Abstract
Toxoplasma gondii infection induces a robust CD8 T-cell immunity that is critical for keeping chronic infection under control. In studies using animal models, it has been demonstrated that the absence of this response can compromise the host ability to keep chronic infection under check. Therapeutic agents that facilitate the induction and maintenance of CD8 T-cell response against the pathogen need to be developed. In the last decade, major strides in understanding the development of effector and memory response, particularly in viral and tumor models, have been made. However, factors involved in the generation of effector or memory response against T. gondii infection have not been extensively investigated. This information will be invaluable in designing immunotherapeutic regimens needed for combating this intracellular pathogen that poses a severe risk for pregnant women and immunocompromised individuals.
Keywords: CD8 T cells, IFN-γ, IL-12, IL-15, immunotherapy, infection, Tcm, Tem, Toxoplasma gondii
Toxoplasma gondii, an obligate intracellular parasite of the phylum Apicomplexa infects a wide variety of warm-blooded animals including humans [1]. Besides complications during pregnancy, Toxoplasma-infected immunocompetent adults remain asymptomatic in most cases [2]. In the intermediate host, Toxoplasma undergoes stage conversion between the rapidly multiplying tachyzoite that is thought to be responsible for acute toxoplasmosis and the slowly replicating, relatively quiescent, primarily encysted bradyzoite stage that can persist possibly for life [3,4]. However, with loss of immune competence as observed in HIV/AIDS or immunosuppressive therapies, the parasite reactivates back to presumably tachyzoite stage and causes the development of toxoplasmic encephalitis and death of the host [5]. Although the incidence of toxoplasma encephalitis (TE) among AIDS patients declined considerably in the USA owing to prophylactic treatment and HAART therapy, it still remains a significant problem in developing countries [6].
Historically, T. gondii population structure has been divided into three clonal types based on genetic characteristics [7]. However, recent studies have demonstrated that this population structure is much more diverse in places such as rural Africa and South America where it has been associated with increased risk of primary and reactive ocular toxoplasmosis [7]. Moreover, recent work has now correlated T. gondii infection with increased risk of dementia and Alzheimer’s in the elderly [8]. Therefore, beyond TE in immunocompromised individuals, immunocompetent adults may be at a higher risk for Toxoplasma-related complications.
Apart from their toxicity, current drugs are only efficacious against the tachyzoite stage of this protozoan and not against the encysted bradyzoite stage [3]. Although live-attenuated strains of the parasite have been successfully used to vaccinate mice, they are not considered sufficiently safe for use in humans [9–12]. Hence, understanding the mechanisms responsible for immune control of parasites as well as parasite reactivation is critical for evolving strategies for the development of immunotherapeutic agents against the pathogen.
Immune protection against many intracellular pathogens including viruses, bacteria and protozoa is provided by a robust cell-mediated immunity [13,14]. T cells, macrophages, NK cells and cytokines such as IFN-γ, TNF-α and nitric oxide are the key effector components of this protective immune response [5,15–17]. However, the development of this potent T-cell immunity is critically dependent on appropriate interactions with antigen-presenting cells (APCs), such as dendritic cells, both via cell surface receptor mediated interactions and a conducive cytokine milieu namely IL-12 and IL-15 [18–21]. This activation leads to the proliferation, differentiation and acquisition of effector functions of the antigen-specific T cells [22]. The effector functions of activated antigen-specific T cells include secretion of cytokines IFN-γ and TNF-α and cytotoxicity, which promote further development of adaptive immunity and pathogen control.
As demonstrated by Frenkel et al. using the hamster model, humoral immunity provides little protection against acute toxoplasmosis [23]. Adoptive transfer experiments by this group using injection of intact or lysed splenocytes from immunized animals to naive recipients revealed that upon challenge, only recipients that received intact splenocytes were protected. This suggested that protection against T. gondii is primarily dependent on cell-mediated immune response. Later, using an antibody-based depletion strategy, it was demonstrated that both CD4 and CD8 T cells were important for control of infection with the CD8 T-cell subset playing the dominant role [24]. Further studies by Suzuki et al., using IFN-γ depletion and CD8 T-cell adoptive transfer approach, identified IFN-γ as a major mediator of resistance against toxoplasmosis [15]. Subsequently, Khan et al. developed the first antigen-specific CD8 T-cell clones capable of killing T. gondii tachyzoites in vitro via their cytotoxic activity [25]. These studies combined suggest that the critical effector mechanisms involved in controlling T. gondii infection include IFN-γ production and cytotoxicity mediated by CD8 T cells. Subsequent studies have reinforced the paradigm that CD8 T cells are the dominant effector cell responsible for the control of long-term infection with CD4 T cells playing an important secondary role [26,27]. Since these seminal observations were made, the complex multifactorial steps involved in CD8 activation, effector function acquisition and memory/effector differentiation during T. gondii infection have been elucidated with greater clarity. However, many questions regarding this process still remain to be addressed. In this article, we present current knowledge of how this multistep process leads to the activation of CD8 T-cell response to T. gondii and how it modulates the potency of this infection. In addition, we discuss how this knowledge regarding T. gondii-specific CD8 T-cell response development can be exploited for the development of immunotherapeutic agents.
Antigen presentation & priming of CD8 T-cell immunity
As a first step for activation of CD8 T-cell response, cell–cell interaction between APCs and CD8 T cells is needed [28]. Subsequently, recognition of antigenic peptide presented by APCs in context of MHC class I by CD8 T cells is required [28]. Early studies using inbred and outbred mouse strains demonstrated that differences in the class I haplotype could influence the outcome of T. gondii infection, suggesting that beyond variations in inflammatory responses, a genetic component could influence the control of the parasite [29–31]. Studies conducted using animals with deleted or mutated Ld allele demonstrated that mice such as BALB/c that possess this allele were protected while those that do not (C56BL/6, B10 or CBA/J) exhibit susceptibility to infection [32–34]. Experiments using mice expressing human MHC class I transgenes showed similar allelic dependence for the control of T. gondii [35]. Hence, MHC class I haplotype is an important determinant for the generation of protective CD8 T cells in response to T. gondii infection. Therefore, identification of class I-restricted T. gondii-specific epitopes would be helpful in generating vaccines that mount an effective CD8 response. Several studies have identified Toxoplasma surface antigens and secreted proteins such as the rhoptry, dense granule and microneme proteins as T-cell antigens [12,36–40]. However, whether epitopes contained in such antigens indeed correspond to dominant T. gondii-specific CD8 T-cell response in Toxoplasma-infected mice (i.e., without vaccination or immunization) had not been established until recently. In 2008, Blanchard et al. identified the decapeptide HPGSVNEFDF (HF10) from the GRA6 dense granule protein as the dominant, naturally processed protein recognized by CD8 T cells during T. gondii infection in BALB/c (H-2d) mice [41]. This was followed by the discovery of two more H2-Ld-restricted epitopes, SPMNGGYYM and IPAAAGRFF, from the dense granule protein GRA4 and rhoptry protein ROP7, respectively [42]. Interestingly, while H-2Ld/GRA4-reactive CD8 T cells peaked during the acute phase, H-2Ld/ROP7-reactive CD8 T cells peaked during chronic phase, suggesting parasite stage-specific recognition of endogenous T. gondii-derived CD8 T-cell epitopes. Subsequently, Wilson et al. identified a novel H-2Kb-restricted epitope, SVLAFRRL, derived from TGD057, a protein of unknown function [20]. Hence, the current challenge lies in identifying dominant human MHC class I-restricted epitopes for T. gondii antigens. Preliminary work from McLeod’s laboratory has identified several epitopes from T. gondii antigens restricted by HLA-A02, a supertype family that is present in 50% of the human population [43]. Using a pool of such peptides, they showed that immunized HLA-A*0201 transgenic mice were protected against Toxoplasma challenge. However, this protection was only partial and it remains to be established whether a similar pool of peptides can prevent TE in chronically infected individuals.
