Abstract
Accurate and reliable assessment tools are needed in transplantation. The objective of this prospective, multicenter study was to determine the associations of the alpha and pi iso-enzymes of glutathione S-transferase (GST), measured from perfusate solution at the start and end (base and post) of kidney allograft machine perfusion, with subsequent delayed graft function (DGF). We also compared GST iso-enzyme perfusate levels from discarded versus transplanted kidneys. A total of 428 kidneys were linked to outcomes as recorded by the United Network of Organ Sharing. DGF, defined as any dialysis in the first week of transplant, occurred in 141 recipients (32%). Alpha and pi-GST levels significantly increased during machine perfusion. The adjusted relative risks (95% confidence interval) of DGF with each log-unit increase in base and post pi-GST were 1.14 (1.0-1.28) and 1.33 (1.02-1.72), respectively. Alpha-GST was not independently associated with DGF. There were no significant differences in GST values between discarded and transplanted kidneys, though renal resistance was significantly higher in discarded kidneys. We found pi-GST at the end of machine perfusion to be independently associated with DGF. Further studies should elucidate the utility of GST for identifying injured kidneys with regard to organ allocation, discard and recipient management decisions.
Keywords: Perfusion pumping, kidney injury, ischemia, biomarker
Introduction
Assessing kidney quality prior to transplantation is an important step in optimizing deceased-donor kidney utilization and allograft outcomes. Accurate quality assessment can improve the allocation process by avoiding both transplantation of poor-quality kidneys and discard of viable kidneys. Current strategies to evaluate donor kidney quality rely primarily on a combination of donor characteristics and pre-implantation biopsy, but the ability of these models to predict allograft outcomes remains imprecise (1, 2). Increasing utilization of kidneys at higher risk for poor outcomes, such as those from expanded-criteria donors (ECD) and donors after circulatory death (DCD), has heightened the need for more accurate and effective tools to determine donor kidney quality. Compared to standard-criteria kidneys, ECD and DCD kidneys have higher rates of delayed graft function (DGF) (3, 4), which often leads to prolonged hospital stays and higher transplant costs (5). Though the relationship is not definitive among DCD kidney recipients (6, 7), in general, other kidney transplant recipients who develop DGF are at increased risk of acute rejection and premature graft loss (8, 9). Thus, evaluating the extent of renal damage occurring throughout the organ preservation process could provide valuable information about kidney allograft viability and ultimately help guide organ allocation and transport as well as early recipient management.
Machine perfusion has been shown to minimize ischemic injury, reduce discard rates and improve allograft outcomes compared with static cold storage (10-12). Machine perfusion continuously flushes cold preservation solution through the kidney, which decreases metabolic demand via cold temperatures and also provides cellular nutrients and removes toxic metabolites (13). In addition to its probable therapeutic benefits, this modality holds significant promise as an opportunity to assess kidney viability itself. As such, the U.S. saw a 37% increase in the use of machine perfusion between 2007 and 2010 (14). Several studies have shown that abnormal perfusion parameters (e.g. high renal resistance or low perfusate flow) are associated with increased kidney discard rates (12, 15). Though not universally accepted as screening criteria, many institutions routinely assess kidney quality via machine pumping characteristics (16, 17). In addition to these parameters, biochemical markers related to renal physiologic/metabolic responses to ischemic injury can be measured from the preservation solution (i.e., perfusate) (18), which may enhance opportunities for kidney quality and viability assessment throughout the preservation process.
There are conflicting reports regarding the ability of various perfusate biomarkers to assess kidney quality and predict graft outcomes (19). As highlighted in our recent systematic review, one of the most promising biomarkers is glutathione S-transferase (GST). The GST protein family is composed of preformed cytosolic enzymes that are important for the detoxification of free radicals in many human tissues, including renal tubular cells. Whereas serum GST is more likely reflective of hepatocellular damage, elevated urine GST occurs during renal injury and has been associated with acute kidney injury in several patient settings (20). As pumped kidneys may undergo extended periods of ischemic injury, high concentrations of GST in perfusate may reflect kidney damage during organ preservation/transport and even help predict outcomes like DGF. As a potential advantage over measuring total GST concentrations, GST iso-enzyme levels may allow us to better pinpoint the intrarenal location of injury since alpha-GST is primarily released by damaged proximal tubule cells and pi-GST by the distal tubule (21). Despite renewed interest in perfusate GST, its clinical utility in kidney transplantation has yet to be elucidated (22, 23). We therefore conducted a prospective, multicenter cohort study to assess the associations of perfusate GST iso-enzymes with the development of DGF in the setting of deceased-donor kidney transplantation.
Methods
Study Design
This prospective, multicenter observational, translational study was conducted via collaboration between four organ procurement organizations (OPOs) and five academic institutions. Yale University served as the sample and data coordinating center (DCC). The scientific review committees at each OPO and the institutional review boards at each academic site approved the study. Consent for research was obtained according to individual OPO guidelines.
Study Population
Participating OPOs enrolled deceased kidney donors between May 2010 and April 2012. Donors were included if at least one kidney was machine perfused and transplanted. Recipients of single kidneys with at least one GST value were included. Kidneys were excluded if no perfusate samples were obtained. We also excluded kidneys that were pumped en bloc, since it would not be possible to determine the contribution of each individual kidney to the biomarkers concentrations in the perfusate samples.
Donor Management and Sample Collection
Donor management and machine perfusion protocols were carried out by OPO personnel according to individual OPO protocols. In short, the LifePort Kidney Transporter (Organ Recovery Systems, Itasca, IL) was used for all individually perfused kidneys. Kidneys were pumped using pulsatile flow without additives other than 1 liter of KPS-1 solution at a targeted pressure of 30mmHg and targeted temperature of 4°C. Perfusionists at each OPO could adjust the pressure according to their protocols. Kidneys with multiple arteries were preferentially placed on a single cannula when possible. Perfusate samples were collected directly from the perfusion apparatus at two time-points: one 10 ml sample within minutes of starting machine perfusion (once the perfusionist was confident the pump setup was functioning properly, defined as “base”) and a second 10 ml sample just before the kidney left the control of the OPO (“post”). Samples were transported on ice to the individual OPOs and stored at −80°C until monthly batch shipments to the coordinating center. One OPO temporarily stored samples at −20°C for <2 weeks prior to batch shipments. The timing of sample collections as well as pump duration until the machine left the control of the OPO were recorded by OPO personnel participating in the study. Samples were subsequently processed at the DCC following a single controlled thaw, separated into 1 ml barcoded aliquots, and then stored at −80°C without the addition of protease inhibitors until biomarker measurement.
