Abstract
Chronic infectious diseases such as HIV, HBV, and HCV, among others, cause severe morbidity and mortality globally. Progressive decline in CD8 functionality, survival, and proliferative potential—a phenomenon referred to as CD8 exhaustion—is believed to be responsible for poor pathogen control in chronic infectious diseases. While the role of negative inhibitory receptors such as PD-1 in augmenting CD8 exhaustion has been extensively studied, the role of positive costimulatory receptors remains poorly understood. In this review, we discuss how one such costimulatory pathway, CD40–CD40L, regulates CD8 dysfunction and rescue. While the significance of this pathway has been extensively investigated in models of autoimmunity, acute infectious diseases, and tumor models, the role played by CD40–CD40L in regulating CD8 exhaustion in chronic infectious diseases is just beginning to be understood. Considering that monotherapy with blocking antibodies targeting inhibitory PD-1-PD-L1 pathway is only partially effective at ameliorating CD8 exhaustion and that humanized CD40 agonist antibodies are currently available, a better understanding of the role of the CD40–CD40L pathway in chronic infectious diseases will pave the way for the development of more robust immunotherapeutic and prophylactic vaccination strategies.
Keywords: PD-1, CD8 exhaustion, CD40, infectious disease
I. INTRODUCTION
Initiation of adaptive immunity is a complex multi-step process. Apart from TCR–pMHC interaction and cytokine milieu, other secondary signals are also essential for optimal T-cell response.1 Among the accessory signals important for development of robust T-cell response, the role of co-stimulatory molecules, like B7/CD28 and TNF/TNFR family members, has been well established.2–4 Co-stimulatory receptor CD40, a member of the TNF receptor superfamily, was initially characterized on B cells. CD40 is mostly expressed on antigen-presenting cells (APCs) like dendritic cells (DCs), monocytes,5 as well as non-hematopoietic cells such as fibroblasts and epithelial cells.6 Its ligand CD40L (CD154) is preferentially expressed on activated T cells, especially the CD4 subset,7 and in an inflammatory setting is also induced on monocytic cells,5 NK cells,8 mast cells and basophils.9 CD40–CD40L interaction plays a major role in CD4 B-cell collaboration, and abrogation of this pathway leads to the failure of germinal center formation, memory B-cell activation, and somatic hypermutation.10–12
The signaling cascade induced downstream of CD40–CD40L signaling has been studied in depth, especially in dendritic cells, and it is known to be important for their activation.13 Engagement of CD40 by CD40L promotes the clustering of CD40 and induces recruitment of adaptor proteins known as TNFR-associated factors (TRAFs) to the cytoplasmic domain of CD40.14 The cytoplasmic domain of CD40 contains two independent membrane TRAF binding domains—a TRAF6-binding proximal domain and a distinct distal domain that binds TRAF1, 2, 3, 4, and 5.15 TRAF-2 and TRAF-6 appear to play particularly important roles in CD40 signaling.16,17 Although it was previously believed that these two molecules have overlapping roles in CD40 signaling, it has been revealed subsequently that unique roles for these two molecules also exist.15,18 The TRAF proteins activate different signaling pathways, including NFκb, MAPK, and JAK-3.14,19,20 While these signaling pathways downstream of the CD40–CD40L interaction in DCs are somewhat similar to those activated by other receptors such as Toll-like receptors and RANK–RANKL,21 certain functions due to CD40–CD40L interplay are unique.
Appropriate activation of professional APCs is important for the development of robust T-cell immunity. This is highlighted by the critical role played by CD40–CD40L signaling in inducing DC maturation, such as up-regulation of MHC and costimulatory molecules—a process essential for optimal antigen presentation.22–24 The importance of CD40–CD40L signaling is further emphasized by the observation that this pathway plays a significant role in DC-mediated production of IL-12, a pivotal cytokine for driving Th1 response.25,26 While initially believed to be only involved in the generation of thymus-dependent humoral immunity, currently, there is clear evidence that the CD40–CD40L pathway is required for the development of cell-mediated immunity in certain models.13 This is emphasized by the important role played by this pathway in eliciting immunity against certain pathogens, tumors and particularly alloantigens. While most studies dissecting the role of CD40–CD40L in cell-mediated immunity have focused on auto-immune models, a clearer picture of the role played by CD40–CD40L on CD8 T cells in infectious disease models is beginning to emerge.
A hallmark of robust immunity against intracellular pathogens is the development of a potent CD8 T-cell response characterized by low apoptosis, rapid proliferative potential, and polyfunctionality.27 During acute infections, such T cells clear the pathogen, eventually leading to the development of antigen-independent memory T cells that exhibit the following cardinal features: the capacity to mount to rapid recall response and reactivate effector functions upon re-encountering antigens.27,28 While the requirement (or lack thereof) of CD40–CD40L in mounting the CD8 primary and recall responses has been extensively investigated in acute infection models, the role of this pathway on CD8 response in chronic models has not been thoroughly addressed. In contrast to acute infections, during certain chronic infections, antigen-specific CD8 T cells become functionally impaired and even get deleted.
Persistence of such antigen-specific T cells exhibiting poor effector functions, suboptimal recall response, and inferior antigen-independent homeostatic proliferation is referred to as exhaustion. Loss of such effector functionality results in uncontrolled pathogen burden. Most studies in this field have focused on CD8 T-cell exhaustion. However, as demonstrated in a malaria model, CD4 T-cell exhaustion can also result in disease pathogenesis.29 In this review, we discuss the role of CD40–CD40L signaling on CD8 T cells during chronic infections and its potential immunotherapeutic applications in alleviating T-cell exhaustion.