Role of costimulatory molecules in the development of CD8 T-cell immunity against T. gondii
Apart from T-cell receptor–peptide–MHC interaction, costimulatory molecules play a critical role in adequate CD8 T-cell activation [44,45]. Costimulation occurs mainly via interaction of two families of proteins, the immunoglobulin (Ig) superfamily members B7/CD28 and the TNF receptor/TNF superfamily of proteins [46]. Early investigations of costimulatory molecules in T. gondii models using human peripheral blood mononuclear cells demonstrated that blockade of both CD80 and CD86 reduced T-cell activation [47]. Subsequently, studies in mouse models demonstrated that CD28, a receptor for CD80 and CD86, was dispensable for protective immunity against the parasite despite reduced IFN-γ production by T cells [48]. Interestingly, when infected mice were re-infected with the RH strain of the parasite, CD28−/− mice, as opposed to wild-type animals, rapidly succumbed to the infection. Since a potent recall response is considered a hallmark of memory CD8 T cells, these data suggest that lack of CD28 impairs the development of memory T cells [49]. Using an antibody-based approach, CD40 and CD40L were shown to play an important role in the in vitro activation of human T cells in response to T. gondii [50]. However, in vivo studies using mouse models demonstrated that while this pathway affected macrophages, it did not downregulate T-cell responses, suggesting that the CD40–CD40L pathway is dispensable for robust T-cell response [51]. Since CD40–CD40L pathways were not found to be critical for primary T-cell responses against Toxoplasma in mice, other pathways were investigated for their role in T-cell activation against this pathogen. These studies focused on the CD28 homolog, inducible costimulator protein (ICOS) [52]. However, unlike CD28, ICOS is an inducible costimulatory molecule for T-cell activation [52]. Although ICOS−/− mice elicited a poor CD4 T-cell response, the CD8 T-cell immunity remained unaffected during acute toxoplasmosis [53]. This suggests that neither ICOS–ICOSL signaling or differential CD4 T-cell help affects primary CD8 response during acute toxoplasmosis. Interestingly, over 90% of brain-resident CD8 T cells expressed ICOS during chronic phase [54]. In allograft rejection models, it has been shown that delayed but not early blockade of ICOS–ICOSL reduces effector CD8 development, suggesting a possible role of this pathway postpriming [55]. However, in Toxoplasma models, it remains to be addressed whether the ICOS–ICOSL pathway has a differential effect on CD8 T cells in lymphoid versus nonlymphoid tissue or during the acute/chronic phase. The development of a potent CD8 T-cell response with minimal immunopathology involves a delicate balance between positive signal from costimulatory receptors and negative signal from inhibitory receptors [56]. However, the role of the latter family of receptors on CD8 T-cell response against T. gondii has not been thoroughly investigated. Early work on these receptors focused on cytotoxic T lymphocyte antigen (CTLA)-4, a receptor expressed on activated CD8 T cells that binds to CD80 and CD86, albeit with much higher affinity than CD28 [57]. Ex vivo treatment of splenocytes from Toxoplasma-infected mice with anti-CD3 and CTLA-4-Ig was found to significantly reduce IFN-γ production in the spleen but not in the brain [48]. However, in our laboratory, using a similar protocol for brain mononuclear cell preparation, we have noted that apart from leukocytes, other cell types do not survive well in vitro [Bhadra R & Khan IA, Unpublished Observations]. Hence, for more thorough understanding of the role of CTLA-4 on brain resident CD8 T cells in the endogenous microenvironment, in vivo blockade with anti- CTLA-4 antibody needs to be performed. Apart from CTLA-4, PD-1, a negative regulator of activated T cells, has been shown to be upregulated on CD8 T cells from Toxoplasma-infected C57BL/6 mice during the chronic phase [58]. Although the significance of the PD-1–PD-L1 pathway in mediating CD8 dysfunction in chronic viral models is well established, the role of this pathway in the T. gondii model of infection remains to be extensively investigated [59,60]. Overall, the mechanism relating to the differential role of various costimulatory and inhibitory molecules in activating CD8 T cells and modulating their function is poorly understood. Hence, a more thorough understanding of these receptors in T. gondii models is required for their potentially critical application as adjuvants during vaccination or other immunotherapeutic interventions. As such, using high-throughput approaches such as microarrays in combination with conditional knockouts and bone marrow chimeras, we may be able to better indentify and characterize new targets of costimulation or coinhibition on CD8 T cells and elucidate the CD8 intrinsic and extrinsic role of these molecules in mediating their activation, differentiation and effector function acquisition.
Role of cytokines in the development of CD8 T-cell immunity against T. gondii
Once initial CD8 T-cell activation takes place, their expansion and differentiation is modulated by the cytokine milieu [61]. During T. gondii challenge, neutrophils (polymorphonuclear neutrophils), macrophages, dendritic cells and several inflammatory cell types infiltrate into the site of infection and exert their function by providing nitric oxide, cytokines and chemokines [20,62–69]. A critical cytokine produced by these cells during the acute phase of infection is IL-12, which is necessary for optimal induction of the primary CD8 T-cell response [21,70–72]. The biologically active form of IL-12, IL-12p70 is composed of two subunits: the constitutively expressed IL-12p35 and the inducible IL-12p40 [73]. IL-12p70 produced during T. gondii infection can induce CD8 T cells to produce IFN-γ, differentiate, proliferate and expand [70,71,74,75]. The levels of IL-12p70 heterodimer and IL-12p40 production elicited by T. gondii is strain dependent and this can potentially cause differences in the robustness of CD8 responses [76]. Despite the critical role of IL-12 in mediating a potent CD8 T-cell immunity against this parasite, the addition of IL-23, a cytokine structurally similar to IL-12p70, does not affect T-cell-mediated IFN-γ production [77]. Moreover, CD8 T cells in Toxoplasma-infected p19−/− (IL-23-deficient) mice do not exhibit reduced activation vis-à-vis wild-type controls. Apart from IL-12, IL-27 (an IL-12-related cytokine) has been recently shown to play a critical role in eliciting IFN-γ production by CD8 T cells during acute toxoplasmosis [78]. However, whether IL-27 plays any role in long-term CD8 response to T. gondii remains to be established. Interestingly, in the same study, the authors found that CD8 intrinsic IFN-γ receptor signaling is not required for optimal IFN-γ production by CD8 T cells in vivo.
