) | C17B—H17B | 0.9300 |
| C4A—C5A | 1.382 (4) | C18B—H18B | 0.9300 |
| C4A—C9A | 1.374 (4) | C19B—H19B | 0.9300 |
| C5A—C6A | 1.380 (3) | C20B—H20B | 0.9300 |
| C6A—C7A | 1.372 (4) | C21B—H21B | 0.9300 |
| C7A—C8A | 1.374 (4) | C23B—H23A | 0.9700 |
| C8A—C9A | 1.380 (3) | C23B—H23B | 0.9700 |
| C11A—C12A | 1.488 (4) | C24B—H24A | 0.9600 |
| C2A—H2A1 | 0.9700 | C24B—H24B | 0.9600 |
| C2A—H2A2 | 0.9700 | C24B—H24C | 0.9600 |
| O1A···O3A | 2.669 (3) | C10A···H23Bix | 3.0100 |
| O1A···N1A | 2.701 (3) | C15B···H21B | 2.8500 |
| O1A···O1Ai | 3.032 (3) | C16B···H5A | 3.0200 |
| O1A···C2Ai | 3.150 (3) | C16B···H9Avi | 3.0300 |
| O2A···C9A | 3.272 (3) | C18B···H8Avi | 3.0100 |
| O3A···C14Bii | 3.418 (4) | C19B···H6A | 3.0300 |
| O3A···O1A | 2.669 (3) | C19B···H8Avi | 3.0200 |
| O4A···N2A | 2.644 (3) | C21B···H5A | 3.0400 |
| O5B···N3B | 2.724 (3) | C22B···H11B | 3.0100 |
| O5B···O7B | 2.667 (3) | C24B···H19Bx | 3.0800 |
| O5B···O5Bii | 3.024 (3) | H2A1···O7Bii | 2.6200 |
| O5B···C14Bii | 3.163 (3) | H2A2···O3Ai | 2.6600 |
| O6B···C21B | 2.976 (3) | H2A2···O1Ai | 2.5500 |
| O7B···O5B | 2.667 (3) | H5A···O6B | 2.7700 |
| O8B···N4B | 2.638 (3) | H5A···C16B | 3.0200 |
| O1A···H2A2i | 2.5500 | H5A···C21B | 3.0400 |
| O2A···H24Biii | 2.7200 | H6A···C5Aiv | 3.0900 |
| O2A···H20Biv | 2.8100 | H6A···C19B | 3.0300 |
| O3A···H2A2i | 2.6600 | H7A···H12Cviii | 2.5800 |
| O3A···H11B | 2.6500 | H7A···H24Aviii | 2.5400 |
| O3A···H11A | 2.6800 | H8A···C18Bix | 3.0100 |
| O3A···H14Aii | 2.5900 | H8A···C19Bix | 3.0200 |
| O5B···H14Bii | 2.5700 | H9A···N4Bix | 2.7700 |
| O6B···H12Bv | 2.6600 | H9A···C16Bix | 3.0300 |
| O6B···H5A | 2.7700 | H11A···O3A | 2.6800 |
| O6B···H21B | 2.6500 | H11B···C22B | 3.0100 |
| O7B···H11B | 2.9100 | H11B···O3A | 2.6500 |
| O7B···H23A | 2.6900 | H11B···O7B | 2.9100 |
| O7B···H2A1ii | 2.6200 | H12A···H19Bviii | 2.5900 |
| O7B···H14Bii | 2.6500 | H12B···O6Bxi | 2.6600 |
| O7B···H23B | 2.6400 | H12C···H7Aviii | 2.5800 |
| N1A···O1A | 2.701 (3) | H14A···O3Aii | 2.5900 |
| N2A···O4A | 2.644 (3) | H14B···O5Bii | 2.5700 |
| N3B···O5B | 2.724 (3) | H14B···O7Bii | 2.6500 |
| N4B···O8B | 2.638 (3) | H17B···N4B | 2.6600 |
| N4B···H17B | 2.6600 | H18B···C5Aviii | 3.0400 |
| N4B···H9Avi | 2.7700 | H19B···C24Bx | 3.0800 |