II. CD40–CD40L PATHWAY AND CD8 RESPONSE ACTIVATION
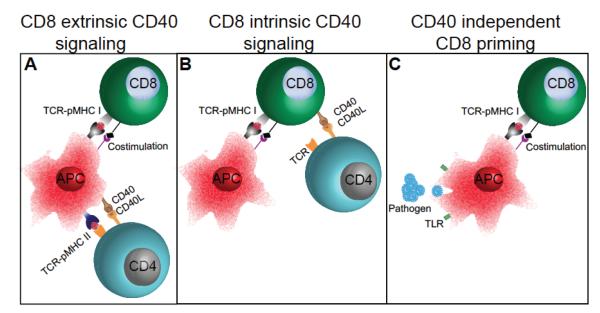
CD40–CD40L interaction plays a major role in CD4 T-cell–mediated B-cell response. However, this pathway is also important for rendering CD4 help to CD8 T cells.30,31 This is especially emphasized by the observation that agonistic CD40 antibody treatment can substitute for CD4 T-cell function in preventing latent herpesvirus reactivation.32 Based on various experimental observations, multiple models have been proposed to explain the mechanism of CD4 help to CD8 T cells. Because CD40 is preferentially expressed on APCs, it has been postulated that CD4 help to CD8 T cells is indirect. According to this model, CD40L-expressing CD4 T cells activate APCs by engaging CD40 (on APCs).33–35 Such “licensed” APCs then prime robust CD8 activation (Figure 1).
FIGURE 1.
Role of the CD40–CD40L pathway during the CD8 primary response. Multiple models have proposed to explain the role of the CD40–CD40L pathway in mediating CD4 help to CD8 T cells. (A) The first model suggests that CD40L-bearing CD4 T cells fully activate or license APC by engaging CD40 on APC. Such licensed or highly activated DC can then prime CD8 T cells. (B) The second model suggests that CD8 T cells either simultaneously or sequentially interact with antigen-presenting cells and CD40L-expressing CD4 T cells, which permits full activation of CD8 T cells. (C) However, in acute infectious disease models, CD4 T-cell help is dispensable for the CD8 primary response and CD8-intrinsic CD40 signaling plays a minimal role in mediating CD8-response development. For certain infections, even CD8-extrinsic CD40 signaling is not required for optimal CD8 response. This may be due to pathogen-mediated activation of TLRs on APCs and consequent inflammatory milieu, which may overcome the need for CD4 help or CD40 signaling.
This paradigm has been challenged by a study in the HY-TCR transgenic model.36 It was demonstrated that, akin to B cells, CD40-expressing CD8 T cells directly receive help from CD4 T cells via CD40–CD40L interaction. However, it is unclear whether initial naïve CD8 T-cell activation and subsequent memory CD8 T-cell development have differential dependence on CD40 signaling (Figure 1). A recent report using bone marrow chimeras where CD40 is selectively expressed by APCs or HY antigen-specific CD8 T cells demonstrated that CD40 in a context-dependent manner has distinct roles in mediating the CD8 primary response, CD8 memory development, and recall response.37,38 In the absence of CD40 on DCs, the CD8 primary response and memory development is predominantly affected, but recall response remains relatively unscathed. In contrast, when CD8 T cells are deficient in CD40 signaling, the CD8 primary response and memory development are only modestly affected, but recall response is severely compromised.
In contrast, the CD8 primary response in several infectious diseases is not only independent of CD8 intrinsic CD40 signaling. For certain infections such as toxoplasmosis, the CD8 primary response is independent of CD8 extrinsic CD40 signaling as well.30,33,39 Similarly, the CD8 recall response in a Listeria model is independent of CD8 expressed CD40.39 Why is CD8 intrinsic CD40 signaling dispensable for development of CD8 immunity in infectious disease models? In contrast to the HY-TCR transgenic model, in which the antigen encounter occurs in a relatively non-inflammatory setting, in infectious diseases, TCR-pMHC interaction occurs in a highly inflammatory environment.
Pathogens elicit robust induction of Toll-like receptors as well as engagement of other pattern recognition receptors that contribute to the inflammatory milieu (Figure 1).35 Hence, in a highly inflammatory environment, due to the overabundance of cytokines such IL-12, IL-15, and IFNα/β, as well as the elevated expression of multiple co-stimulatory molecules such as 4-1BB, OX40, and CD70, among others, dependence on CD8-intrinsic CD40 signaling for CD8 response may be circumvented.32 However, most observations regarding the role of CD40 signaling on pathogen-specific CD8 T cells were made using acute infectious disease models.
Incidentally, in a chronic viral model, Fuse et al. demonstrated that, in the absence of CD4 help, presumably due to deficient CD40 signaling, development of memory CD8 T cells specific for a gamma herpes virus epitope is compromised.40 Moreover, in a polyoma virus model, costimulation requirements for antiviral CD8 T cells vary between acute and chronic phases of infection.41 Taken together, these findings suggest that the role of CD40 signaling on CD8 T cells during chronic infections may be different than those noted in acute infectious diseases. Considering that CD8 exhaustion occurs in several clinically relevant chronic infections, a better understanding of the role of the CD40–CD40L pathway in regulating CD8 exhaustion is imperative for the development of improved therapeutic interventions.