Several cytokines, other than IL-12-related cytokines, are important for CD8 T-cell function in response to T. gondii infection. This includes members of the common cytokine receptor γ-chain (γc) cytokines such as IL-2, IL-7 and IL-15 [79]. Unlike other pathogens, T. gondii does not induce a potent IL-2 response [80–82]. Hence, it is not surprising that in mice deficient in CD4 T cells, which is considered to be the primary source of IL-2, primary CD8 response during acute toxoplasmosis remains unaffected [27]. Paradoxically T. gondii-infected IL-2−/− mice exhibited poor CD8 response activation [83]. While in the former study, the animals were orally infected, in the latter, mice were challenged via the intraperitoneal route. These apparently contradictory observations suggest a possible differential dependence on IL-2, based on the route of infection. Hence, the effect of CD4 T cells or IL-2 on the maintenance of CD8 T-cell memory as well as programming and differentiation of CD8 T-cells effectors need to be investigated more thoroughly using both oral and intraperitoneal routes of infection.
Very little work has been carried out to investigate the function of IL-7 in the development of CD8 T-cell responses against T. gondii. However, an early study from our group reported that exogenous treatment with the cytokine can augment cytotoxic CD8 T-cell response against infection [80]. Moreover, in a very recent study we reported that in absence with IL-15, endogenous IL-7 plays a critical role in the development of T. gondii-specific CD8 T-cell memory precursors via expression of antiapoptotic protein Bcl-2 [79]. By contrast, the role of related γc family cytokine IL-15 in the maintenance of CD8 T-cell memory response against Toxoplasma infection has been established by the studies conducted in our laboratory [18,79,84–86]. Moreover, during the acute response, it plays a critical role in regulating CD8 burst size but not per cell function [79]. Interestingly, IL-15−/− animals survive as well as wild-type mice when infected with T. gondii, suggesting that there may be some redundancy in its functions [85,87]. As such, when IL-15−/− mice are depleted of IL-7, they exhibit extremely rapid mortality [79]. Recently, IL-21 (another γc family member) was demonstrated to modulate the quality and potency of CD8 T-cell response in both acute and chronic viral models of infection [88,89]. Thus, it will be very important to address the role of this cytokine during Toxoplasma infection in susceptible and resistant mouse strains.
Apart from the aforementioned cytokines, IL-10, a downregulatory cytokine, is induced during T. gondii infection [90]. Interestingly, the major source of this cytokine during acute toxoplasmosis is not Foxp3-expressing regulatory CD4 T cells but rather conventional T-bet+Foxp3−CD4 T cells [91]. Recent studies have demonstrated that while IL-10 downregulates CD8 response during chronic viral infection, it does not suppress CD8 immunity during acute viral infection [89,92]. In contrast to these findings, Gazzinelli et al. noted upregulation of CD8 mediated TNF-α but not IFN-γ production in IL-10-deficient B6 mice during acute toxoplasmosis [90]. Incidentally, unlike viral models such as lymphocytic choriomeningitis virus, CD8 response against Toxoplasma is dependent on IL-12 [93]. As IL-10−/− mice overproduce IL-12p70, increased TNF-α production by CD8 T cells in Toxoplasma-infected IL-10−/− mice may be reflective of IL-12-mediated increase in the number of antigen-specific CD8 T cells or augmented per cell functionality or both [90]. Future studies with wild-type/IL-10R−/− mixed bone marrow-chimerized mice are needed to address whether this augmented CD8 response in IL-10R−/− mice is indeed due to differential CD8 intrinsic IL-10 signaling. In agreement with observations in viral models, the blockade of IL-10 in chronically infected BALB/c mice resulted in an increased number of CD8 T cells in the brain with concurrent decrease in T. gondii burden [94]. However, IL-10 blockade during acute or chronic toxoplasmosis in susceptible mice strains causes severe immunopathology and rapid mortality [Bhadra R & Khan IA, Unpublished Data] [91]. Hence, this potential risk must be taken into consideration while designing any immunotherapeutic strategy utilizing a IL-10-blocking regimen to boost the CD8 response against T. gondii.
Differentiation of effector & memory CD8 T-cell subpopulations
After activation of naive CD8 T cells during an infection, they expand over 104-fold and differentiate into a heterogeneous population of effector cells [95,96]. This population is composed of terminally differentiated effector cells termed short-lived effector cells (SLECs) and cells that are destined to become long-lived memory cells termed memory precursor effector cells (MPECs) [95,96]. Phenotypically, SLECs can be discriminated from MPECs based on the expression of KLRG1 and lack of IL-7Rα expression on the former [96]. Both SLECs and MPECs exhibit effector functions and low proliferative capacity [96,97]. However, as MPECs further develop into mature memory CD8 T cells, they become self-renewing and undergo homeostatic proliferation [96]. After this initial expansion and differentiation, the majority of activated CD8 T cells undergoes a process termed contraction where the terminally differentiated SLECs undergo apoptosis leaving behind memory T cells [95]. Many factors including, antigen avidity and affinity, costimulation and the level of inflammation can influence CD8 T-cell differentiation [44,61,98]. The mechanisms governing these processes are being investigated in viral and bacterial model systems and only now are beginning to be addressed in the field of T. gondii [96].
Seminal work from Joshi et al. using Listeria monocytogenes reported that master transcription factor T-bet acts as a rheostat whereby under highly inflammatory conditions namely IL-12, T-bet upregulation results in preferential CD8 differentiation to SLECs as opposed to MPEC [97]. Similarly, a recent study using the T. gondii model has demonstrated a critical role for IL-12 in mediating T-bet-mediated generation of KLRG1+ CD8 effector T cells [93]. Although IL-12 is important for SLEC differentiation in vivo, a study using lymphocytic choriomeningitis virus model demonstrated that their survival is critically dependent on IL-15 [97].
Memory CD8 T cells are critical for long-term protection against many intracellular pathogens [96,99]. These long-lived cells can be further broadly categorized into two subsets: central memory and effector memory CD8 T (Tem) cells [100]. Effector Tem cells are characterized by high cytotoxicity, lack of expression of lymphoid homing molecules such as CCR7 and CD62L and persistence primarily in nonlymphoid tissue [61,101]. Central memory T (Tcm) cells on the other hand, reside mainly in secondary lymphoid tissue, express high levels of IL-7Rα, CCR7 and CD62L and are less cytotoxic than Tem cells [61,100]. However, these cells exhibit increased antigen sensitivity and self-renewal and mediate rapid and robust recall responses vis-à-vis the effector memory population. In various infectious disease models including HIV, development of a potent Tcm subset has been linked with improved outcome, suggesting that one of the goals of any vaccination regimen targeting CD8 T-cell-based immunity should be generation of a robust Tcm subset [102,103]. While the differential role of Tcm and Tem in the control of T. gondii infection remains to be thoroughly investigated, a recent study by Wilson et al. suggests that high levels of IL-12, while beneficial for effector and effector memory CD8 populations, have detrimental effect on number and function of tgd057-specific CD8 Tcm cells [20]. As parasite virulence can alter the levels of IL-12 produced during infection, one potential mechanism of immune escape may be via overproduction of IL-12, thereby inhibiting development of the Tcm subset, which may result in deficient control of chronic infection [104].
