Abstract
Aim of study
Sodium nitroprusside-enhanced CPR, or SNPeCPR, consists of active compression-decompression CPR with an impedance threshold device, abdominal compression, and intravenous sodium nitroprusside (SNP). We hypothesize that SNPeCPR will improve post resuscitation left ventricular function and neurological function compared to standard (S) CPR after 15 min of untreated ventricular fibrillation in a porcinemodel of cardiac arrest.
Methods
Pigs (n = 22) anesthetized with isoflurane underwent 15 min of untreated ventricular fibrillation, were then randomized to 6 min of S-CPR (n = 11) or SNPeCPR (n = 11) followed by defibrillation. The primary endpoints were neurologic function as measured by cerebral performance category (CPC) score and left ventricular ejection fraction.
Results
SNPeCPR increased 24-hour survival rates compared to S-CPR (10/11 versus 5/11, p = 0.03) and improved neurological function (CPC score 2.5± 1, versus 3.8 ± 0.4, respectively, p = 0.004). Left ventricular ejection fractions at 1, 4 and 24 hours after defibrillation were 72± 11, 57± 11.4 and 64 ± 11 with SNPeCPR versus 29 ± 10, 30 ± 17 and 39 ± 6 with S-CPR, respectively (p < 0.01 for all).
Conclusions
In this pig model, after 15 min of untreated ventricular fibrillation, SNPeCPR significantly improved 24-hour survival rates, neurologic function and prevented post-resuscitation left ventricular dysfunction compared to S-CPR.
Keywords: Cardiopulmonary resuscitation, Left ventricular function, Neurological function, Survival, Vasodilation
1. Introduction
Cardiopulmonary resuscitation (CPR) rates have remained disappointingly low over the past half-century with only minimal improvements in neurologically intact survival.1 Vasoconstrictors such as epinephrine are commonly used in standard (S) CPR according to current AHA guidelines to increase blood pressure, however they have not been shown to improve long-term outcomes, and may reduce microcirculatory blood flow and left ventricular function.2 Historically, studies have demonstrated that when ventricular fibrillation (VF) is untreated for more than 10 min, the rate of return of spontaneous circulation (ROSC) as well as short and long term survival are severely reduced.3 In animal models, current non-invasive methods of resuscitating untreated VF duration of longer than 12–13 min prohibit long-term neurological recovery.4,5
Sodium nitroprusside (SNP) enhanced CPR, or SNPeCPR, is a new, clinically relevant, method of resuscitation that consists of: (1) active compression-decompression CPR with an impedance threshold device (ACD CPR+ITD) which lowers intracranial pressure during chest decompression, enhances venous return, improves vital organ blood flow, and improves short and long term survival compared to standard CPR (S-CPR) in animal and clinical trials;6,7 (2) high dose intravenous sodium nitroprusside which vasodilates the coronary and cerebral vascular beds and decreases peripheral vascular resistance; and 3) manual lower abdominal compression which mechanically increases resistance to descending aortic blood flow, augments venous blood return to the heart, thereby effectively redistributing blood flow to the thorax and brain.8,9
Previous animal studies have demonstrated that SNPeCPR, applied in a common clinical scenario after eight min of untreated VF with up to 25 min of continuous CPR, can maintain heart and brain viability and significantly improve carotid blood flow, end-tidal CO2 (ETCO2), ROSC, and 24-h survival with good neurological function compared to S-CPR.10 Furthermore, SNPeCPR has been shown to improve ROSC rates after prolonged VF and pulseless electrical activity (PEA) arrest compared to treatment with S-CPR and American Heart Association resuscitation recommendations.11
The effects of SNPeCPR on 24-h neurological recovery and function as well as left ventricular function after prolonged untreated VF cardiac arrest have not yet been investigated. Based upon the multiple mechanisms of action of SNPeCPR, we hypothesize that this novel approach will prevent post-resuscitation left ventricular dysfunction and improve 24-h survival rates with favorable neurological function when compared with traditional S-CPR after 15 min of untreated VF cardiac arrest.
2. Methods
Our protocol was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified, experienced research team in Yorkshire female farm pigs weighing 36 ± 2 kg. A certified and licensed veterinarian provided a blinded neurological assessment in the 24-h survival experiments.
2.1. Preparatory phase
The anesthesia, surgical preparation, data monitoring, and recording procedures used in this study have been described previously.10 We employed aseptic surgical conditions, using initial sedation with intramuscular ketamine (7 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) followed by inhaled isoflurane at a dose of 0.8 to 1.2%. Pigs were intubated with a size 7.0 endotracheal tube. The animal’s temperature was maintained at 37.5 ± 0.5°C, with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN). Central aortic blood pressure was recorded continuously with a micromanometer-tipped (Mikro-Tip Transducer, Millar Instruments, Houston, TX) catheter placed at the beginning of the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. All animals received an intravenous heparin bolus (100 units/kg). An ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, NY) was placed to the right internal carotid artery to record blood flow (mL/min). The animals were then ventilated with room air, using a volume-control ventilator (Narcomed, Telford, PN), with a tidal volume of 10 mL/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mmHg and PaO2 of 80 mmHg (blood oxygen saturation >95%), as measured from arterial blood (Gem 3000, Instrumentation Laboratory, Lexington, MA) to adjust the ventilator as needed. Surface electrocardiographic tracings were continuously recorded. All data were recorded with a digital recording system (BIOPAC MP 150, BIOPAC Systems, Inc., CA). ETCO2, tidal volume, min ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (CO2SMO Plus, Novametrix Medical Systems, Wallingford, CN).