III. ROLE OF THE CD40–CD40L PATHWAY IN CD8 T-CELL RESPONSE IN CHRONIC INFECTIONS
A. CD40 AND CD8 T-Cell Exhaustion in Chronic Infections
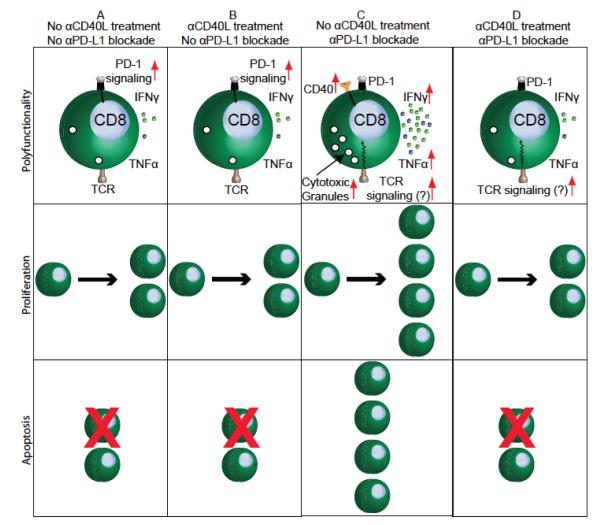
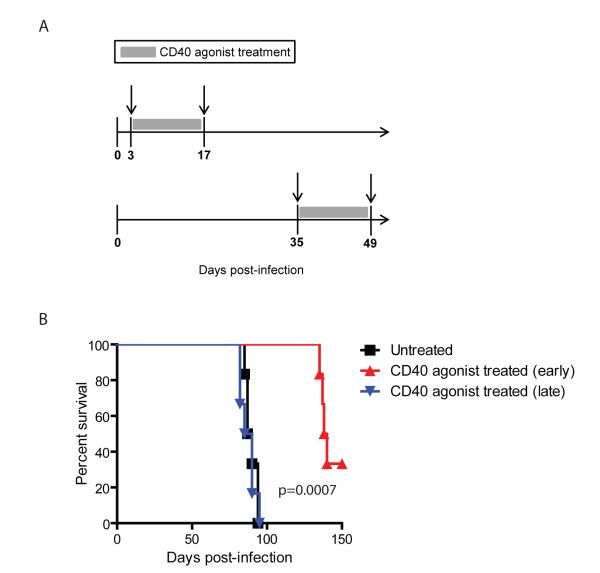
The role of the CD40–CD40L pathway during acute infections has been extensively investigated. However, the significance of this pathway in chronic infection models remains understudied. CD8 exhaustion as reported in chronic viral models such as LCMV (lymphocytic choriomeningitis virus), HIV (human immunodeficiency virus), SIV (Simian immunodeficiency virus), HCV (hepatitis C virus), and HBV (hepatitis B virus) involves a hierarchical loss of functions (cytokine, proliferation, and cytotoxicity) and, in extreme cases, CD8 T cells can be physically deleted.42 Recent studies have demonstrated that this paradigm is not restricted to chronic viral infections alone but can be extended to parasitic diseases caused by Toxoplasma, Leishmania, and Plasmodium.28,43 Studies in these models have demonstrated that inhibitory receptors, especially the PD-1–PD-L1 pathway, play a pivotal role in mediating CD8 T-cell dysfunction. Significantly, in vivo blockade of these inhibitory molecules is sufficient to rescue exhausted CD8 T cells and control pathogen burden. It has been postulated that interplay of signals between costimulatory and inhibitory receptors play an important role in T-cell exhaustion.44 However, the role of costimulatory receptors in mediating CD8 dysfunction or, for that matter, rescue of exhausted CD8 T cells has not been extensively studied. A recent study from our laboratory identified CD40 as one of the costimulatory molecules highly upregulated on PD-1–expressing CD8 T cells in αPD-L1–treated mice chronically infected with Toxoplasma gondii.45 In agreement with previous studies, in the absence of αPD-L1 treatment, blockade of CD40–CD40L interaction alone had no major impact on CD8 response. Significantly, in chronically infected animals dually treated with αPD-L1 and αCD40L, CD40–CD40L blockade abrogated the ameliorative effects of αPD-L1 treatment (Figure 2). The combined data demonstrate that the CD40–CD40L pathway plays a profound role during rescue of exhausted CD8 T cells. Additionally, the data imply that elevated PD-1–PD-L1 signaling or its downstream effects dampen CD40 expression on CD8 T cells and possibly other cell types and that blockade of this interaction upregulates CD40 signaling. Paradoxically in dually treated mice, despite blockade of PD-1–PD-L1 signaling, CD40 expression on CD8 T cells was not elevated. In future studies, it will be interesting to address whether dampened CD40 expression on CD8 T cells in dually treated mice is also dependent on CD8 extrinsic or CD8 intrinsic CD40 signaling or both. Although data from the aforementioned study suggests that CD40–CD40L signaling does not play a role in mediating the CD8 primary response during chronic toxoplasmosis, ongoing experiments in our laboratory suggest otherwise. This notion is supported by preliminary observations in our laboratory showing that treatment of T. gondii-challenged animals with agonistic CD40 antibody during the first 2 weeks of infection prevent host mortality. However, if the treatment is delayed to 5–6 weeks post-infection, improved survival is not observed (Figure 3). Considering that CD8 T cells play a critical role in mediating long-term protection against Toxoplasma,46 this potentially suggests that, depending on the temporal context, CD40–CD40L may have a role in mediating long-term CD8 immunity and in preventing CD8 exhaustion. Alternatively, it is possible that, during the chronic phase of infection, exhausted CD8 T cells may have down-regulated expression of CD40 or other molecules involved in CD40 transduction or both; as a result, they are incapable of responding to agonist CD40 antibody treatment. Addressing these possibilities will be critical for better understanding the applicability of CD40 as an adjuvant in immunotherapeutic or prophylactic setting against chronic infectious diseases.
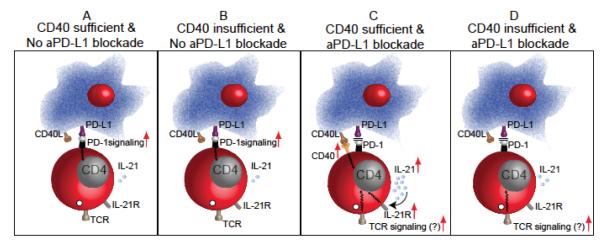
FIGURE 2.
CD40 signaling plays a critical role during rescue of exhausted CD8 T cells during chronic infection. (A) Exhausted CD8 T cells in untreated chronically infected mice exhibit minimal CD40 exp-ression, poor polyfunctionality, low proliferation and high apoptotic potential. (B) Transient blockade of chronically infected animals with αCD40L does not exacerbate CD8 exhaustion. This finding suggests that the CD40–CD40L pathway does not play a major role during the chronic phase of infection. (C) While blockade of the PD-1–PD-L1 pathway augments CD40 expression on CD8 T cells and rescues CD8 polyfunctionality, proliferation, and survival, (D) co-administration of αCD40L antibody abrogates the ameliorative effects of αPD-L1 treatment. Combined, these findings imply that positive costimulatory signals such as CD40–CD40L play a critical role during the rescue of exhausted CD8 T cells. ‘CD8 T cell rescue,’ as mentioned in the figure or text refers to restoration of CD8 T-cell function during chronic infection with blocking antibodies against inhibitory receptors (such as αPD-L1).
FIGURE 3.
Effect of CD40 agonist on survival of T. gondii-infected animals. (A) Wild-type mice were infected perorally with T. gondii cysts. Beginning on either day 3 or week 5 post-infection, mice were treated with CD40 agonist antibody (FGK115) or PBS twice weekly for a 2-week period. (B) Mice were monitored for survival, and data represent two experiments with at least 6 mice per group.