Effector role of CD8 T cells during T. gondii infection
As mentioned previously IFN-γ is critical for mediating protective immunity to T. gondii and during chronic infection, CD8 T cells are a major source of this cytokine, which is essential for controlling parasite reactivation [15,26]. IFN-γ acts on surrounding cells, including macrophages, which in turn inhibit the proliferation of the parasite via induction of inducible nitric oxide synthetase (iNOS), reactive oxygen species (ROS) and indoleamine 2,3-deoxygenase (IDO) [67,105–112]. Moreover, IFN-γ via modulation of p47GTPases regulates the survival of the parasite in activated macrophages [113]. Thus, IFN-γ is likely to have two roles in controlling the parasite; initially during acute infection to reduce parasite numbers and then during chronic infection to exert immune pressure sufficient enough to maintain the parasites in the encysted bradyzoite stage (Figure 1). In agreement with this notion, IFN-γ −/− mice or IFN-γ depletion of acutely infected wild-type mice results in rapid mortality due to uncontrolled replication of the parasite [15,114]. Similarly, anti-IFN-γ treatment of chronically infected mice results in reactivation of encysted parasites [115,116]. CD8 T cells can also provide effector function via production of other cytokines including TNF-α and through their cytolytic abilities [117]. The role of TNF-α in control of T. gondii infection was shown early on to be synergistic with IFN-γ [7]. A second study demonstrated that when infected orally, depletion of TNF-α led to the death of susceptible C57BL/6 mice [28]. An additional study investigating TE in Toxoplasma-infected mice revealed that TNF-α depletion of chronically infected mice caused reactivation of T. gondii as rapidly as neutralization of IFN-γ suggesting that this cytokine played an equally important role in control of chronic T. gondii infection [5]. Taken together, these studies reveal an important function of TNF-α in the control of T. gondii parasites and development of TE in mice. In viral and bacterial model systems, CD8 T cells have been shown to produce copious amounts of TNF-α [118,119]. Although Gazzinelli et al. demonstrated that CD8 T cells can indeed produce TNF-α during acute toxoplasmosis, the role of CD8-produced TNF-α during acute and chronic infection remains to be definitively addressed [90].
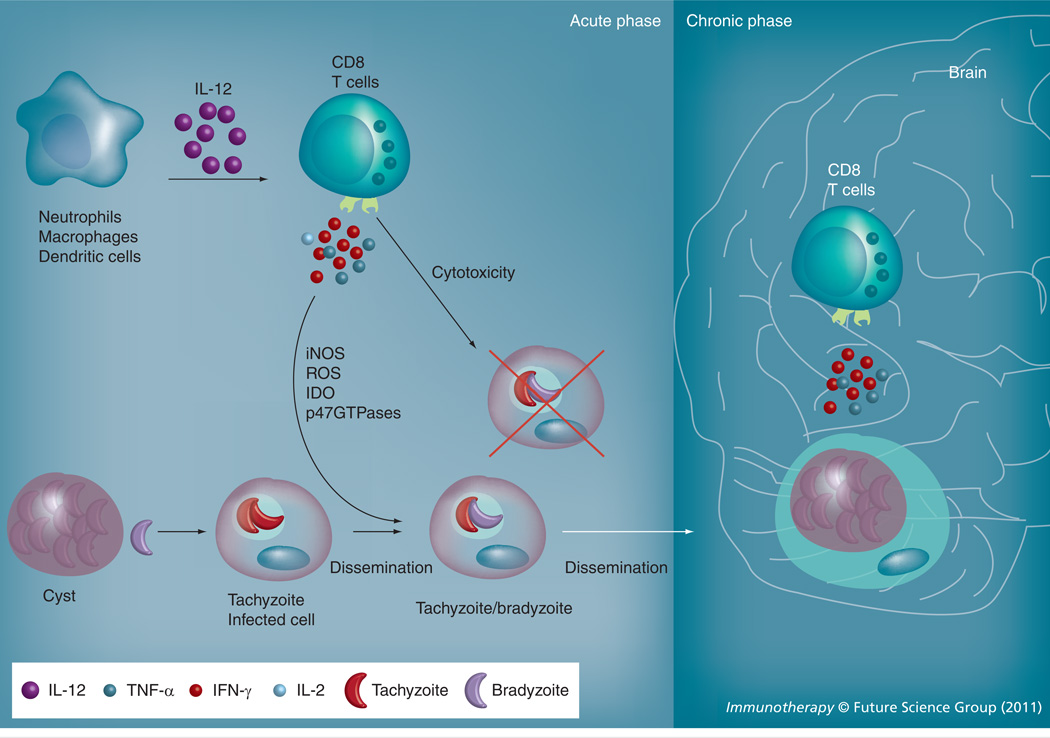
Figure 1. CD8 T-cell effector mechanisms during acute and chronic toxoplasmosis.
During the acute phase, CD8 T cells primarily via the production of IFN-γ and possibly TNF-α and cytotoxicity-associated molecules such as perforin and granzyme B inhibit parasite replication and cause conversion of the parasite from tachyzoite stage to bradyzoite stage. IFN-γ elicits the production of NO, ROS and IDO and activates p47GTPases that augment parasite clearance. During the chronic phase, CD8-produced IFN-γ and possibly TNF-α is vital for maintaining the parasites in the encysted stage.
IDO: Indoleamine 2,3-deoxygenase; iNOS: Inducible nitric oxide synthetase; ROS: Reactive oxygen species.