Data Collection
Donor Variables
Detailed transplant and donor clinical characteristics were abstracted by study staff from OPO donor charts. Pump parameters (perfusion flow, renal resistance, and pump duration) were abstracted from perfusion records. Additional transplant and donor variables were obtained from the United Network for Organ Sharing (UNOS) database. We calculated the kidney donor risk index (KDRI) as described by Rao et al. based on the following donor characteristics: age, gender, race, height, weight, history of hypertension and/or diabetes and terminal creatinine (24, 25). We converted the KDRI score for each donor, as per convention (24), to obtain the kidney donor profile index (KDPI).
Recipient Variables
Recipients of kidneys from enrolled donors were identified from the UNOS database, and all recipient characteristics and graft outcomes were defined according to UNOS standards.
Outcome Variable
DGF was defined by UNOS as receipt of any dialysis in the first week of transplantation.
Laboratory Measurements
EKF Diagnostics (parent company of Argutus Medical, Dublin, Ireland) measured perfusate samples (blinded to all clinical information) for alpha-GST and pi-GST in duplicates using standard enzyme-linked immunosorbent assays as previously described (20, 26). Of note, alpha and pi-GST were undetectable in stock (unused) kidney perfusate solutions. Lower detection limits were 1.9 ug/L for alpha-GST and 1.3 ug/L for pi-GST. The coefficient of variation was ≤10% for both assays.
Statistical Analysis
Descriptive statistics were reported as mean ± standard deviation or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. Alpha and pi-GST perfusate concentrations, as well as recipient characteristics and clinical outcomes, were compared between those that developed DGF and those that did not using the Wilcoxon rank sum test for continuous variables, given the non-normal distribution of these variables, and Pearson’s Chi-Square test for categorical variables.
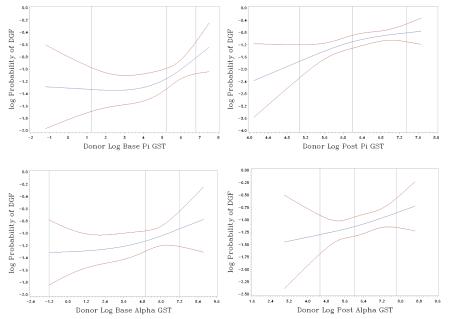
We used a log-binomial model for the probability of DGF to test the association between GST and DGF and to estimate the log relative risk (RR) of DGF per unit change in the biomarker concentration. We visually evaluated whether the raw GST iso-enzyme concentration or the natural logarithm of GST were linearly related to the response variable expressed as the log-link function of the risk of DGF using spline knots placed at the quartiles of GST or loge(GST). Three knots were sufficient to capture the patterns of the relationship (Appendix 1) (27, 28). Because loge(GST) appeared to have a linear relationship with DGF, we conducted log-binomial regression to determine the RR for DGF per unit increase in GST iso-enzyme concentration. GST levels were modeled as continuous variables (per 1 unit, per 100 units, and per loge unit), adjusting for cold ischemia time in hours, renal resistance in mmHg/ml/min, and the donor characteristics that comprise the KDRI (i.e., age in years, Black race, DCD status, hypertension, diabetes, stroke as cause of death, terminal serum creatinine in mg/dl, height in cm, and weight in kg – note, hepatitis C status not included because only one donor was seropositive). We performed further adjustment with the addition of the following recipient factors, which have been shown to associate with DGF (29): Black race, male gender, previous kidney transplant, diabetes as the cause of end stage renal disease, need for pretransplant blood transfusion, number of human leukocyte antigen mismatches, body mass index in kg/m2, and duration of dialysis before transplant in months. To facilitate convergence for parameter estimation, we used the COPY method as described by Deddens and Petersen (30); however, the fully adjusted model with recipient characteristics would not converge given several observations were missing renal resistance. Thus, renal resistance was not included in the fully adjusted model. We accounted for the cluster effect of paired kidneys from the same donor via generalized estimating equations for the log-binomial model (31). We determined that the minimal sample size needed for our analyses was 376 recipients. This was calculated to achieve 80% study power at a type I error of 0.05 to detect a RR of at least 1.2 for the development of DGF per standard deviation change in biomarker value based on an outcome prevalence of 30% at the mean biomarker value.
Additionally, we used receiver-operating characteristic curve analysis to determine the discriminatory ability for the individual perfusate biomarkers for predicting DGF in our cohort as assessed by the area under the curve (AUC). We also calculated other important test characteristics (sensitivity, etc.) for the individual perfusate biomarkers at different log-value cutoffs for predicting DGF. To determine overall predictive accuracy of the various multivariable models, we used C-statistics (analogous to AUCs in this context).
Secondary analyses were performed to compare GST values and perfusion parameters in discarded versus transplanted kidneys. For descriptive purposes, we considered any of the following UNOS-reported reasons for organ discard to represent presumed poor kidney quality: “biopsy findings,” “diseased organ,” “poor organ function,” or recipient “list exhausted”). The method used to accommodate missing data for all analyses was exclusion. All statistical tests and confidence intervals were two-sided with a significance level of 0.05. We used SAS 9.3 statistical software for Windows (SAS Institute, Cary, NC) for all analyses.
Results
Study Cohort
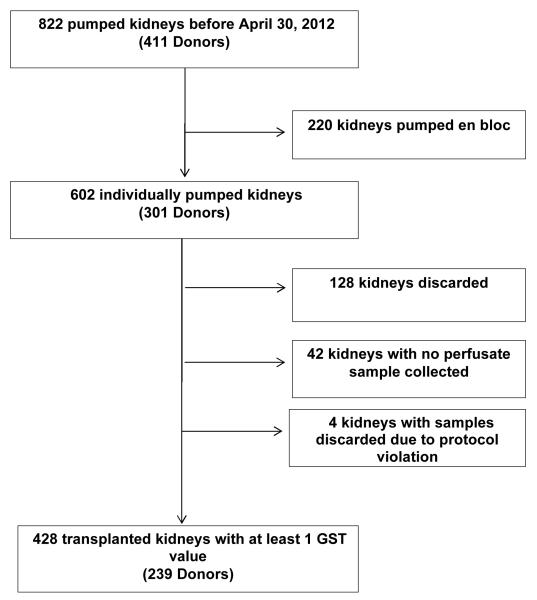
The study flow diagram is presented in Figure 1. A total of 428 kidneys with GST values were linked to recipients after pre-specified exclusions. Baseline recipient and donor characteristics are presented in Table 1. DGF occurred in 141 recipients (32%). No recipient characteristics, apart from serum creatinine at transplant, were significantly different in the DGF group compared with the non-DGF group. For donor or kidney characteristics, the only significant differences in DGF versus non-DGF groups were DCD status (44% vs. 28%, P<0.01) and kidney pump duration (12.8±5.4 vs. 11.7±5.9 hours, P=0.05). Of note, cold ischemia time and KDPI were not statistically different by DGF status.