2.2. Measurements and recording
Thoracic aortic pressure, right atrial pressure, ETCO2, and carotid blood flow were continuously recorded. Coronary perfusion pressure (CPP) during CPR was calculated from the mean arithmetic difference between right-atrial pressure and aortic pressure during the decompression phase. Carotid artery blood flow was reported in mL/sec.
2.3. Experimental protocol
After the surgical preparation was complete, oxygen saturation on room air was greater than 95%, and ETCO2 was stable between 35 and 42 mmHg for five min, VF was induced by delivering direct intracardiac current via a temporary pacing wire (Daig Division, St Jude Medical, Minnetonka, MN). The ventilator was disconnected from the endotracheal tube. Standard and active compression–decompression CPR were performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described.10 Uninterrupted chest compressions at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anterior–posterior chest diameter were provided. During CPR, asynchronous positive-pressure ventilations were delivered with a manual resuscitator bag. The fraction of inspired oxygen was 1.0, the tidal volume was maintained at ~10 mL/kg and the respiratory rate was 10 breaths/min.
During SNPeCPR, manual abdominal binding was performed to provide approximately 40 lbs of force to the lower half of the abdomen.10
The investigators were blinded to hemodynamics during CPR. Blinding of ACD CPR and abdominal binding was not possible.
2.4. Protocol
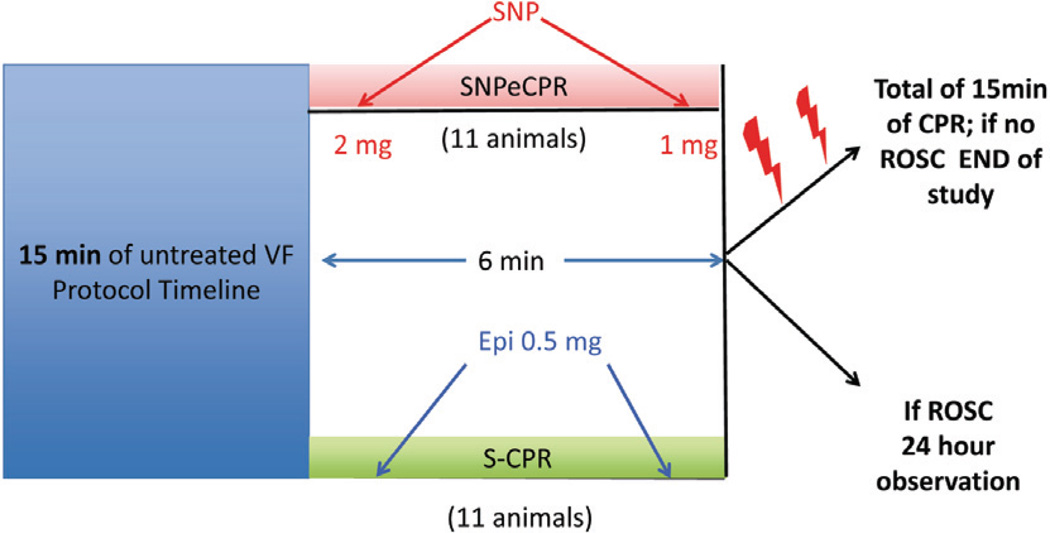
Following 15 min of untreated VF, 22 pigs were prospectively randomized to either 6 min of S-CPR (n = 11) or SNPeCPR (n = 11). Epinephrine was administered to the S-CPR control group in 0.5 mg (~15 mcg/kg) boluses at min 1 and 4 whereas SNP was delivered into the jugular vein in the SNPeCPR group as a 2 mg bolus at min 1 and a second 1 mg bolus at min 4 of CPR. (Fig. 1) Defibrillation was delivered with 150 J biphasic shocks at min 6 of CPR. If SNPeCPR-treated animals did not achieve ROSC with the first 2 shocks, 0.5 mg (~15 mcg/kg) intravenous epinephrine was given and shocks were delivered again after 1 min.
Figure 1.
Schematic of experimental protocol. SNPeCPR: sodium nitroprusside-enhanced CPR; S-CPR: standard CPR; VF: ventricular fibrillation; ROSC: return of spontaneous circulation; SNP: Sodium Nitroprusside; Epi: Epinephrine.