CD8 T cells have been shown to directly kill extracellular tachyzoites of T. gondii in culture cells [25]. In vitro studies demonstrated that antigen-specific cytotoxic activity towards T. gondii-infected cells was exhibited by human CD8 T cells [120,121]. Despite the findings from these studies, the role of this cytotoxic function of CD8 T cell in controlling T. gondii infection is not completely understood. Although perforin knockout mice vaccinated with the attenuated mutant ts-4 displayed severely defective CTL activity against T. gondii-infected target cells, these mice survived infection with a virulent strain [122]. A subsequent study using adoptive transfer of CD8 T cells from wild-type, IFN-γ −/− or perforin−/− mice demonstrated that perforin-mediated cytotoxicity was dispensable for prevention of TE in resistant mouse strain [123]. By contrast, another study using Toxoplasma-susceptible mice demonstrated that adoptive transfer of immune CD8 T cells into infected severe combined immunodeficiency (SCID) mice (treated with sulfadiazine to establish chronic infection) removed encysted parasites in the brain via a perforin-dependent mechanism [124]. However, a recent paper by Schaeffer et al. demonstrating that CD8 T cells do not directly interact with intact Toxoplasma cysts in the brain, raises the question of whether donor CD8 T cells are actually lysing intact cysts in drug-treated SCID mice [125]. This notion is further supported by observations in sulfadiazine-treated IRF8−/− mice (IRF8−/− do not produce IL-12p40 and do not upregulate IFN-γ) where an increase in number and size of parasitic foci in the brain is noted over time [126,127]. This potentially suggests that drug treatment in immunodeficient mouse strains is unable to completely prevent cyst rupture and parasite release. Based on these two papers, it is likely that in drug-treated SCID mice, cysts intermittently rupture and release parasites that are then killed by donor CD8 T cells before they can form new cysts. Over time, this would result in cyst reduction as observed by Suzuki et al. [124]. Hence, in future studies, it will be important to keep this possibility in mind. Apart from perforin-mediated cytolysis, recent work by Jordan et al. using cps1–1 strain of the parasite demonstrates that a perforin and Fas–FasL independent pathway may play a subsidiary role in CD8-mediated cytotoxicity [128]. It will be critical to explore this pathway thoroughly in future studies. Taken together, IFN-γ seems to be the key effector cytokine during both acute and chronic phases of infection. However, the role of TNF-α and cytotoxicity associated molecules such as granzyme B and perforin in mediating CD8 effector functions during toxoplasmosis needs to be investigated further (Figure 1). Moreover, how transcription factors other than T-bet such as eomesodermin, BLIMP, Run×3 and Bcl-6, which have been shown to play a role in other model systems and regulate development as well as differentiation and function of CD8 T cells during T. gondii infection, need to be addressed [129–133]. Hitherto, a major hurdle in investigating these questions thoroughly was the lack of information regarding dominant CD8 epitopes. However, the advent of new tools such as MHC class I tetramers, T-cell receptor transgenic mice and ovalbumin expressing transgenic parasites will permit dissection of effector mechanisms of various CD8 subsets and their correlation with immune protection during acute and chronic phases of infection with much greater clarity [20,41,42,134,135].
Conclusion
There is little debate that CD8 T cells are critical for long-term control of toxoplasmosis. Hence, for a vaccination strategy to be successful against this pathogen, a potent CD8 T-cell response needs to be elicited. Considering the prevalence of toxoplasmosis in developing countries, any vaccine production strategy cannot utilize costly and labor-intensive approaches such as peptide-loaded dendritic cell or ex vivo CD8 manipulation for instance. Preliminary studies from the McLeod laboratory have shown partial efficacy of Toxoplasma peptide pool immunization in HLA-A*0201 transgenic mice [43]. However, a major obstacle that needs to be surmounted is the identification of dominant MHC class I-restricted epitopes for T. gondii recognized by CD8 T cells during acute and chronic toxoplasmosis in humans. Considering that Toxoplasma is a eukaryote with a complex lifecycle, it is likely that for any vaccination regimen to be efficacious, multiple epitopes will need to be targeted [3,4]. Moreover, depending on whether vaccination has prophylactic or therapeutic purpose, peptide pool and adjuvant selection may be different. For example, an adjuvant that induces high-levels of IL-12 or inflammation may be ideal for therapeutic vaccination strategies as it would elicit a potent effector response (Figure 2). On the other hand, use of such a regimen for a prophylactic vaccine will not be the best solution as it may not elicit a robust long-lived Tcm response (Figure 2). In viral infections including HIV, T-cell polyfunctionality (i.e., the capacity to exhibit multiple effector mechanisms upon antigen encounter) has been identified as one of the most important immune correlates of protection [118,136–138]. While the role of CD8 poly-functionality in Toxoplasma model has not been thoroughly investigated, any vaccine design targeting this parasite must take this parameter into consideration. A recent study using vaccination against SIV further reinforces this paradigm [139]. In that report, the frequency of polyfunctional CD8 T cells rather than antigen-specific CD8 T-cell frequency correlated with immune protection. Incidentally, the authors found that vaccination with Toll-like receptor (TLR) agonists along with IL-15 elicited the most potent polyfunctional CD8 responses. While TLR agonists (TLR2, 3 and 9) enhance CD8 response by activating and maturing dendritic cells, IL-15 promotes the generation of high-avidity, long-lived CD8 T cells and increases homeostatic proliferation of memory CD8 T cells. The efficacy of a similar regimen needs to be experimented in vaccination protocols against T. gondii. Overall, a better understanding of immune correlates of a robust, long-lived CD8 response is critical for the development of successful vaccine against Toxoplasma.
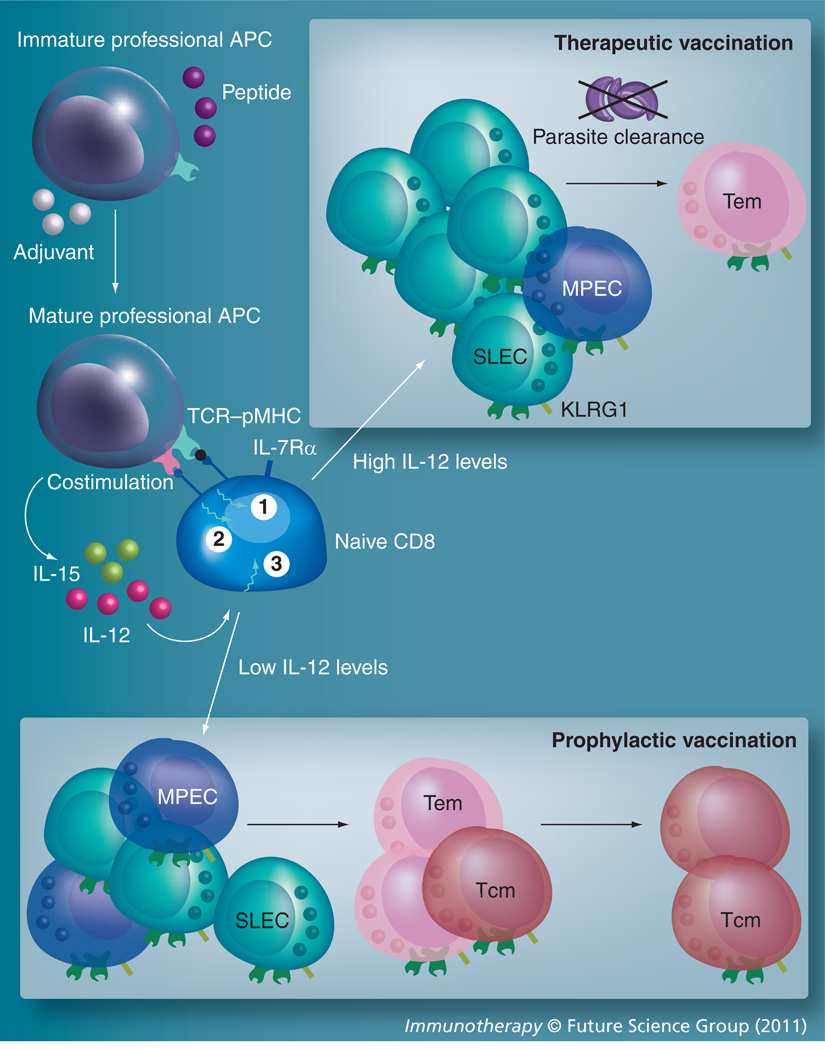
Figure 2. Vaccination strategy targeting CD8 T-cell response against Toxoplasma.