| C2A···O1Ai | 3.150 (3) | H19B···H12Aviii | 2.5900 |
| C7A···C21Biv | 3.596 (4) | H19B···H24Cx | 2.5200 |
| C8A···C8Avii | 3.562 (4) | H20B···O2Aiv | 2.8100 |
| C9A···O2A | 3.272 (3) | H21B···O6B | 2.6500 |
| C14B···O3Aii | 3.418 (4) | H21B···C15B | 2.8500 |
| C14B···O5Bii | 3.163 (3) | H21B···C7Aiv | 3.0000 |
| C18B···C18Bviii | 3.544 (5) | H23A···O7B | 2.6900 |
| C21B···O6B | 2.976 (3) | H23B···O7B | 2.6400 |
| C21B···C7Aiv | 3.596 (4) | H23B···C10Avi | 3.0100 |
| C5A···H6Aiv | 3.0900 | H24A···H7Aviii | 2.5400 |
| C5A···H18Bviii | 3.0400 | H24B···O2Axii | 2.7200 |
| C7A···H21Biv | 3.0000 | H24C···H19Bx | 2.5200 |
| C1A—O1A—C2A | 114.7 (2) | H12B—C12A—H12C | 109.00 |
| C10A—O4A—C11A | 116.1 (2) | C11A—C12A—H12A | 110.00 |
| C13B—O5B—C14B | 114.6 (2) | C11A—C12A—H12B | 109.00 |
| C22B—O8B—C23B | 116.02 (19) | C11A—C12A—H12C | 109.00 |
| C3A—N1A—C4A | 121.4 (2) | H12A—C12A—H12B | 109.00 |
| N2A—N1A—C3A | 123.2 (2) | H12A—C12A—H12C | 110.00 |
| N2A—N1A—C4A | 115.21 (19) | O5B—C13B—N4B | 127.0 (2) |
| N1A—N2A—C1A | 116.5 (2) | O5B—C13B—C22B | 112.3 (2) |
| N4B—N3B—C16B | 114.74 (19) | N4B—C13B—C22B | 120.5 (2) |
| C15B—N3B—C16B | 122.9 (2) | O5B—C14B—C15B | 114.3 (2) |
| N4B—N3B—C15B | 122.32 (19) | O6B—C15B—N3B | 124.3 (2) |
| N3B—N4B—C13B | 117.6 (2) | O6B—C15B—C14B | 120.6 (2) |
| O1A—C1A—N2A | 126.9 (3) | N3B—C15B—C14B | 115.0 (2) |
| O1A—C1A—C10A | 112.7 (2) | N3B—C16B—C17B | 119.1 (2) |
| N2A—C1A—C10A | 120.1 (2) | N3B—C16B—C21B | 119.9 (2) |
| O1A—C2A—C3A | 114.0 (2) | C17B—C16B—C21B | 121.0 (2) |
| N1A—C3A—C2A | 114.7 (2) | C16B—C17B—C18B | 119.4 (3) |
| O2A—C3A—C2A | 121.8 (3) | C17B—C18B—C19B | 119.5 (3) |
| O2A—C3A—N1A | 123.5 (2) | C18B—C19B—C20B | 120.9 (2) |
| C5A—C4A—C9A | 121.1 (2) | C19B—C20B—C21B | 119.7 (3) |
| N1A—C4A—C5A | 119.4 (2) | C16B—C21B—C20B | 119.5 (2) |
| N1A—C4A—C9A | 119.5 (2) | O7B—C22B—O8B | 125.9 (3) |
| C4A—C5A—C6A | 119.0 (3) | O7B—C22B—C13B | 122.5 (2) |
| C5A—C6A—C7A | 120.0 (3) | O8B—C22B—C13B | 111.6 (2) |
| C6A—C7A—C8A | 120.7 (2) | O8B—C23B—C24B | 107.2 (2) |
| C7A—C8A—C9A | 119.8 (3) | O5B—C14B—H14A | 109.00 |
| C4A—C9A—C8A | 119.4 (3) | O5B—C14B—H14B | 109.00 |
| O3A—C10A—O4A | 125.7 (3) | C15B—C14B—H14A | 109.00 |
| O3A—C10A—C1A | 122.2 (3) | C15B—C14B—H14B | 109.00 |