B. CD40–CD40L-Mediated Regulation of CD8 Proliferation and Apoptosis in Chronic Infection Models
One of the hallmarks of exhausted CD8 T cells is their elevated apoptotic potential concomitant with poor proliferative potential.47–49 Previous studies have demonstrated that CD40 has both pro- and anti-apoptotic functions, depending on cellular and environmental context.50 In agreement with this notion, CD40-deficient CD8 T cells in the wild-type CD40−/− (WT:CD40 KO) chimeras, chronically infected with Toxoplasma, exhibited modestly reduced apoptosis.45 However, proliferation in these mutant CD8 T cells was dampened. This finding suggests that CD8-intrinsic CD40 signaling regulates proliferation and apoptosis during chronic infection. However, this effect is modest at best, since the WT:CD40 KO ratio of CD8 T cells in both naïve and chronically infected chimeras remained similar. In contrast, in the context of exhausted CD8 rescue, CD8 intrinsic CD40 signaling appears to play a profound role. Irrespective of CD40 sufficiency, αPD-L1 treatment reduced CD8 apoptosis and increased proliferation of both WT and CD40KO CD8 T cells in the chimeras. Although CD40 sufficient CD8 T cells expanded nearly two-fold more than CD40 KO CD8 T cells due to increased proliferation of WT CD8 T cells, these CD40 sufficient CD8 T cells were also more apoptotic than CD40 KO counterparts, albeit by a modest degree. This finding perhaps highlights the importance of careful considerations when including agonistic CD40 as part of an immunotherapeutic vaccination regimen. Due to increased CD8 apoptosis caused by CD40 signaling, the benefit caused by increased proliferation may be outweighed by sharply elevated apoptosis, especially in scenarios involving agonistic CD40 treatment. This may explain why agonistic CD40 antibody treatment, instead of boosting CD8 response, resulted in immune suppression in LCMV model.51
How does CD40 signaling differentially regulate CD8 survival and proliferation in the context of CD8 rescue? A recent study has revealed that PD-1 expression on T cells inhibits T-cell proliferation by down-regulating Akt and Ras pathways.52 Incidentally, CD40 is a potent activator of both Akt and Ras pathways.53,54 In the context of exhausted CD8 rescue, whether a similar CD40-induced pathway results in augmented proliferation of CD40-sufficient CD8 T cells remains to be investigated. As mentioned earlier, CD40 can induce pro- and anti-apoptotic molecules. A previous study demonstrated that CD40-activated human B-cell lymphocytes are highly susceptible to Fas-induced apoptosis.55 Considering that PD-1-expressing memory CD8 subsets in HIV infection are also highly susceptible to Fas-induced apoptosis,56 it will be interesting to investigate whether a combinatorial therapy with αPD-L1, αCD40, and αFasL elicits a better outcome than therapy with αPD-L1 alone or αPD-L1 and αCD40 together.
C. CD40–CD40L-Mediated Regulation CD8 Polyfunctionality in Chronic Infection Models
Several studies have demonstrated the critical role of T-cell polyfunctionality (i.e., the capacity of single cell to exhibit multiple functions) in mediating protective immune response.57,58 The importance of this subset is further emphasized by the observation that improved protective response in HIV-infected non-progressors does not correlate with the number of antigen-specific T cells but rather with the abundance of virus-specific polyfunctional CD8 T cells.59 T-cell exhaustion results in progressive attrition of CD8 polyfunctionality.28 However, the role of CD40 signaling in regulating CD8 polyfunctionality has not been extensively investigated in chronic infection models of CD8 exhaustion. Studies using Toxoplasma-challenged αCD40L-treated (during chronic phase) mice or wild-type/CD40 KO mixed bone marrow chimera have revealed that attrition of polyfunctionality during CD8 exhaustion is independent of CD8-intrinsic or -extrinsic CD40 signaling.45 However, αPD-L1–dependent rescue of CD8 functionality is highly dependent on CD8 intrinsic and to a certain extent CD8 extrinsic CD40 signaling. Interestingly, independent of PD-1–PD-L1 or CD40–CD40L signaling, IL-2 production by exhausted Toxoplasma-specific CD8 T cells could not be revived. Ongoing studies in our laboratory are investigating whether this is due to coexpression of other inhibitory receptors on Toxoplasma-specific CD8 T cells.
D. CD40–CD40L-Mediated Regulation of CD8 Transcription in Chronic Infection Models
The pivotal role of transcription factors in modulating differentiation, survival, and function of CD8 T cells has been well established.28 T-box transcription factors T-bet and Eomesodermin (Eomes), NFAT,60 BATF,61 and zinc finger transcription factor Blimp-1 play critical roles in regulating not only functional CD8 T cells but CD8 exhaustion as well.62–67 However, how the CD40–CD40L pathway modulates the expression of these transcription factors in the context of CD8 exhaustion or rescue of exhausted CD8 T cells has not been extensively investigated. Preliminary studies performed in our laboratory reveal that, in chronically infected Toxoplasma-challenged mice, CD8-intrinsic or -extrinsic CD40 signaling does not play a role in regulating T-bet or Eomes level.45 However, during the rescue of exhausted CD8 T cells via αPD-L1 treatment, both T-bet and Eomes levels are upregulated in a CD40-dependent manner. While upregulation of Eomes on CD8 T cells in the context of CD8 rescue is independent of CD8 intrinsic CD40 signaling, augmented T-bet expression is strongly dependent on CD8 intrinsic CD40 signaling.
In acute viral infection models, during the effector phase, CD8 T cells express high levels of T-bet; during the memory phase, they express higher levels of Eomes.66 A recent study in LCMV clone 13 (chronic) demonstrated that Eomeshi CD8 T cells, despite higher granzyme B levels during primary infection, have a phenotype consistent with more severe exhaustion: these cells produce lower cytokine levels and express higher levels of PD-1.64 In contrast, T-bethi CD8 T cells exhibit higher production of cytokines and lower levels of PD-1. Considering the differential role of T-bet and Eomes in acute and chronic infection models, it will be critical to ascertain the role of these molecules during the rescue of exhausted CD8 response.