Figure 1.
Study Flow Diagram
Table 1.
Characteristics of Kidney Transplant Recipients and Allografts by DGF status
| Recipient Characteristics 1 | ALL (N=428) |
DGF (N=141) |
Non DGF (N=287) |
P 2 | |
|---|---|---|---|---|---|
| Age, years | 54.0 (15.4) | 55.4 (13.0) | 53.3 (16.4) | 0.56 | |
| Male gender | 271 (63%) | 97 (69%) | 174 (61%) | 0.10 | |
| Black race | 178 (42%) | 66 (47%) | 112 (39%) | 0.13 | |
| Hispanic ethnicity | 71 (17%) | 27 (19%) | 44 (15%) | 0.32 | |
| Cause of ESRD | Diabetes | 121 (28%) | 44 (31%) | 77 (27%) | 0.44 |
| Hypertension | 128 (30%) | 44 (31%) | 84 (29%) | ||
| Glomerulonephritis | 75 (18%) | 18 (13%) | 57 (20%) | ||
| Graft failure | 30 (7%) | 9 (6%) | 21 (7%) | ||
| Other or unknown | 74 (17%) | 26 (18%) | 48 (17%) | ||
| HLA Mismatch Level | 0 | 11 (3%) | 2 (1%) | 9 (3%) | 0.07 |
| 1 | 5 (1%) | 3 (2%) | 2 (1%) | ||
| 2 | 11 (3%) | 3 (2%) | 8 (3%) | ||
| 3 | 54 (13%) | 12 (9%) | 42 (15%) | ||
| 4 | 106 (25%) | 28 (20%) | 78 (27%) | ||
| 5 | 153 (36%) | 57 (40%) | 96 (33%) | ||
| 6 | 88 (21%) | 36 (26%) | 52 (18%) | ||
| Peak PRA Class1 | Missing or <10% | 399 (93%) | 134 (95%) | 265 (92%) | 0.50 |
| 11- 80% | 18 (4%) | 5 (4%) | 13 (5%) | ||
| >80% | 11 (3%) | 2 (1%) | 9 (3%) | ||
| Peak PRA% | 17 [4–80] | 9 [1–36] | 25 [8.5–83.5] | 0.12 | |
| Peak PRA Class2 | Missing or <10% | 400 (93%) | 135 (96%) | 265 (92%) | 0.18 |
| 11- 80% | 16 (4%) | 5 (4%) | 11 (4%) | ||
| >80% | 12 (3%) | 1 (1%) | 11 (4%) | ||
| Peak PRA% | 62 [11–84] | 39 [10–65] | 66.5 [40–84.5] | 0.27 | |
| Pre-transplant blood transfusion | 82 (19%) | 29 (21%) | 53 (18%) | 0.60 | |
| Cold ischemia time, hours | 18.37 (6.08) | 18.83 (6.15) | 18.15 (6.04) | 0.38 | |
| Preemptive transplant | 36 (8%) | 7 (5%) | 29 (10%) | 0.12 | |
| Allograft (Donor) Characteristics | |||||
| Age, years | 46.8 (14.0) | 47.2 (13.6) | 46.6 (14.2) | 0.96 | |
| Male gender | 275 (64%) | 180 (63%) | 95 (67%) | 0.35 | |
| Black race | 73 (17%) | 28 (20%) | 45 (16%) | 0.28 | |
| Hispanic ethnicity | 98 (23%) | 74 (26%) | 24 (17%) | 0.04 | |
| DCD | 141 (33%) | 62 (44%) | 79 (28%) | 0.001 | |
| ECD | 131 (31%) | 39 (28%) | 92 (32%) | 0.35 | |
| Hypertension | 180 (42%) | 54 (38%) | 126 (44%) | 0.27 | |
| Diabetes | 40 (9%) | 11 (8%) | 29 (10%) | 0.44 | |
| Terminal serum creatinine, mg/dl | 1.11 (0.69) | 1.2 (0.85) | 1.07 (0.6) | 0.85 | |
| Height, cm | 170.4 (11.2) | 170.2 (12.3) | 170.5 (10.6) | 0.91 | |
| Weight, kg | 82.0 (21.2) | 83.2 (24.3) | 81.5 (19.6) | 0.69 | |
| Pump duration, hours 3 | 12.1 (5.7) | 12.8 (5.4) | 11.8 (5.9) | 0.05 | |
| Kidney donor profile index, % 3 | 74.1 (19.7) | 76.1 (17.7) | 73.1 (20.6) | 0.33 | |
Values are mean (standard deviation), median [interquartile range], or n (%). DGF, delayed graft function; ESRD, endstage renal disease; HLA, human leukocyte antigen; PRA, panel reactive antibody; DCD, donation after cardiac death; ECD, expanded criteria donor.
No characteristics were missing in more than 5 participants except for pump duration, which was not available in 124 kidneys
Kruskal-Wallis test for continuous variables and Chi-Square test for categorical variables.
From the time the kidney was placed on pump to the time the machine left the control of the organ procurement organization
http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf
Associations of Perfusate GST, Pump Parameters and the outcome of DGF
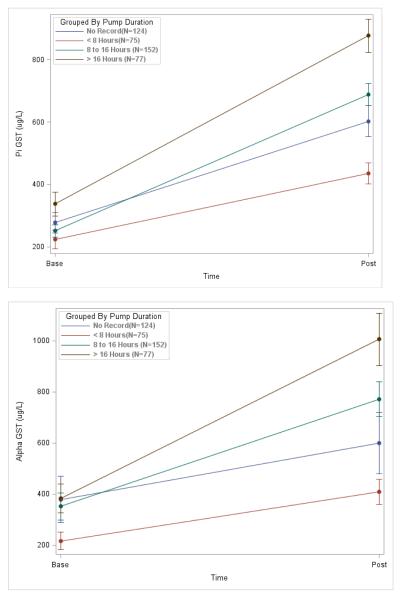
Overall, alpha and pi-GST levels significantly increased during machine perfusion from base to post time-points. This relationship was observed for varying lengths of pump durations demonstrating a progressive release of both of these biomarkers during the pumping process (Figure 2). Table 2 lists the median [interquartile range] for all of the biomarkers in the entire cohort and by DGF status. Pi-GST concentrations were significantly higher in the DGF group at both base and post time-points. Delta pi-GST (i.e., the difference between base and post values) was also greater in the DGF group. For alpha-GST, however, only the post time-point was significantly higher in the DGF group—neither base alpha-GST nor delta alpha-GST were significantly different by DGF status. For machine pump parameters, kidneys that developed DGF had higher resistance and lower flow at 4 hours, with differences approaching statistical significance. At no other time-point (1, 2, or 8 hours) were either of these parameters significantly different by DGF status. Of note, resistance was strongly correlated with flow (Appendix 2). The perfusate biomarkers were also significantly correlated, but there were no strong correlations between the perfusate biomarkers and either pump parameter.