Any vaccination strategy against Toxoplasma must involve appropriate selection of adjuvant and peptide pools such that there is optimal TCR–peptide MHC interaction (signal 1), costimulation (signal 2) and cytokine milieu (signal 3) during CD8 priming. In addition to peptide pool used, adjuvant selection may need to be altered depending on whether it is a prophylactic or therapeutic vaccine. Adjuvants that are highly inflammatory will result in preferential CD8 differentiation to effector lineage that may be ideal for therapeutic vaccination. Conversely, adjuvants that elicit comparatively lower inflammation will result in robust Tcm development, which may be optimal for prophylactic vaccination regimens. APC: Antigen-presenting cell; MPEC: Memory precursor effector cell; SLEC: Short-lived effector cell; Tem: T effector memory; Tcm: Central T memory; TCR: T-cell receptor.
Future perspective.
Understanding the generation of long-term CD8 T-cell responses is central to the development of immunotherapeutic agents against T. gondii infection. However, at present, a major limitation in this field is the lack of information regarding epitopes involved in the elicitation of this response in humans. In recent years, studies in this direction have already begun and we anticipate dominant human MHC-restricted epitopes will be identified in the near future. This will enable us to determine and quantitate effector and memory CD8 T-cell response generated during natural infection and various vaccination protocols. The information from these studies will provide an important breakthrough in the understanding of factors involved in the generation of long-term CD8 T-cell immunity against complex intracellular parasites in humans and permit the development of improved immunotherapeutic regimens against this parasite.
Executive summary.
-
▪
Toxoplasma gondii is an obligate intracellular parasite that causes morbidity and mortality in immunocompromised individuals and also poses a risk to the fetus of pregnant women.
-
▪
Although the parasite induces a strong innate and adaptive immune response, CD8 T cells are major effector cells responsible for long-term protection with CD4 T cells playing an important secondary role.
-
▪
While CD4 T cells are not essential for the induction of primary CD8 T-cell immunity, they play an important role in the maintenance of long-term response.
-
▪
Effector response of CD8 T cells is dependent on their ability to secrete IFN-γ and exhibit cytolytic activity against parasite-infected cells.
-
▪
Factors involved in the development of long-term (effector or central memory) CD8 T-cell response against T. gondii infection remain insufficiently studied.
-
▪
Studies related to the parasite antigens involved in the elicitation of CD8 T-cell immunity against the pathogen are limited.
-
▪
Cytokines such as IL-12, IL-7 and IL-15 are important for the induction of primary effector CD8 T-cell immunity. However, among these three cytokines, only IL-15 appears to be critical for the maintenance of long-term CD8 T-cell immune response.
-
▪
Immunotherapeutic agents that induce appropriate T-cell receptor–peptide MHC interaction, cytokine (IL-12, IL-7 and IL-15) and costimulatory milieu during the priming phase, thereby ensuring the development of polyfunctional effector and memory CD8 response, will serve as successful vaccine candidates.
Acknowledgments
Research in the laboratory of IA Khan cited in this article was supported by grants from the NIH (AI-33325).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 1998;28(7):1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 2.Wallon M, Liou C, Garner P, Peyron F. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. BMJ. 1999;318(7197):1511–1514. doi: 10.1136/bmj.318.7197.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons RE, Mcleod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18(5):198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 4.Matrajt M, Donald RG, Singh U, Roos DS. Identification and characterization of differentiation mutants in the protozoan parasite Toxoplasma gondii. Mol. Microbiol. 2002;44(3):735–747. doi: 10.1046/j.1365-2958.2002.02904.x. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 1993;151(7):3672–3681. [PubMed] [Google Scholar]
- 6.Vidal JE, Hernandez AV, De Oliveira AC, Dauar RF, Barbosa SP, Jr, Focaccia R. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS. 2005;19(10):626–634. doi: 10.1089/apc.2005.19.626. [DOI] [PubMed] [Google Scholar]
- 7.Sibley Ld, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-α triggers antitoxoplasmal activity of IFN-γ primed macrophages. J. Immunol. 1991;147(7):2340–2345. [PubMed] [Google Scholar]
- 8.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr. Bull. 2007;33(3):745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gigley JP, Fox BA, Bzik DJ. Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect. Immun. 2009;77(12):5380–5388. doi: 10.1128/IAI.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokosuka T, Kobayashi W, Sakata-Sogawa K, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C θ translocation. Immunity. 2008;29(4):589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escajadillo A, Frenkel JK. Experimental toxoplasmosis and vaccine tests in Aotus monkeys. Am. J. Trop. Med. Hyg. 1991;44(4):382–389. doi: 10.4269/ajtmh.1991.44.382. [DOI] [PubMed] [Google Scholar]
- 12.Jongert E, Roberts CW, Gargano N, Forster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem. Inst. Oswaldo Cruz. 2009;104(2):252–266. doi: 10.1590/s0074-02762009000200019. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann SH. Cell-mediated immunity: dealing a direct blow to pathogens. Curr. Biol. 1999;9(3):R97–R99. doi: 10.1016/s0960-9822(99)80059-1. [DOI] [PubMed] [Google Scholar]
- 14.Thorne KJ, Blackwell JM. Cell-mediated killing of protozoa. Adv. Parasitol. 1983;22:43–151. doi: 10.1016/s0065-308x(08)60461-3. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 16.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl Acad. Sci. USA. 1997;94(25):13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter CA, Ellis-Neyer L, Gabriel KE, et al. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J. Immunol. 1997;158(5):2285–2293. [PubMed] [Google Scholar]
- 18.Kasper LH, Matsuura T, Fonseka S, Arruda J, Channon JY, Khan IA. Induction of γδ T cells during acute murine infection with Toxoplasma gondii. J. Immunol. 1996;157(12):5521–5527. [PubMed] [Google Scholar]
- 19.Aliberti J, Jankovic D, Sher A. Turning it on and off: regulation of dendritic cell function in Toxoplasma gondii infection. Immunol. Rev. 2004;201:26–34. doi: 10.1111/j.0105-2896.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DC, Grotenbreg Gm, Liu K, et al. Differential regulation of effector- and central-memory responses to Toxoplasma gondii infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog. 2010;6(3):E1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan IA, Matsuura T, Kasper LH. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 1994;62(5):1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Lier RA, Ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat. Rev. Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel JK. Adoptive immunity to intracellular infection. J. Immunol. 1967;98(6):1309–1319. [PubMed] [Google Scholar]
- 24.Suzuki Y, Remington JS. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 1988;140(11):3943–3946. [PubMed] [Google Scholar]
- 25.Khan IA, Smith KA, Kasper LH. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J. Immunol. 1988;141(10):3600–3605. [PubMed] [Google Scholar]
- 26.Suzuki Y, Remington JS. The effect of anti-IFN-γ antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J. Immunol. 1990;144(5):1954–1956. [PubMed] [Google Scholar]
- 27.Casciotti L, Ely KH, Williams ME, Khan IA. CD8(+)-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4(+) T cells. Infect. Immun. 2002;70(2):434–443. doi: 10.1128/IAI.70.2.434-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson LL. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect. Immun. 1992;60(5):1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo FG, Williams DM, Grumet FC, Remington JS. Strain-dependent differences in murine susceptibility to toxoplasma. Infect. Immun. 1976;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mcleod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J. Immunol. 1989;142(9):3247–3255. [PubMed] [Google Scholar]