| O4A—C10A—C1A | 112.1 (2) | H14A—C14B—H14B | 108.00 |
| O4A—C11A—C12A | 107.0 (2) | C16B—C17B—H17B | 120.00 |
| C3A—C2A—H2A1 | 109.00 | C18B—C17B—H17B | 120.00 |
| C3A—C2A—H2A2 | 109.00 | C17B—C18B—H18B | 120.00 |
| O1A—C2A—H2A2 | 109.00 | C19B—C18B—H18B | 120.00 |
| O1A—C2A—H2A1 | 109.00 | C18B—C19B—H19B | 120.00 |
| H2A1—C2A—H2A2 | 108.00 | C20B—C19B—H19B | 120.00 |
| C6A—C5A—H5A | 121.00 | C19B—C20B—H20B | 120.00 |
| C4A—C5A—H5A | 120.00 | C21B—C20B—H20B | 120.00 |
| C5A—C6A—H6A | 120.00 | C16B—C21B—H21B | 120.00 |
| C7A—C6A—H6A | 120.00 | C20B—C21B—H21B | 120.00 |
| C8A—C7A—H7A | 120.00 | O8B—C23B—H23A | 110.00 |
| C6A—C7A—H7A | 120.00 | O8B—C23B—H23B | 110.00 |
| C9A—C8A—H8A | 120.00 | C24B—C23B—H23A | 110.00 |
| C7A—C8A—H8A | 120.00 | C24B—C23B—H23B | 110.00 |
| C4A—C9A—H9A | 120.00 | H23A—C23B—H23B | 109.00 |
| C8A—C9A—H9A | 120.00 | C23B—C24B—H24A | 109.00 |
| C12A—C11A—H11A | 110.00 | C23B—C24B—H24B | 110.00 |
| C12A—C11A—H11B | 110.00 | C23B—C24B—H24C | 109.00 |
| O4A—C11A—H11A | 110.00 | H24A—C24B—H24B | 109.00 |
| O4A—C11A—H11B | 110.00 | H24A—C24B—H24C | 109.00 |
| H11A—C11A—H11B | 109.00 | H24B—C24B—H24C | 110.00 |
| C2A—O1A—C1A—N2A | −24.5 (4) | N4B—N3B—C15B—O6B | −176.9 (2) |
| C2A—O1A—C1A—C10A | 161.4 (3) | C15B—N3B—C16B—C21B | 48.9 (4) |
| C1A—O1A—C2A—C3A | 36.4 (4) | N3B—N4B—C13B—O5B | −0.1 (4) |
| C11A—O4A—C10A—O3A | 3.7 (4) | N3B—N4B—C13B—C22B | 174.4 (2) |
| C11A—O4A—C10A—C1A | −174.9 (2) | N2A—C1A—C10A—O3A | −172.7 (3) |
| C10A—O4A—C11A—C12A | −180.0 (3) | N2A—C1A—C10A—O4A | 6.0 (4) |
| C13B—O5B—C14B—C15B | 35.2 (4) | O1A—C1A—C10A—O4A | −179.4 (2) |
| C14B—O5B—C13B—N4B | −22.8 (4) | O1A—C1A—C10A—O3A | 1.9 (4) |
| C14B—O5B—C13B—C22B | 162.3 (2) | O1A—C2A—C3A—O2A | 156.5 (3) |
| C22B—O8B—C23B—C24B | −178.7 (2) | O1A—C2A—C3A—N1A | −25.5 (4) |
| C23B—O8B—C22B—C13B | −176.4 (2) | N1A—C4A—C5A—C6A | 179.9 (2) |
| C23B—O8B—C22B—O7B | 2.4 (4) | C5A—C4A—C9A—C8A | −1.3 (4) |
| N2A—N1A—C3A—O2A | 179.0 (3) | C9A—C4A—C5A—C6A | 0.9 (4) |
| N2A—N1A—C3A—C2A | 1.0 (4) | N1A—C4A—C9A—C8A | 179.7 (2) |
| C4A—N1A—N2A—C1A | −171.4 (2) | C4A—C5A—C6A—C7A | −0.5 (4) |
| C3A—N1A—C4A—C9A | −78.9 (3) | C5A—C6A—C7A—C8A | 0.4 (4) |
| N2A—N1A—C4A—C5A | −73.3 (3) | C6A—C7A—C8A—C9A | −0.8 (4) |