Figure 2.
Alpha and pi-GST levels at the beginning and end of perfusion by strata of pump duration. Differences between base and post values were significant (p-values <0.001) for both biomarkers and all pump duration strata.
Table 2.
Biomarker Concentrations in Allograft Pump Perfusate by DGF Status
| Variable 1 | ALL (N=428) |
DGF (N=141) |
Non DGF (N=287) |
P 2 |
|---|---|---|---|---|
| Base pi-GST, ug/L | 191 [65–356] | 244 [76–399] | 176 [61–336] | 0.03 |
| Post pi-GST, ug/L | 498 [321–905] | 653 [394–980] | 446 [299–870] | 0.002 |
| Delta pi-GST, ug/L3 | 314 [145–601] | 368 [196–610] | 296 [124–595] | 0.05 |
| Base alpha-GST, ug/L | 147 [43–394] | 154 [66–413] | 140 [38–373] | 0.25 |
| Post alpha-GST, ug/L | 414 [218–800] | 506 [226–865] | 372 [215–748] | 0.06 |
| Delta alpha-GST, ug/L3 | 203 [81–481] | 220 [103–544] | 189 [69–406] | 0.07 |
| Renal resistance at 4 hrs, mmHg/ml/min |
0.23 [0.17–0.29] | 0.23 [0.19–0.32] | 0.22 [0.17–0.28] | 0.12 |
| Perfusate flow at 4 hrs, ml/min | 114 [94–132] | 111 [91–131] | 115 [99–133] | 0.13 |
Values are medians [interquartile range]. DGF, delayed graft function; GST, Glutathione S-transferase
For all perfusate biomarkers, base values were missing in 10 kidneys and post values were missing in 59 kidneys. Renal resistance and perfusate flow were missing in 106 kidneys.
Wilcoxon rank sum test (DGF vs. non-DGF)
Difference in post minus base values
Stratifying by donor type (DCD vs. brain death), the difference in post pi-GST concentration by DGF status approached statistical significance for DCD kidneys (P=0.06), but not for non-DCD kidneys (P=0.11). There were no statistically significant interactions between the biomarkers and donor type on the development of DGF [e.g., P=0.64 for Loge(post pi-GST)xDCD].
In multivariable analyses, KDRI variables, cold ischemia time, renal resistance and recipient factors modestly attenuated the associations between perfusate GST and DGF (Table 3). Post pi-GST had the strongest independent association with DGF at an adjusted RR of 1.36 (1.14–1.63) per log-unit increase. Base pi-GST had an adjusted RR of 1.05 (0.96–1.16) per log-unit increase. Delta pi-GST was not independently associated with DGF. No alpha-GST values (base, post or delta) nor total GST, obtained by adding alpha and pi-GST values together, were independently associated with DGF. The AUC for predicting DGF with post pi-GST alone and in the fully adjusted model revealed modest discriminatory utility at 0.6 (0.54–0.66) and 0.71 (0.65–0.78), respectively. Test characteristics for the perfusate biomarkers are provided in Appendix 3, and complete information about RRs for the adjustment variables in each model is provided in Appendix 4.
Table 3.
Association of Perfusate Biomarkers with DGF
| Biomarker | Time-point | Relative Risk (95% confidence interval) for DGF | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for donor variables only1 |
Adjusted for donor & transport variables 2 |
Adjusted for donor, transport & recipient variables 3 |
||
| Pi-GST | Base | 1.10 (0.98–1.22) | 1.07 (0.96–1.19) | 1.14 (1.01–1.27) | 1.05 (0.96–1.16) |
| Post | 1.41 (1.13–1.77) | 1.28 (1.02–1.61) | 1.36 (1.05–1.75) | 1.36 (1.14–1.63) | |
| Alpha-GST | Base | 1.06 (0.97–1.15) | 1.04 (0.96–1.13) | 1.09 (1.00–1.18) | 1.03 (0.96–1.11) |
| Post | 1.14 (0.97–1.33) | 1.03 (0.88–1.22) | 1.09 (0.93–1.26) | 1.11 (0.97–1.26) | |
Perfusate biomarkers were natural log-transformed and added individually to all models (i.e., perfusate biomarkers were not combined during multivariable adjustment). DGF, delayed graft function; GST, glutathione S-transferase.
Donor variables used for adjustment: age in years, Black race, DCD status, hypertension, diabetes, stroke as cause of death, terminal serum creatinine in mg/dl, height in cm, and weight in kg
Includes donor variables listed above plus cold ischemia time in hours and renal resistance at 4 hours in mmHg/ml/min
Includes all variables listed above (except renal resistance) plus the following recipient variables: Black race, male gender, previous kidney transplant, diabetes as the cause of end stage renal disease, need for pretransplant blood transfusion, number of human leukocyte antigen mismatches, body mass index in kg/m2, and duration of dialysis before transplant in months
We assessed the effect of perfusion duration on the association between post pi-GST and DGF via stratified analysis with three duration categories (<8, 8-16, and >16 hours). The RR estimate for DGF with each unit increase in Loge(post pi-GST) were comparable for each category in the range of 1.3 to 1.6. Multivariable analyses involving other covariates were not performed due to relatively low event rates within each duration category.
Associations of Perfusate GST and the outcome of Kidney Discard
There were 128 individually-perfused kidneys that were not transplanted, and 116 of these discarded kidneys had available GST values, out of which 30 had a UNOS-reported reason for discard. Presumed poor kidney quality (combining similarly themed reasons), was the most common justification given for discarding the kidney (60%). The UNOS-reported reasons for discard in the other kidneys were anatomical/vascular damage, potential recipient issues at transplant and “other”. While renal resistance was significantly higher and perfusate flow lower in discarded kidneys compared with transplanted kidneys, perfusate GST values were essentially no different, or even lower, in discarded kidneys (Table 4). Biomarker values were available in 32 kidney pairs in which one was transplanted and the other discarded from the same donor. DGF occurred in 8 of these transplanted kidneys (25%), but there were no significant differences in median GST values between transplanted and discarded donor kidney pairs.
Table 4.