E. CD40–CD40L-Mediated Regulation of CD4 Help in Chronic Infection
CD4 T cells play a critical role in modulating CD8 T-cell response. Whereas the CD8 primary response in acute infection models seems independent of CD4 help, “unhelped” CD8 T cells are unable to mediate robust recall response in viral as well as nonviral models.68,69
Significantly, depletion of CD4 T cells in chronic viral models of CD8 exhaustion results in more pronounced functional defects in CD8 T cells.70–72 This potentially suggests that, unlike acute infections during chronic infections, CD4 T cells play a significant role in regulating the CD8 primary response. This notion is further supported by the observation that adoptive transfer of antigen-specific CD4 T cells partially rescued exhausted CD8 T cells and augmented germinal center reactions during chronic LCMV infection.73 Adoptive transfer of CD4 T cells not only elevated the number of antigen specific CD8 T cells but also increased their functionality, resulting in improved viral control. Incidentally, CD40–CD40L-mediated CD4 help is especially important for memory CD8 T-cell function during chronic LCMV infection.74
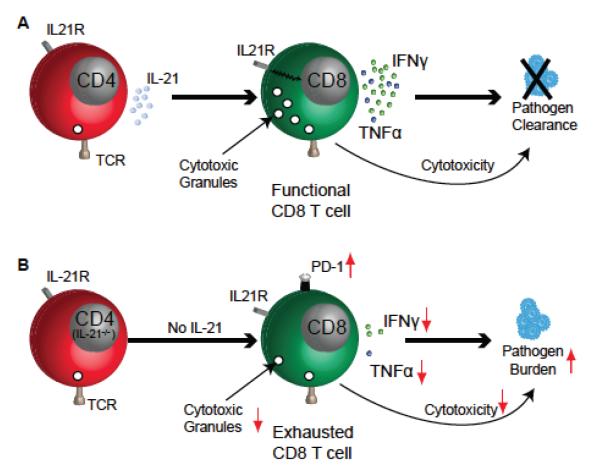
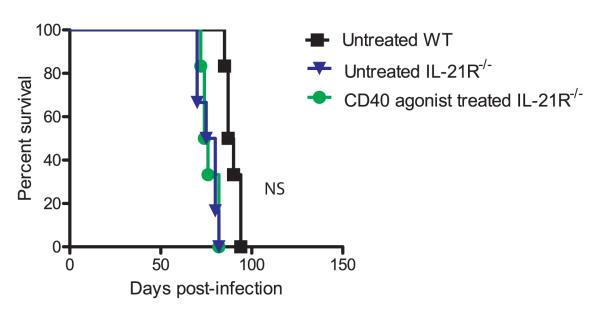
Recent studies have demonstrated that IL-21–IL-21R signaling plays a critical role in sustaining CD8 response during chronic viral infections but not during acute infections.75,76 In the absence of IL-21 signaling, LCMV-specific CD8 T cells fail to exhibit optimal proliferation and cytokine production during chronic infection, resulting in poor pathogen control (Figure 4). Incidentally, CD4 T cells have been shown to be the major producer of IL-21 during chronic viral infection.75,76 Although the role of IL-21 against T. gondii has not been extensively investigated, preliminary studies demonstrate that, during chronic toxoplasmosis, CD4 T cells produce minimal IL-21.45 Whereas αPD-L1 treatment strongly augments the frequency of IL-21–producing CD4 T cells, this ameliorative effect even in untreated mice is strongly dependent on CD4-intrinsic CD40 signaling (Figure 5). However, which CD4 subset is the primary producer of IL-21 in αPD-L1–treated Toxoplasma-challenged mice is currently unknown. A previous study has demonstrated that CD40 triggering results in up-regulation of IL-21R on chronic lymphocytic leukemia B cells.77 Hence, it is hardly surprising that, during chronic Toxoplasma infection, T-cell–intrinsic CD40 signaling plays a significant role in positively regulating IL-21R expression both on CD4 and CD8 T cells, especially in the context of αPD-L1 treatment.45 The critical role of CD40/CD40L signaling in regulating the IL-21–IL-21R pathway is further emphasized by the observation that the ameliorative effect of agonist CD40 antibody treatment on long-term survival of Toxoplasma-challenged wild-type mice is lost in IL-21R−/− mice (Figure 6).
FIGURE 4.
IL-21 production controls exhaustion of CD8 T cells during chronic infection. (A) In chronic viral models, IL-21 production by CD4 T cells prevents CD8 deletion, resists attrition of polyfunctionality, ultimately resulting in viral clearance. (B) However, in the absence of IL-21 production, CD8 T cells become severely exhausted and exhibit poor polyfunctional CD8 response, resulting in poor viral control and pathogen persistence.
FIGURE 5.
The CD40-CD40L pathway plays a critical role in CD4 T-cell–mediated IL-21 production during the rescue of exhausted T-cell response. (A) During chronic infections like toxoplasmosis, minimal IL-2 is produced by CD4 T cells in untreated animals. (B) Additionally these cells exhibit low levels of IL-21R and CD40 expression. Considering the low CD40 expression on CD4 T cells in untreated mice, it is not surprising that abrogation of CD40–CD40L signaling has, at best, a modest effect on CD4 T-cell–mediated IL-21 production or IL-21R expression. (C) Interestingly, αPD-L1 treatment not only augmented CD40 expression on CD4 T cells in chronically infected animals but also increased the expression of IL-21 and IL-21R on CD4 T cells. (D) However, in the absence of CD40–CD40L signaling, CD4 T cells in αPD-L1–treated animals failed to dramatically augment IL-21 or IL-21R, suggesting that the CD40–CD40L pathway plays a critical role in regulating IL-21 production and IL-21R expression on CD4 T cells in the context of T-cell rescue.
FIGURE 6.
Effect of CD40 agonist treatment on survival of T. gondii-infected IL-21R−/− mice. Wild-type and IL-21R−/− mice were infected perorally with T. gondii cysts. Beginning on day 3 post-infection, mice were treated with CD40 agonist antibody (FGK115) or PBS twice weekly for a 2-week period. Mice were monitored for survival, and data represent two experiments with at least 6 mice per group.