- 31.Deckert-Schluter M, Schluter D, Schmidt D, Schwendemann G, Wiestler Od, Hof H. Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect. Immun. 1994;62(1):221–228. doi: 10.1128/iai.62.1.221-228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown Cr, Mcleod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 1990;145(10):3438–3441. [PubMed] [Google Scholar]
- 33.Suzuki Y, Joh K, Kwon OC, Yang Q, Conley FK, Remington JS. MHC class I gene(s) in the D/L region but not the TNF-α gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 1994;153(10):4649–4654. [PubMed] [Google Scholar]
- 34.Brown CR, Hunter CA, Estes RG, et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85(3):419–428. [PMC free article] [PubMed] [Google Scholar]
- 35.Brown CR, David CS, Khare SJ, Mcleod R. Effects of human class I transgenes on Toxoplasma gondii cyst formation. J. Immunol. 1994;152(9):4537–4541. [PubMed] [Google Scholar]
- 36.Kur J, Holec-Gasior L, Hiszczynska-Sawicka E. Current status of toxoplasmosis vaccine development. Expert Rev. Vaccines. 2009;8(6):791–808. doi: 10.1586/erv.09.27. [DOI] [PubMed] [Google Scholar]
- 37.Beghetto E, Nielsen HV, Del Porto P, et al. A combination of antigenic regions of Toxoplasma gondii microneme proteins induces protective immunity against oral infection with parasite cysts. J. Infect. Dis. 2005;191(4):637–645. doi: 10.1086/427660. [DOI] [PubMed] [Google Scholar]
- 38.Khan IA, Ely KH, Kasper LH. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 1994;152(4):1856–1860. [PubMed] [Google Scholar]
- 39.Reichmann G, Dlugonska H, Fischer HG. Characterization of TgROP9 (p36), a novel rhoptry protein of Toxoplasma gondii tachyzoites identified by T cell clone. Mol. Biochem. Parasitol. 2002;119(1):43–54. doi: 10.1016/s0166-6851(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 40.Scorza T, D’souza S, Laloup M, et al. A GRA1 DNA vaccine primes cytolytic CD8(+) T cells to control acute Toxoplasma gondii infection. Infect. Immun. 2003;71(1):309–316. doi: 10.1128/IAI.71.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchard N, Gonzalez F, Schaeffer M, et al. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 2008;9(8):937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frickel EM, Sahoo N, Hopp J, et al. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J. Infect. Dis. 2008;198(11):1625–1633. doi: 10.1086/593019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cong H, Mui EJ, Witola WH, et al. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine. 2011;29(4):754–762. doi: 10.1016/j.vaccine.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 45.Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 46.Sharpe AH. Mechanisms of costimulation. Immunol. Rev. 2009;229(1):5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subauste CS, De Waal Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J. Immunol. 1998;160(4):1831–1840. [PubMed] [Google Scholar]
- 48.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 1999;163(6):3354–3362. [PubMed] [Google Scholar]
- 49.Zammit Dj, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J. Immunol. 1999;162(11):6690–6700. [PubMed] [Google Scholar]
- 51.Reichmann G, Walker W, Villegas EN, et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 2000;68(3):1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshinaga SK, Whoriskey JS, Khare Sd, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 53.Wilson Eh, Zaph C, Mohrs M, et al. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J. Immunol. 2006;177(4):2365–2372. doi: 10.4049/jimmunol.177.4.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villegas EN, Lieberman LA, Mason N, et al. A role for inducible costimulator protein in the CD28-independent mechanism of resistance to Toxoplasma gondii. J. Immunol. 2002;169(2):937–943. doi: 10.4049/jimmunol.169.2.937. [DOI] [PubMed] [Google Scholar]
- 55.Harada H, Salama AD, Sho M, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J. Clin. Invest. 2003;112(2):234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Croft M. The evolving crosstalk between co-stimulatory and co-inhibitory receptors: HVEM-BTLA. Trends Immunol. 2005;26(6):292–294. doi: 10.1016/j.it.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 58.Wilson EH, Harris TH, Mrass P, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30(2):300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blattman JN, Greenberg PD. PD-1 blockade: rescue from a near-death experience. Nat. Immunol. 2006;7(3):227–228. doi: 10.1038/ni0306-227. [DOI] [PubMed] [Google Scholar]
- 60.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 2000;165(8):4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 63.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ - and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 1999;189(7):1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aliberti J, Reis E Sousa C, Schito M, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8 α+ dendritic cells. Nat. Immunol. 2000;1(1):83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 65.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 2001;69(8):4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 2001;167(11):6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 67.Borges JS, Johnson WDJR. Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J. Exp. Med. 1975;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Remington JS, Krahenbuhl JL, Mendenhall JW. A role for activated macrophages in resistance to infection with Toxoplasma. Infect. Immun. 1972;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robben PM, Laregina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 2005;201(11):1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl Acad. Sci. USA. 1993;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994;153(6):2533–2543. [PubMed] [Google Scholar]
- 72.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen Toxoplasma gondii. J. Immunol. 2000;165(2):628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 73.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 74.Cooper Am, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28(1):33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Scharton-Kersten T, Caspar P, Sher A, Denkers EY. Toxoplasma gondii: evidence for interleukin-12-dependent and-independent pathways of interferon-γ production induced by an attenuated parasite strain. Exp. Parasitol. 1996;84(2):102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 76.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 2004;172(6):3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 77.Lieberman LA, Cardillo F, Owyang AM, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 2004;173(3):1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 78.Mayer KD, Mohrs K, Reiley W, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-γ production by CD8 T cells during infection. J. Immunol. 2008;180(2):693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 79.Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One. 2010;5(5):E10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kasper LH, Matsuura T, Khan IA. IL-7 stimulates protective immunity in mice against the intracellular pathogen Toxoplasma gondii. J. Immunol. 1995;155(10):4798–4804. [PubMed] [Google Scholar]
- 81.Haque S, Khan I, Haque A, Kasper L. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect. Immun. 1994;62(7):2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Candolfi E, Hunter CA, Remington JS. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect. Immun. 1994;62(5):1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villegas EN, Lieberman LA, Carding SR, Hunter CA. Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of γ interferon. Infect. Immun. 2002;70(9):4757–4761. doi: 10.1128/IAI.70.9.4757-4761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan IA, Casciotti L. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 1999;163(8):4503–4509. [PubMed] [Google Scholar]