Perfusate Biomarker Concentration by Organ Discard Status
| Biomarker |
Discarded Kidneys (N=128) |
Transplanted & DGF (N=141) |
Transplanted & Non-DGF (N=287) |
P-value (Discard vs. DGF) |
P-value (Discard vs. Non-DGF) |
|---|---|---|---|---|---|
| Base pi-GST, ug/L | 188 [42–337] | 243 [76.3–398.8] | 176 [61–335] | 0.07 | 0.90 |
| Post pi-GST, ug/L | 580 [353–965] | 652 [394–980] | 446 [298–870] | 0.34 | 0.12 |
| Base alpha-GST, ug/L | 93 [16–270] | 153 [66.3–412.5] | 140 [38–373] | 0.004 | 0.03 |
| Post alpha-GST, ug/L | 303 [158–723] | 506 [225.6–865] | 372 [215–747.5] | 0.02 | 0.22 |
| Renal resistance at 4 hrs, mmHg/ml/min |
0.29 [0.21–0.4] | 0.23 [0.19–0.32] | 0.22 [0.17–0.29] | <0.001 | <0.001 |
| Perfusate flow at 4 hrs, ml/min |
93 [71–118] | 110 [91–131] | 114 [98–131] | <0.001 | <0.001 |
Values are medians [interquartile range]. DGF, delayed graft function; GST, glutathione S-transferase. P-values were obtained by Wilcoxon rank sum tests comparing discarded kidneys with transplanted kidneys that either did or did not develop DGF as indicated.
Discussion
This is the first multicenter, prospective study of perfusate biomarkers in the United States. Since the 1970s, several investigators have suggested that GST may be useful for predicting allograft outcomes in deceased-donor kidney transplantation (19). While our study corroborates prior observations regarding associations between this perfusate biomarker and the development of DGF, our data reveal that the iso-enzyme pi-GST may be even more relevant than total GST. We found that all univariate assessments for pi-GST (base, post and delta) were associated with DGF, and after adjusting for traditional risk factors, we found a 36% increased risk for the development of DGF with each log-unit increase in the concentration of perfusate pi-GST at the end of machine perfusion. In secondary analyses of discarded versus transplanted kidneys, we also found that the traditional perfusion parameters were significantly worse in kidneys that were discarded, whereas the perfusate biomarkers values were not significantly different.
Current kidney viability assessment methods are limited in their ability to predict allograft outcomes (1, 2). Adding biomarkers to these models may ultimately offer superior information. Whereas early studies focused on univariate associations, analyses from a recent European randomized-controlled trial of machine perfusion showed that perfusate GST at the end of machine perfusion was independently associated with DGF (23). Our findings are consistent with the results from this trial but go further in clarifying the potential utility of pi-GST, a marker of distal tubule injury, over that of alpha-GST, a marker of proximal tubule injury. Only the post time-point for alpha-GST was significantly different by DGF status, but the association did not persist after multivariable adjustment. An exciting extension of these results, which challenges common teaching and requires further pre-clinical investigation, is the possibility that the degree of injury to the distal tubule during periods of ischemia may have a bigger impact on outcomes like DGF than injury affecting the proximal tubule, which may have better regenerative capacity (32).
Another implication from the current study is that the development of accurate biomarkers could ultimately allow for timely and repeatable quantification during the preservation process. As evidenced by generally increasing GST levels from base to post time-points and the association of delta pi-GST with the development of DGF in this cohort, ongoing ischemic injury in the first few hours of machine perfusion could be monitored via changes in perfusate GST iso-enzyme levels. The European machine perfusion trial suggested that overall GST levels do not continue to increase substantially past 4 to 6 hours of pumping (23). Nonetheless, dynamic information about allograft injury based on changes in perfusate biomarker levels may facilitate preservation management by directing pump parameter adjustments during the first few hours of organ transport. Perfusate biomarker analysis may ultimately facilitate clinical studies to enhance current perfusion technology and lead to innovations in machine perfusion overall.
The vast majority of discarded kidneys, which would have been included in this cohort if transplanted, did not have UNOS-reported reasons for discard; however, presumed poor kidney quality appears to be the most common justification for not transplanting these organs. Our analyses suggest perfusion parameters like resistance and flow may be influencing organ acceptance patterns. In addition, our findings of no significant differences in perfusate biomarkers between kidney pairs, where one was discarded and the other transplanted, suggests that some of these discarded kidneys may have been viable for transplantation. Perfusate biomarkers did not predict organ discard in this cohort. Nonetheless, given the clear need to reduce unnecessary organ refusals, a more forward-thinking hypothesis is that biomarkers like perfusate pi-GST might differentiate between recoverable versus unrecoverable kidney injury more precisely than current perfusion parameters. Many OPOs and prospective transplant centers pay close attention to perfusion parameters at the time of organ offer (12, 15-17). Consequently, most observational studies that assess the utility of perfusion parameters in predicting allograft outcomes are limited by potential selection bias and confounding by indication, and the current study is no different in this regard. The European machine perfusion trial was undoubtedly less affected by this type of selection bias given the blinded and paired-kidney design of the study. Subsequent analyses of the trial found high renal resistance at the end of machine perfusion to be an independent risk factor for both DGF and one-year graft failure (33). As other investigators have also suggested (34), additional studies are urgently needed to discern the role of perfusion parameters in the decision process for allograft selection and organ discard, and we believe the potential utility of perfusate biomarkers of kidney injury deserves further investigation as well.
The generalizability of this multicenter cohort and its relatively large sample size are important study strengths. We also accounted for the clustering effect of donors (shared donor characteristics across two kidneys) and adjusted for KDRI variables, pump parameters, cold ischemia time and important recipient variables. However, there are some limitations to consider. First, this was an observational study utilizing the UNOS database for the outcome of DGF. Also, while improved generalizability from a multicenter design was advantageous, our analyses could not account for all potential differences in OPO perfusion practices, particularly with regard to differences in perfusate collection times for post samples. This may have affected changes in perfusate biomarker concentrations during machine pumping. In addition, our collection of only two samples, rather than multiple serial samples, did not allow for a full description of the kinetics of biomarker release during machine perfusion. There could also be other biases associated with different perfusion practices. For example, pumping may be continued in relatively poor-quality kidneys for therapeutic purposes. Nonetheless, higher perfusate pi-GST levels conveyed increased risk for DGF across different pumping durations in this cohort. Lastly, despite adjusting for multiple donor, transplant and recipient variables, the potential for residual confounding still exists.
Before implementing novel biomarkers into clinical practice, the transplant community should demonstrate which, if any, of these markers perform reliably over several phases of development. While desirable, it is unlikely that a single biomarker will sufficiently predict outcomes on its own for use as the sole prognostic tool prior to transplant, as evidenced by modest AUCs for the biomarkers studied here. Thus, proposed biomarkers should only be considered useful in assessing kidney quality if adding them to currently utilized clinical models actually improves the predictive value of those models.