IL-21 can be produced by activated CD4 T cells, including follicular helper cells (Tfh).78,79 Tfh cells regulate the germinal center B-cell reaction, which is necessary for the generation of high-affinity antibody responses that play a critical role in pathogen clearance in multiple models, including Plasmodium.80–83 As shown in the former model, rescue of exhausted CD4 T-cell response via PD-1 and LAG-3 blockade was directly associated with elevated frequency of Tfh and augmented plasma cell differentiation.29 CXCR5 is one of the quintessential Tfh markers. Interestingly, CD4 T cells that express high levels of CXCR5 also exhibit elevated levels of CD40L.84 Thus, it is hardly surprising that, in the absence of CD40–CD40L signaling, CD4 T cells fail to efficiently differentiate into Tfh and show reduced germinal center formation.81 However, the role of CD40-CD40L signaling in regulating Tfh development during chronic infection has not been extensively addressed. Blockade of CD4-intrinsic CD40 signaling sharply reduced the expression of Tfh markers (CXCR5, PD-1, and Bcl-6) during chronic toxoplasmosis.45 A previous study has demonstrated that blockade of PD-1–PD-L1 signaling during immunization with keyhole limpet hemocyanin or helminth antigen augments Tfh development.85 Consistent with those findings, blockade of PD-1–PD-L1 during chronic toxoplasmosis resulted in upregulated Tfh frequency. However, this ameliorative effect was partially dependent on CD4-intrinsic CD40 signaling.45 A recent study has demonstrated that the production of IL-6 during the later phase of chronic infection with LCMV clone 13 is critical for boosting Tfh response and viral control.82 Considering that CD40 has been shown to induce IL-6 production, it will be critical to address the role of CD40–CD40L-induced IL-6 expression in augmenting Tfh response during chronic infection.86,87
Regulatory T cells (Treg) play a critical role in controlling immunopathology and mediating unresponsiveness to self-antigens.88 Treg induction has been implicated as a mechanism of pathogen escape in several chronic infectious disease models.89,90 Recent studies have demonstrated that, during chronic infection with Friend’s retrovirus or LCMV clone 13, depletion of regulatory T cells rescues exhausted CD8 T cells.91 Furthermore, depletion of Tregs in combination with αPD-L1 treatment was more efficacious in controlling viral burden. This finding potentially suggests that Tregs represent a viable target for ameliorating CD8 exhaustion. A recent study has shown that absence of PD-1–PD-L1 signaling promotes the development of Foxp3-expressing T follicular regulatory (Tfr) cells that are known to suppress Tfh.92,93 Hence, it is alternatively possible that increased efficacy of combinatorial therapy (i.e., αPD-L1 and Foxp3+ T-cell depletion) vis-à-vis monotherapy with αPD-L1 may be due to augmented development of suppressive Tfr in animals treated with αPD-L1 alone. While Tfrs are known to down-modulate B cells and Tfh response, their role in regulating T-cell exhaustion during chronic infections remains uncharacterized.93
DCs play a critical role in Treg development.94 Incidentally, DCs expressing low levels of CD40 have been shown to promote Treg generation in a Leishmania donovani model.95 Similarly, inhibition of the CD40–CD40L pathway on graft-specific CD4 T cells results in increased Tregs.96 This potentially suggests that augmenting CD40 signaling via agonistic CD40 antibody treatment during the early phase of chronic infection may be an ideal strategy for preventing Treg development and CD8 exhaustion. However, considering that the CD40–CD40L pathway has been shown to induce Tregs in other models systems via IL-2 dependent mechanism, it is likely that the effect of CD40 signaling on Treg development may be highly dependent on the environmental and temporal contexts.97,98 Hence, in models in which CD8 exhaustion is associated with Treg development, careful analysis must be made before including agonistic CD40 antibody as an immunotherapy.
IV. CONCLUSION
While the role of CD40–CD40L on T cells has been widely investigated in models of autoimmunity and to a considerable extent in acute infectious disease and tumor models, the role of this pathway in chronic infectious disease models has remained underexplored. The current review highlights the recent advances in understanding the role of CD40 signaling on CD8 T cells in chronic infectious diseases. Considering that monotherapy with αPD-L1 only rescues a subset of the exhausted CD8 T cells in these models,47,48 it is highly likely that a multi-pronged immunotherapy approach that includes agonistic CD40 antibody as well as the blocking antibodies against multiple inhibitory receptors will elicit a better outcome. Several humanized CD40 agonist antibodies undergoing phase 1 clinical trials in cancer patients have yielded promising results.99–101 However, whether superior outcomes in such patients correlate with improved CD8 response is currently unknown. Although the studies discussed here emphasize the role played by the CD40–CD40L pathway during rescue of exhausted CD8 response, the molecular underpinnings of this mechanism remain underexplored. A recent study in a Listeria (acute infection) model has demonstrated that TRFA6, one of the molecules involved in CD40 signal transduction, regulates CD8 memory development.102 However, whether the CD40-dependent “rescue” effect is dependent on TRAF6 signaling remains to be determined. Similarly, how far the ameliorative effects of CD40 signaling overlap with IL-21 signaling needs to be investigated. Additionally, as mentioned earlier, CD40 expression on dendritic cells plays a critical role in T-cell response development.103 How CD40–CD40L signaling on dendritic cells regulate T-cell exhaustion remains uncharacterized. Nevertheless, the critical role played by the CD40–CD40L pathway during the rescue of exhausted CD8 T cells, as well as reinvigoration of Tfh response in murine models of infectious disease, make a strong case for including agonist CD40 antibodies in immunotherapeutic vaccinations against chronic infections.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grant AI-33325 to I.A.K.