- 85.Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc. Natl Acad. Sci. USA. 2006;103(17):6635–6640. doi: 10.1073/pnas.0506180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan IA, Moretto M, Wei XQ, Williams M, Schwartzman JD, Liew FY. Treatment with soluble interleukin-15Rα exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J. Exp. Med. 2002;195(11):1463–1470. doi: 10.1084/jem.20011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lieberman LA, Villegas EN, Hunter CA. Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect. Immun. 2004;72(11):6729–6732. doi: 10.1128/IAI.72.11.6729-6732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 2010;185(8):4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 91.Jankovic D, Kullberg MC, Feng CG, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Natl Acad. Sci. USA. 2010;107(7):3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-γ production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J. Immunol. 2008;180(9):5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 94.Deckert-Schluter M, Buck C, Weiner D, et al. Interleukin-10 downregulates the intracerebral immune response in chronic Toxoplasma encephalitis. J. Neuroimmunol. 1997;76(1–2):167–176. doi: 10.1016/s0165-5728(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 95.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol. Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bullock TN, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 2000;164(5):2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- 99.Jordan KA, Hunter CA. Regulation of CD8+ T cell responses to infection with parasitic protozoa. Exp. Parasitol. 2010;126(3):318–325. doi: 10.1016/j.exppara.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 101.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 102.Vaccari M, Trindade CJ, Venzon D, Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J. Immunol. 2005;175(6):3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- 103.Seaman MS, Peyerl FW, Jackson SS, et al. Subsets of memory cytotoxic T lymphocytes elicited by vaccination influence the efficiency of secondary expansion. in vivo. J. Virol. 2004;78(1):206–215. doi: 10.1128/JVI.78.1.206-215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schade B, Fischer HG. Toxoplasma gondii induction of interleukin-12 is associated with acute virulence in mice and depends on the host genotype. Vet. Parasitol. 2001;100(1–2):63–74. doi: 10.1016/s0304-4017(01)00484-8. [DOI] [PubMed] [Google Scholar]
- 105.Sharma SD, Catterall JR, Remington JS. Parasiticidal activity of macrophages against. Toxoplasma. Methods Enzymol. 1986;132:626–637. doi: 10.1016/s0076-6879(86)32046-9. [DOI] [PubMed] [Google Scholar]
- 106.Remington JS, Merigan TC. Interferon: protection of cells infected with an intracellular protozoan (Toxoplasma gondii) Science. 1968;161(843):804–806. doi: 10.1126/science.161.3843.804. [DOI] [PubMed] [Google Scholar]
- 107.Krahenbuhl JL, Remington JS. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect. Immun. 1971;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pfefferkorn ER, Pfefferkorn Lc. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 1976;39(3):365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 110.Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-γ. J. Immunol. 1985;134(3):1619–1622. [PubMed] [Google Scholar]
- 111.Anderson SE, Jr, Remington JS. Effect of normal and activated human macrophages on Toxoplasma gondii. J. Exp. Med. 1974;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J. Immunol. 1990;144(7):2725–2729. [PubMed] [Google Scholar]
- 113.Butcher BA, Greene RI, Henry SC, et al. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect. Immun. 2005;73(6):3278–3286. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scharton-Kersten TM, Wynn TA, Denkers EY, et al. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1996;157(9):4045–4054. [PubMed] [Google Scholar]
- 115.Gazzinelli R, XU Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 1992;149(1):175–180. [PubMed] [Google Scholar]
- 116.Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J. Immunol. 1989;143(6):2045–2050. [PubMed] [Google Scholar]
- 117.Badovinac VP, Harty JT. Detection and analysis of antigen-specific CD8+ T cells. Immunol Res. 2001;24(3):325–332. doi: 10.1385/IR:24:3:325. [DOI] [PubMed] [Google Scholar]
- 118.Rehr M, Cahenzli J, Haas A, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J. Virol. 2008;82(7):3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sutherland JS, Young JM, Peterson KL, et al. Polyfunctional CD4+ and CD8+ T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J. Immunol. 2010;184(11):6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- 120.Montoya JG, Lowe KE, Clayberger C, et al. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to Toxoplasma gondii-infected cells. Infect. Immun. 1996;64(1):176–181. doi: 10.1128/iai.64.1.176-181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Purner MB, Berens RL, Nash PB, et al. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 1996;64(10):4330–4338. doi: 10.1128/iai.64.10.4330-4338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denkers EY, Yap G, Scharton-Kersten T, et al. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 1997;159(4):1903–1908. [PubMed] [Google Scholar]
- 123.Wang X, Kang H, Kikuchi T, Suzuki Y. γ interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun. 2004;72(8):4432–4438. doi: 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki Y, Wang X, Jortner Bs, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 2010;176(4):1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schaeffer M, Han SJ, Chtanova T, et al. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J. Immunol. 2009;182(10):6379–6393. doi: 10.4049/jimmunol.0804307. [DOI] [PubMed] [Google Scholar]
- 126.Jost C, Reiter-Owona I, Liesenfeld O. The timing of sulfadiazine therapy impacts the reactivation of latent Toxoplasma infection in IRF-8−/− mice. Parasitol. Res. 2007;101(6):1603–1609. doi: 10.1007/s00436-007-0700-y. [DOI] [PubMed] [Google Scholar]
- 127.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J. Exp. Med. 1997;186(9):1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jordan KA, Wilson EH, Tait ED, et al. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to. Toxoplasma gondii. Infect. Immun. 2009;77(9):3894–3901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 130.Rutishauser RL, Martins GA, Kalachikov S, et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009;206(1):51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J. Immunol. 2004;173(2):883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 133.Yoshida K, Sakamoto A, Yamashita K, et al. Bcl6 controls granzyme B expression in effector CD8+ T cells. Eur. J. Immunol. 2006;36(12):3146–3156. doi: 10.1002/eji.200636165. [DOI] [PubMed] [Google Scholar]
- 134.Kirak O, Frickel EM, Grotenbreg GM, Suh H, Jaenisch R, Ploegh HL. Transnuclear mice with predefined T cell receptor specificities against Toxoplasma gondii obtained via SCNT. Science. 2010;328(5975):243–248. doi: 10.1126/science.1178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tait ED, Jordan KA, Dupont CD, et al. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J. Immunol. 2010;185(3):1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almeida JR, Sauce D, Price DA, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113(25):6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodrigue-Gervais IG, Rigsby H, Jouan L, et al. Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection. J. Immunol. 2010;184(6):3134–3144. doi: 10.4049/jimmunol.0902522. [DOI] [PubMed] [Google Scholar]
- 138.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J. Exp. Med. 2007;204(6):1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sui Y, Zhu Q, Gagnon S, et al. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc. Natl Acad. Sci. USA. 2010;107(21):9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]