In summary, pi-GST measured from preservation solution in machine pump perfusion for deceased-donor kidney transplantation is independently associated with DGF. Our findings reveal the potential for perfusate biomarkers to be incorporated into clinical practice for assessing kidney quality during the organ preservation process, which would likely be facilitated by further point-of-care development of the most promising biomarkers (35). Besides GST, more recently described biomarkers of acute kidney injury, such as neutrophil gelatinase-associated lipocalin, interleukin-18, and kidney injury molecule-1 should be studied in the context of kidney allograft machine perfusion given mounting evidence in other transplant as well as native kidney settings (18, 36). While the ever-growing disparity between organ supply and demand in kidney transplant is well known, the renewed popularity of machine perfusion may help to narrow this gap to some degree. Novel methods like perfusate biomarker analysis deserve further systematic investigation to enhance this preservation technique, assess allograft quality and ultimately predict allograft as well as recipient outcomes in deceased-donor kidney transplantation.
Acknowledgements
We thank the dedicated clinical and other support personnel at the following organ procurement organizations for their active participation in this study: Gift of Life Philadelphia, the New York Organ Donor Network, the Michigan Organ and Tissue Donation Program, and the New Jersey Sharing Network. We especially thank our study collaborators: Isabel Butrymowicz, Rowena Kemp, Angela Yu and Katherine Xiu from Yale University; Adam Mussell and Daniel Leidy from the University of Pennsylvania; and William Reitsma from the New Jersey Sharing Network. This work was supported by the National Institutes of Health grant RO1DK-93770, a Roche Organ Transplantation Research Foundation Award, and the Health Resources and Services Administration contract 234-2005-37011C. CRP is also supported by NIH grant K24DK090203. The biomarker assays were donated by EKF Diagnostics. The sponsors did not participate in data analysis, interpretation of results or preparation of this manuscript. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. Dr. Hall, Mr. Hong and Dr. Parikh had full access to all data and take responsibility for its integrity and the accuracy of all analyses.
Abbreviations
- AUC
Area under the curve
- DCC
Data coordinating center
- DCD
Donation after cardiac death
- DGF
Delayed graft function
- ECD
expanded-criteria donor
- GST
Glutathione S-transferase
- KDRI
Kidney donor risk index
- KDPI
Kidney donor profile index
- OPO
Organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- RR
Relative risk
- UNOS
United Network for Organ Sharing
Appendix 1. Estimated spline transformation with 95% confidence intervals
Appendix 2. Spearman correlation between GST and Flow/Resistance
| Biomarker | Post pi-GST |
Base alpha-GST |
Post alpha-GST |
Renal resistance 4 at hrs |
Perfusate flow at 4 hrs |
|---|---|---|---|---|---|
| Base pi-GST | r=0.44 p<0.001 |
r=0.82 p<0.001 |
r=0.29 p<0.001 |
r=0.02 p=0.76 |
r=−0.06 p=0.27 |
| Post pi-GST | r=0.26 p<0.001 |
r=0.57 p<0.001 |
r=0.12 p=0.04 |
r=−0.17 p=0.004 |
|
| Base alpha-GST | r=0.52 p<0.001 |
r=0.03 p=0.58 |
r=−0.07 p=0.23 |
||
| Post alpha-GST | r=0.11 p=0.07 |
r=−0.1 p=0.11 |
|||
|
Renal resistance at 4 hrs |
r=−0.86 p<0.001 |
Shown here are the Spearman correlation coefficients and associated p-values between each perfusate biomarker and 4-hour perfusion parameter. GST, glutathione S-transferase.
Appendix 3. Cutoff values and test characteristics for predicting DGF using log-transformed perfusate biomarkers
| Unadjusted perfusate biomarkers for predicting DGF |
Cutoff value 1 |
Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
|
Loge(Base pi-GST) AUC 0.57 (0.51–0.63) |
5.53 | 0.50 | 0.65 | 0.42 | 0.73 |
| 2.69 | 0.90 | 0.12 | 0.33 | 0.70 | |
| 6.37 | 0.13 | 0.90 | 0.38 | 0.68 | |
|
Loge(Post pi-GST) AUC 0.6 (0.54–0.66) |
6.11 | 0.69 | 0.51 | 0.42 | 0.77 |
| 5.61 | 0.90 | 0.20 | 0.36 | 0.81 | |
| 7.09 | 0.19 | 0.90 | 0.49 | 0.69 | |
|
Loge(Base alpha-GST) AUC 0.54 (0.48–0.59) |
4.21 | 0.75 | 0.36 | 0.37 | 0.75 |
| 2.47 | 0.90 | 0.14 | 0.34 | 0.74 | |
| 6.49 | 0.14 | 0.90 | 0.42 | 0.68 | |
|
Loge(Post alpha-GST) AUC 0.56 (0.50–0.62) |
0.26 | 0.60 | 0.53 | 0.39 | 0.72 |
| 4.19 | 0.90 | 0.15 | 0.35 | 0.75 | |
| 6.89 | 0.15 | 0.90 | 0.43 | 0.68 |
DGF, delayed graft function; PPV, positive predictive value; NPV, negative predictive value.
From top down for each biomarker, these are the log values that correspond with the optimal cutoff (yielding the largest sum of sensitivity + specificity), the cutoff that yields a sensitivity of 0.9, and the cutoff that yields a specificity of 0.9.