ABBREVIATIONS
- APC
antigen presenting cells
- DC
dendritic cells
- TRAFs
TNFR-associated factors
- Tfh
follicular helper cells
- Tfr
T follicular regulatory cells
- Treg
regulatory T cells
REFERENCES
- 1.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Ann Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 2.Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, Shaw AS, Weiss A, Dustin ML. The immunological synapse and CD28-CD80 interactions. Nature Immunol. 2001 Dec;2(12):1159–66. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 3.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009 May;229(1):152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell biol. 1999 Feb;77(1):1–10. doi: 10.1046/j.1440-1711.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 5.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukocyte Biol. 2000 Jan;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Gormand F, Briere F, Peyrol S, Raccurt M, Durand I, Ait-Yahia S, Lebecque S, Banchereau J, Pacheco Y. CD40 expression by human bronchial epithelial cells. Scand J Immunol. 1999 Apr;49(4):355–61. doi: 10.1046/j.1365-3083.1999.00510.x. [DOI] [PubMed] [Google Scholar]
- 7.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. PNAS USA. 2006 Jul 11;103(28):10717–22. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jyothi MD, Khar A. Regulation of CD40L expression on natural killer cells by interleukin-12 and interferon gamma: its role in the elicitation of an effective antitumor immune response. Cancer Immunol Immunother: CII. 2000 Dec;49(10):563–72. doi: 10.1007/s002620000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs BF. Human basophils as effectors and immunomodulators of allergic inflammation and innate immunity. Clin Exper Med. 2005 Jul;5(2):43–9. doi: 10.1007/s10238-005-0064-5. [DOI] [PubMed] [Google Scholar]
- 10.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. PNAS USA. 1992 Jul 15;89(14):6550–4. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siepmann K, Skok J, van Essen D, Harnett M, Gray D. Rewiring of CD40 is necessary for delivery of rescue signals to B cells in germinal centres and subsequent entry into the memory pool. Immunology. 2001 Mar;102(3):263–72. doi: 10.1046/j.1365-2567.2001.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari S, Giliani S, Insalaco A, Al-Ghonaium A, Soresina AR, Loubser M, Avanzini MA, Marconi M, Badolato R, Ugazio AG, Levy Y, Catalan N, Durandy A, Tbakhi A, Notarangelo LD, Plebani A. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. PNAS USA. 2001 Oct 23;98(22):12614–9. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Ann Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 14.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exper Med Biol. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 15.Davies CC, Mak TW, Young LS, Eliopoulos AG. TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells. Molec Cell Biol. 2005 Nov;25(22):9806–19. doi: 10.1128/MCB.25.22.9806-9819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995 Sep 8;269(5229):1424–7. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 17.Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, Wijnands E, Cleutjens J, Zernecke A, Weber C, Ahonen CL, Benbow U, Newby AC, Noelle RJ, Daemen MJ, Lutgens E. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008 May 1;111(9):4596–604. doi: 10.1182/blood-2007-05-088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcipowski KM, Stunz LL, Graham JP, Kraus ZJ, Vanden Bush TJ, Bishop GA. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1) J Biol Chem. 2011 Mar 25;286(12):9948–55. doi: 10.1074/jbc.M110.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saemann MD, Diakos C, Kelemen P, Kriehuber E, Zeyda M, Bohmig GA, Hörl WH, Baumruker T, Zlabinger GJ. Prevention of CD40-triggered dendritic cell maturation and induction of T-cell hyporeactivity by targeting of Janus kinase 3. Am J Transplant. 2003 Nov;3(11):1341–9. doi: 10.1046/j.1600-6143.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Saemann MD, Kelemen P, Zeyda M, Bohmig G, Staffler G, Zlabinger GJ. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. Transplant Proc. 2002 Aug;34(5):1407–8. doi: 10.1016/s0041-1345(02)02907-x. [DOI] [PubMed] [Google Scholar]
- 21.Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis and Rheumatism. 2005 Aug;52(8):2307–12. doi: 10.1002/art.21211. [DOI] [PubMed] [Google Scholar]
- 22.Demangel C, Palendira U, Feng CG, Heath AW, Bean AG, Britton WJ. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infection and Immunity. 2001 Apr;69(4):2456–61. doi: 10.1128/IAI.69.4.2456-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Bergwelt-Baildon M, Maecker B, Schultze J, Gribben JG. CD40 activation: potential for specific immunotherapy in B-CLL. Ann Oncol ESMO. 2004 Jun;15(6):853–7. doi: 10.1093/annonc/mdh213. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes and Infection / Institut Pasteur. 2004 Nov;6(14):1333–8. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000 Oct;13(4):453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nature Immunol. 2006 Jul;7(7):740–6. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 27.Wherry EJ. T cell exhaustion. Nature Immunol. 2011 Jun;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 28.Gigley JP, Bhadra R, Moretto MM, Khan IA. T cell exhaustion in protozoan disease. Trends Parasitol. 2012 Sep;28(9):377–84. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature Immunol. 2012 Feb;13(2):188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infection and Immunity. 2000 Mar;68(3):1312–8. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borrow P, Tough DF, Eto D, Tishon A, Grewal IS, Sprent J, Flavell RA, Oldstone MB. CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J Virology. 1998 Sep;72(9):7440–9. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medzhitov R. Origin and physiological roles of inf lammation. Nature. 2008 Jul 24;454(7203):428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 33.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exper Med. 2003 Dec 1;198(11):1759–64. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998 Jun 4;393(6684):480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011 May 27;34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002 Sep 20;297(5589):2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 37.Meunier S, Rapetti L, Beziaud L, Pontoux C, Legrand A, Tanchot C. Synergistic CD40 signaling on APCs and CD8 T cells drives efficient CD8 response and memory differentiation. J Leukocyte Biol. 2012 Jun;91(6):859–69. doi: 10.1189/jlb.0611292. [DOI] [PubMed] [Google Scholar]
- 38.Bullock TN. Editorial: (CD)40 winks to prevent CD8+ T cell lethargy. J Leukocyte Biol. 2012 Jun;91(6):845–8. doi: 10.1189/jlb.1211650. [DOI] [PubMed] [Google Scholar]
- 39.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004 Mar 15;172(6):3385–9. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 40.Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol. 2009 Apr 1;182(7):4244–54. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kemball CC, Lee ED, Szomolanyi-Tsuda E, Pearson TC, Larsen CP, Lukacher AE. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J Immunol. 2006 Feb 1;176(3):1814–24. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- 42.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exper Med. 2006 Oct 2;203(10):2223–7. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhadra R, Khan IA. Redefining chronic toxoplasmosis—a T cell exhaustion perspective. PLoS Pathogens. 2012 Oct;8(10):e1002903. doi: 10.1371/journal.ppat.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009 Apr;21(2):179–86. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol. 2011 Nov 1;187(9):4421–5. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhadra R, Gigley JP, Khan IA. The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy. 2011 Jun;3(6):789–801. doi: 10.2217/imt.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. PNAS USA. 2008 Sep 30;105(39):15016–21. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhadra R, Gigley JP, Khan IA. PD-1-mediated attrition of polyfunctional memory CD8+ T cells in chronic toxoplasma infection. J Infect Dis. 2012 Jul 1;206(1):125–34. doi: 10.1093/infdis/jis304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. PNAS USA. 2011 May 31;108(22):9196–201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dallman C, Johnson PW, Packham G. Differential regulation of cell survival by CD40. Apoptosis. 2003 Jan;8(1):45–53. doi: 10.1023/a:1021696902187. [DOI] [PubMed] [Google Scholar]