| C4A—N1A—C3A—O2A | 4.0 (4) | C7A—C8A—C9A—C4A | 1.2 (4) |
| C4A—N1A—C3A—C2A | −174.0 (2) | O5B—C13B—C22B—O7B | 2.5 (4) |
| C3A—N1A—N2A—C1A | 13.3 (3) | O5B—C13B—C22B—O8B | −178.7 (2) |
| N2A—N1A—C4A—C9A | 105.7 (3) | N4B—C13B—C22B—O7B | −172.8 (3) |
| C3A—N1A—C4A—C5A | 102.1 (3) | N4B—C13B—C22B—O8B | 6.0 (4) |
| N1A—N2A—C1A—O1A | −1.0 (4) | O5B—C14B—C15B—O6B | 155.0 (3) |
| N1A—N2A—C1A—C10A | 172.8 (2) | O5B—C14B—C15B—N3B | −27.5 (4) |
| C15B—N3B—N4B—C13B | 8.9 (3) | N3B—C16B—C17B—C18B | −178.5 (3) |
| C16B—N3B—N4B—C13B | −170.1 (2) | C21B—C16B—C17B—C18B | −0.3 (5) |
| N4B—N3B—C15B—C14B | 5.8 (4) | N3B—C16B—C21B—C20B | 179.5 (3) |
| C16B—N3B—C15B—O6B | 2.0 (4) | C17B—C16B—C21B—C20B | 1.3 (5) |
| C16B—N3B—C15B—C14B | −175.3 (2) | C16B—C17B—C18B—C19B | −0.7 (5) |
| N4B—N3B—C16B—C17B | 46.1 (3) | C17B—C18B—C19B—C20B | 0.8 (5) |
| N4B—N3B—C16B—C21B | −132.1 (3) | C18B—C19B—C20B—C21B | 0.2 (5) |
| C15B—N3B—C16B—C17B | −132.8 (3) | C19B—C20B—C21B—C16B | −1.2 (4) |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) −x+1, −y+1, −z+1; (iii) x+1, y−1, z; (iv) −x+1, −y, −z; (v) x, y−1, z; (vi) x−1, y, z; (vii) −x+2, −y+1, −z; (viii) −x+1, −y+1, −z; (ix) x+1, y, z; (x) −x, −y+1, −z; (xi) x, y+1, z; (xii) x−1, y+1, z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2A—H2A2···O1Ai | 0.97 | 2.55 | 3.150 (3) | 120 |
| C14B—H14A···O3Aii | 0.97 | 2.59 | 3.418 (4) | 143 |
| C14B—H14B···O5Bii | 0.97 | 2.57 | 3.163 (3) | 120 |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) −x+1, −y+1, −z+1.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: IS5362).
References
- Barbari, M., Kraljevi, S., Grce, M. & Zorc, B. (2003). Acta Pharm. 53, 175–186. [PubMed]
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chopra, D., Mohan, T. P., Rao, K. S. & Guru Row, T. N. (2004). Acta Cryst. E60, o2413–o2414.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Gsell, L. & Maientisch, P. (1998). WO Patent 9806710.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536814011106/is5362sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814011106/is5362Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814011106/is5362Isup3.cml
CCDC reference: 1003020
Additional supporting information: crystallographic information; 3D view; checkCIF report