Appendix 4. Models for predicting DGF using Loge(Post pi-GST)
| Model | Parameter | RR | 95% CI | P |
|---|---|---|---|---|
| Unadjusted biomarker Model AUC = 0.60 (0.5398–0.6603) |
Log(Post pi-GST) | 1.410 | 1.126 −1.7653 | 0.003 |
| Adjusted for donor variables only 1 Model AUC = 0.669 (0.6108–0.7264) |
Log(Post pi-GST) | 1.284 | 1.0237 −1.6113 | 0.031 |
| Donor age (years) | 1.012 | 0.9999 −1.0248 | 0.052 | |
| Donor Black race (vs. non-Black) | 1.282 | 0.8761 −1.8745 | 0.201 | |
| DCD kidney | 1.757 | 1.2266 −2.5162 | 0.002 | |
| Donor history of hypertension | 0.852 | 0.598 −1.2127 | 0.373 | |
| Donor history of diabetes | 0.900 | 0.5447 −1.4866 | 0.680 | |
| Stroke as donor cause of death | 0.904 | 0.6319 −1.2925 | 0.579 | |
| Terminal serum creatinine (mg/dl) | 1.276 | 1.0607 −1.5353 | 0.010 | |
| Donor height (cm) | 0.991 | 0.9778 −1.0044 | 0.185 | |
| Donor weight (kg) | 1.003 | 0.9974 −1.0083 | 0.306 | |
| Adjusted for donor & transport variables2 Model AUC = 0.679 (0.6124–0.7439) |
Log(Post pi-GST) | 1.358 | 1.0525 −1.7513 | 0.019 |
| Pefusion resistance at 4 hrs (mmHg/ml/min) | 2.529 | 1.1915 −5.3666 | 0.016 | |
| Cold ischemia time (hrs) | 0.994 | 0.9646 −1.0251 | 0.716 | |
| Donor age (years) | 1.014 | 1.0002 −1.0285 | 0.047 | |
| Donor Black race (vs. non-Black) | 1.197 | 0.7649 −1.8731 | 0.431 | |
| DCD kidney | 1.715 | 1.1358 −2.5884 | 0.010 | |
| Donor history of hypertension | 0.917 | 0.6253 −1.3443 | 0.657 | |
| Donor history of diabetes | 0.938 | 0.563 −1.5624 | 0.806 | |
| Stroke as donor cause of death | 0.812 | 0.5442 −1.2103 | 0.306 | |
| Terminal serum creatinine (mg/dl) | 1.239 | 0.9987 −1.5365 | 0.051 | |
| Donor height (cm) | 0.998 | 0.9826 −1.0135 | 0.793 | |
| Donor weight (kg) | 1.001 | 0.9944 −1.0083 | 0.704 | |
| Adjusted for donor, transport & recipient variables 3 Model AUC = 0.715 (0.6539–0.7751) |
Log(Post pi-GST) | 1.363 | 1.1374 −1.6324 | 0.001 |
| Cold ischemia time (hrs) | 0.993 | 0.9694 −1.0166 | 0.547 | |
| Donor age (years) | 1.010 | 1.0 −1.0194 | 0.050 | |
| Donor Black race (vs. non-Black) | 1.238 | 0.7644 −2.0054 | 0.385 | |
| DCD kidney | 1.698 | 1.2062 −2.3897 | 0.002 | |
| Donor history of hypertension | 0.737 | 0.5289 −1.0273 | 0.072 | |
| Donor history of diabetes | 1.030 | 0.6755 −1.5712 | 0.890 | |
| Stroke as donor cause of death | 0.781 | 0.5737 −1.0619 | 0.115 | |
| Terminal serum creatinine (mg/dl) | 1.500 | 1.2189 −1.8459 | 0.000 | |
| Donor height (cm) | 0.994 | 0.9817 −1.0057 | 0.300 | |
| Donor weight (kg) | 1.001 | 0.9994 −1.0032 | 0.188 | |
| Recipient Race (Black) | 1.152 | 0.9198 −1.4421 | 0.218 | |
| Recipient male (vs. female) | 1.213 | 0.9571 −1.5382 | 0.110 | |
| Previous transplant | 1.235 | 0.8028 −1.9006 | 0.337 | |
| Diabetes as cause of ESRD | 1.105 | 0.8583 −1.4214 | 0.440 | |
| Recipient blood transfusion pre-transplant | 1.300 | 1.0141 −1.6655 | 0.059 | |
| HLA mismatches (per unit increase) | 1.257 | 1.1129 −1.4196 | 0.000 | |
| Recipient BMI (kg/m2) | 1.041 | 1.0174 −1.0642 | 0.001 | |
| Duration of dialysis (months) | 1.002 | 0.9972 −1.0058 | 0.505 |
DGF, delayed graft function; RR, relative risk; CI, confidence interval; AUC, area under the curve (regression C-statistics used for multivariable models); GST, glutathione S-transferase; DCD, donation after cardiac death; ESRD, end-stage renal disease; HLA, human leukocyte antigen; BMI, body mass index.
Donor variables used for adjustment: age in years, Black race, DCD status, hypertension, diabetes, stroke as cause of death, terminal serum creatinine in mg/dl, height in cm, and weight in kg
Includes donor variables listed above plus cold ischemia time in hours and renal resistance at 4 hours in mmHg/ml/min
Includes all variables listed above (except renal resistance) plus the following recipient variables: Black race, male gender, previous kidney transplant, diabetes as the cause of end stage renal disease, need for pretransplant blood transfusion, number of human leukocyte antigen mismatches, body mass index in kg/m2, and duration of dialysis before transplant in months
Footnotes
Conflict of Interest: None
References
- 1.Louvar DW, Li N, Snyder J, Peng Y, Kasiske BL, Israni AK. “Nature versus Nurture” Study of Deceased-Donor Pairs in Kidney Transplantation. J Am Soc Nephrol. 2009 Jun;20(6):1351–8. doi: 10.1681/ASN.2008070715. PubMed PMID: 19389849. Pubmed Central PMCID: 2689893. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jochmans I, Pirenne J. Graft quality assessment in kidney transplantation: not an exact science yet! Curr Opin Organ Transplant. 2011 Apr;16(2):174–9. doi: 10.1097/MOT.0b013e3283446b31. PubMed PMID: 21383549. Epub 2011/03/09. eng. [DOI] [PubMed] [Google Scholar]
- 3.Hassanain M, Tchervenkov J, Cantarovich M, Metrakos P, Paraskevas S, Keith D, et al. Delayed graft function has an equally bad impact on deceased donor renal graft survival in both standard criteria donors and expanded criteria donors. Transplant Proc. 2009;41:133–4. doi: 10.1016/j.transproceed.2008.10.044. United States. [DOI] [PubMed] [Google Scholar]
- 4.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD--fundamentals for the practicing nephrologist. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:1827–31. doi: 10.2215/CJN.02270409. United States. [DOI] [PubMed] [Google Scholar]
- 5.Marcen R, Orofino L, Pascual J, de la Cal MA, Teruel JL, Villafruela JJ, et al. Delayed graft function does not reduce the survival of renal transplant allografts. Transplantation. 1998 Aug 27;66(4):461–6. doi: 10.1097/00007890-199808270-00008. PubMed PMID: 9734488. [DOI] [PubMed] [Google Scholar]
- 6.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007 Jul;7(7):1797–807. doi: 10.1111/j.1600-6143.2007.01852.x. PubMed PMID: 17524076. Epub 2007/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Farney AC, Rogers J, Zuckerman J, Reeves-Daniel A, Hartmann E, et al. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant. 2011 Mar-Apr;25(2):255–64. doi: 10.1111/j.1399-0012.2010.01241.x. PubMed PMID: 20331689. Epub 2010/03/25. eng. [DOI] [PubMed] [Google Scholar]
- 8.Yarlagadda SG, Coca SG, Formica RN, Jr., Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009 Mar;24(3):1039–47. doi: 10.1093/ndt/gfn667. PubMed PMID: 19103734. [DOI] [PubMed] [Google Scholar]