- 51.Bartholdy C, Kauffmann SO, Christensen JP, Thomsen AR. Agonistic anti-CD40 antibody profoundly suppresses the immune response to infection with lymphocytic choriomeningitis virus. J Immunol. 2007 Feb 1;178(3):1662–70. doi: 10.4049/jimmunol.178.3.1662. [DOI] [PubMed] [Google Scholar]
- 52.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Science Signaling. 2012 Jun 26;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deregibus MC, Buttiglieri S, Russo S, Bussolati B, Camussi G. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003 May 16;278(20):18008–14. doi: 10.1074/jbc.M300711200. [DOI] [PubMed] [Google Scholar]
- 54.Gulbins E, Brenner B, Schlottmann K, Koppenhoefer U, Linderkamp O, Coggeshall KM, Lang F. Activation of the Ras signaling pathway by the CD40 receptor. J Immunol. 1996 Oct 1;157(7):2844–50. [PubMed] [Google Scholar]
- 55.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exper Med. 1995 Nov 1;182(5):1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exper Med. 2006 Oct 2;203(10):2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine- mediated protection against Leishmania major. Nature Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 58.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature Rev Immunol. 2008 Apr;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 59.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. PNAS USA. 2007 Mar 13;104(11):4565–70. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature Med. 2010 Oct;16(10):1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nature Immunol. 2011 Jul;12(7):663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009 Aug 21;31(2):309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012 Nov 30;338(6111):1220–5. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exper Med. 2007 Sep 3;204(9):2015–21. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunol. 2005 Dec;6(12):1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 67.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006 Dec 1;177(11):7515–9. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 68.Bevan MJ. Helping the CD8(+) T-cell response. Nature Rev Immunol. 2004 Aug;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 69.Jordan KA, Wilson EH, Tait ED, Fox BA, Roos DS, Bzik DJ, Dzierszinski F, Hunter CA. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infection Immunity. 2009 Sep;77(9):3894–901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006 Feb 9;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 71.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exper Med. 1998 Dec 21;188(12):2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994 Dec;68(12):8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. PNAS USA. 2011 Dec 27;108(52):21182–7. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, Haining WN, Ahmed R. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity. 2011 Aug 26;35(2):285–98. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009 Jun 19;324(5934):1569–72. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009 Jun 19;324(5934):1576–80. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 77.de Totero D, Meazza R, Zupo S, Cutrona G, Matis S, Colombo M, Balleari E, Pierri I, Fabbi M, Capaia M, Azzarone B, Gobbi M, Ferrarini M, Ferrini S. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood. 2006 May 1;107(9):3708–15. doi: 10.1182/blood-2005-09-3535. [DOI] [PubMed] [Google Scholar]
- 78.Yi JS, Cox MA, Zajac AJ. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes and Infection / Institut Pasteur. 2010 Dec;12(14–15):1111–9. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008 Jul 18;29(1):138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crotty S. Follicular helper CD4 T cells (TFH) Ann Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 81.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exper Med. 2012 Jul 2;209(7):1241–53. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011 Nov 11;334(6057):825–9. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exper Med. 2011 May 9;208(5):987–99. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exper Med. 2000 Dec 4;192(11):1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, Fallon PG. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011 May 15;186(10):5648–55. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 86.Clark EA, Shu G. Association between IL-6 and CD40 signaling. IL-6 induces phosphorylation of CD40 receptors. J Immunol. 1990 Sep 1;145(5):1400–6. [PubMed] [Google Scholar]
- 87.Mann J, Oakley F, Johnson PW, Mann DA. CD40 induces interleukin-6 gene transcription in dendritic cells: regulation by TRAF2, AP-1, NF-kappa B, AND CBF1. J Biol Chem. 2002 May 10;277(19):17125–38. doi: 10.1074/jbc.M109250200. [DOI] [PubMed] [Google Scholar]
- 88.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011 May;241(1):260–8. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. PNAS USA. 2001 Jul 31;98(16):9226–30. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008 Jan;82(1):21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L, Sparwasser T, Hasenkrug KJ, Dittmer U. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. PNAS USA. 2011 Feb 8;108(6):2420–5. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature Immunol. 2012 Dec 16;14(2):152–61. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nature Med. 2011 Aug;17(8):975–82. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012 Mar;5(2):184–93. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 95.Martin S, Agarwal R, Murugaiyan G, Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J Immunol. 2010 Jul 1;185(1):551–9. doi: 10.4049/jimmunol.0902206. [DOI] [PubMed] [Google Scholar]
- 96.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. PNAS USA. 2011 Dec 20;108(51):20701–6. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guiducci C, Valzasina B, Dislich H, Colombo MP. CD40/CD40L interaction regulates CD4+CD25+ T reg homeostasis through dendritic cell-produced IL-2. Eur J Immunol. 2005 Feb;35(2):557–67. doi: 10.1002/eji.200425810. [DOI] [PubMed] [Google Scholar]
- 98.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010 Jan 1;70(1):99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gladue RP, Paradis T, Cole SH, Donovan C, Nelson R, Alpert R, Gardner J, Natoli E, Elliott E, Shepard R, Bedian V. The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice. Cancer Immunol Immunother CII. 2011 Jul;60(7):1009–17. doi: 10.1007/s00262-011-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clinl Oncol. 2007 Mar 1;25(7):876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 101.Tai YT, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, Hideshima T, Chauhan D, Hamasaki M, Ishitsuka K, Richardson P, Treon SP, Munshi NC, Anderson KC. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004 Apr 15;64(8):2846–52. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- 102.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009 Jul 2;460(7251):103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998 Sep 1;161(5):2094–8. [PubMed] [Google Scholar]