- 9.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996 Jun;155(6):1831–40. doi: 10.1016/s0022-5347(01)66023-3. PubMed PMID: 8618268. English. [DOI] [PubMed] [Google Scholar]
- 10.Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009 Aug;13(38):iii–iv. xi–xiv, 1–156. doi: 10.3310/hta13380. PubMed PMID: 19674537. Epub 2009/08/14. eng. [DOI] [PubMed] [Google Scholar]
- 11.Moers C, Smits JM, Maathuis M-HJ, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. New England Journal of Medicine. 2009 Jan 1;360(1):7–19. doi: 10.1056/NEJMoa0802289. PubMed PMID: 19118301. [DOI] [PubMed] [Google Scholar]
- 12.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008 Apr;8(4):783–92. doi: 10.1111/j.1600-6143.2008.02157.x. PubMed PMID: 18294347. English. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MJ, Baicu SC. Cryobiology. Vol. 60. Elsevier Inc; 2010. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective; pp. S20–35. United States: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein MJ, Lubezky N, Yushkov Y, Bae C, Guarrera JV. Innovations in organ donation. The Mount Sinai journal of medicine, New York. 2012 May-Jun;79(3):351–64. doi: 10.1002/msj.21312. PubMed PMID: 22678859. Epub 2012/06/09. eng. [DOI] [PubMed] [Google Scholar]
- 15.Cho YW, Bunnapradist S, Cho ES, Stadtler M, Simmons V, Locke J, et al. Can machine perfusion decrease the likelihood of discard among biopsied kidneys? Transplant Proc. 2008;40:1029–31. doi: 10.1016/j.transproceed.2008.03.062. United States. [DOI] [PubMed] [Google Scholar]
- 16.Kozaki K, Sakurai E, Kubota K, Iwamoto H, Hama K, Narumi Y, et al. Prediction of kidney nonfunction after transplantation with machine perfusion preservation. Transplantation Proceedings. 2000 Mar;32(2):275–6. doi: 10.1016/s0041-1345(99)00955-0. PubMed PMID: 10715415. [DOI] [PubMed] [Google Scholar]
- 17.Talbot D, Shenton BK, Buckley PE, Gok MA. Experiences Learned in the Successful Establishment of a Nonheart Beating Donor Program for Renal Transplantation. The Journal of Urology. 2003;170(4, Part 1):1088–92. doi: 10.1097/01.ju.0000086774.12582.0f. [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008 May;73(9):1008–16. doi: 10.1038/sj.ki.5002729. PubMed PMID: 18094679. English. [DOI] [PubMed] [Google Scholar]
- 19.Bhangoo RS, Hall IE, Reese PP, Parikh CR. Nephrol Dial Transplant. Vol. 27. England: 2012. Deceased-donor kidney perfusate and urine biomarkers for kidney allograft outcomes: a systematic review; pp. 3305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon BA, Koyner JL, Murray PT. Urinary glutathione S-transferases in the pathogenesis and diagnostic evaluation of acute kidney injury following cardiac surgery: a critical review. Current opinion in critical care. 2010 Dec;16(6):550–5. doi: 10.1097/MCC.0b013e32833fdd9a. PubMed PMID: 20930627. Epub 2010/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:2154–65. doi: 10.2215/CJN.00740110. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogland ER, de Vries EE, Christiaans MH, Winkens B, Snoeijs MG, van Heurn LW. The Value of Machine Perfusion Biomarker Concentration in DCD Kidney Transplantations. Transplantation. 2013 Jan 4; doi: 10.1097/TP.0b013e31827908e6. PubMed PMID: 23296150. Epub 2013/01/09. Eng. [DOI] [PubMed] [Google Scholar]
- 23.Moers C, Varnav OC, van Heurn E, Jochmans I, Kirste GR, Rahmel A, et al. The Value of Machine Perfusion Perfusate Biomarkers for Predicting Kidney Transplant Outcome. Transplantation. 2010;90(9):966–73. doi: 10.1097/TP.0b013e3181f5c40c. English. [DOI] [PubMed] [Google Scholar]
- 24.OPTN: Organ Procurement and Transplantation Network [cited 2013 January 17];A Guide to Calculating and Interpreting KDPI. 2012 Available from: http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=81.
- 25.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009 Jul 27;88(2):231–6. doi: 10.1097/TP.0b013e3181ac620b. PubMed PMID: 19623019. Epub 2009/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 26.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010 Dec;5(12):2154–65. doi: 10.2215/CJN.00740110. PubMed PMID: 20798258. Epub 2010/08/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell FE, Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. Journal of the National Cancer Institute. 1988 Oct 5;80(15):1198–202. doi: 10.1093/jnci/80.15.1198. PubMed PMID: 3047407. Epub 1988/10/05. eng. [DOI] [PubMed] [Google Scholar]
- 28.Harrell J, Frank E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. p. 594. [Google Scholar]
- 29.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010 Oct;10(10):2279–86. doi: 10.1111/j.1600-6143.2010.03179.x. PubMed PMID: 20883559. Epub 2010/10/05. eng. [DOI] [PubMed] [Google Scholar]
- 30.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC medical research methodology. 2008;8:9. doi: 10.1186/1471-2288-8-9. PubMed PMID: 18307814. Pubmed Central PMCID: Pmc2292207. Epub 2008/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. Oxford University Press; USA: 2002. [Google Scholar]
- 32.Devarajan P. The strong silent type: the distal tubule to the rescue. Crit Care Med. 2009;37:2129–30. doi: 10.1097/CCM.0b013e3181a60057. United States. [DOI] [PubMed] [Google Scholar]
- 33.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, et al. The Prognostic Value of Renal Resistance During Hypothermic Machine Perfusion of Deceased Donor Kidneys. Am J Transplant. 2011 Oct;11(10):2214–20. doi: 10.1111/j.1600-6143.2011.03685.x. PubMed PMID: 21834917. Epub 2011/08/13. Eng. [DOI] [PubMed] [Google Scholar]
- 34.van Smaalen TC, Hoogland ER, van Heurn LW. Machine perfusion viability testing. Curr Opin Organ Transplant. 2013 Apr;18(2):168–73. doi: 10.1097/MOT.0b013e32835e2a1b. PubMed PMID: 23385886. Epub 2013/02/07. eng. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009 Jul;76(1):108–14. doi: 10.1038/ki.2009.96. PubMed PMID: 19387469. Pubmed Central PMCID: 2737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martensson J, Martling CR, Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. British journal of anaesthesia. 2012 Dec;109(6):843–50. doi: 10.1093/bja/aes357. PubMed PMID: 23048068. Epub 2012/10/11. eng. [DOI] [PubMed] [Google Scholar]