Animals that achieved ROSC were then observed under general anesthesia with isoflurane until hemodynamically stable. Hemodynamic stability was defined as a mean aortic pressure (MAP) of > 55 mmHg without pharmacological support for 10 min and normalization of ETCO2 and acidosis. Animals that had a stable post-ROSC rhythm but were hypotensive (MAP <50 mmHg) received 500 mL of IV normal saline bolus. If MAP was still <50 mmHg they received increments of 0.1–0.2 mg of epinephrine every 5 min until MAP rose above 50 mmHg. If pH was lower than 7.1, one amp (50 mEq) of NaHCO3 was given intravenously. One liter of normal saline at room temperature (18°C) was given over the next 4 h. At that point vascular repair of the internal jugular and the left common femoral artery were then performed. Arterial blood gases were obtained at baseline, at 5 min CPR, and every 30 min following ROSC.
Survivors were given intramuscular analgesic injections of non-steroidal anti-inflammatory medication as previously described and had free access to water and food.10 There was no other post-ROSC medical care provided after the vascular repair.
2.5. Neurological assessment
Twenty-four hours after ROSC, a certified veterinarian, blinded to the intervention, assessed the pigs’ neurological function based upon a cerebral performance category (CPC) scoring system modified for pigs. The veterinarian used clinical signs such as response to opening the cage door, response to noxious stimuli if unresponsive, response to trying to lift the pig, whether the animal could stand, move all four limbs, walk, eat, urinate, defecate, and respond appropriately to the presence of a person walking into the cage. The following scoring system was used: 1 = normal; 2 = slightly disabled; 3 = severely disabled but conscious; 4 = vegetative state; a 5 was given for dead animals in the cage or when they were absent from the cage due to unachievable ROSC.10 A dichotomous assessment of good versus poor outcomes were also evaluated where good outcome was defined as CPC ≤2 and poor outcome was defined as a CPC >2. Post resuscitation care was not blinded since the same team performed CPR and provided post-ROSC care.
2.6. Echocardiographic evaluation of left ventricular function
A transthoracic echocardiogram was obtained on all survivors 1, 4 and 24 h post ROSC. Images were obtained from the right parasternal window that provides similar views as the long and short parasternal windows in humans.12 Ejection fraction (EF) was assessed using Simpson’s method of volumetric analysis.13 An echocardiographer provided independent visual assessment of the ejection fraction. If there was a discrepancy of more than 5% compared with the measured method, a second echocardiographer provided a visual assessment. The EF provided by the second cardiologist was averaged with the closest value of either the first visual assessment or the volumetric analysis to provide the EF for the specific time point. Before echocardiographic evaluation, any inotropic support was stopped for at least 20 min and, if needed, was restarted immediately after the echocardiographic evaluation.
2.7. Statistical analysis
Values were expressed as mean ± standard deviation. Baseline data were compared using a t-test. Hemodynamics and blood gases during CPR were analyzed with two-way ANOVA. A 2-tailed Fischer exact test was used to compare 24-h survival rate. A t-test was used to evaluate mean CPC scores between groups. A p-value of <0.05 was considered statistically significant.
3. Results
There were no baseline differences between treatment groups in any hemodynamic or respiratory parameters.
3.1. CPR hemodynamics
SNPeCPR provided significantly higher internal carotid blood flow during CPR (128 ± 25 vs 35 ± 3 mmHg at 2 min of CPR and 133 ± 26 vs 29.6 ± 4.4 mmHg at 5 min of CPR [p < 0.05 for all]) compared to S-CPR in all animals. There were no significant differences in systolic, diastolic pressures, or CPP between groups at min 5 (Table 1).
Table 1.
Hemodynamics, resuscitation rates, and 24-hour survival.
| Baseline | 2 min CPR | 5 min CPR | Number of shocks to initial ROSC |
1-hour ROSC |
24-hour survival |
|
|---|---|---|---|---|---|---|
| SNPeCPR | ||||||
| SBP | 87.7± 5.6 | 77.8± 3.3* | 86.6± 4.9 | |||
| DBP | 65.2± 3.8 | 28.5± 2.2 | 29.6± 4.3 | |||
| RA | 3.7± 2.1 | 6± 1.4* | 6.4± 2.7 | 3± 2 | 10/11 | 10/11* |
| CPP | 64.9± 4.4 | 22± 2.1 | 23.2± 4.1 | (91%) | ||
| CBF | 166± 41 | 128± 25* | 133± 26* | |||
| S-CPR | ||||||
| SBP | 84.1± 4.4 | 49.7± 5.7 | 73.6± 6.3 | |||
| DBP | 60.4± 3.9 | 18.5± 3.9 | 30.7± 4.1 | |||
| RA | 3.2± 1.1 | 3± 1.3 | 4± 1.0 | 2± 5 | 9/11 | 5/11 |
| CPP | 57.1± 4.2 | 13.7± 4.5 | 26± 3.9 | (45%) | ||
| CBF | 170± 13 | 35± 3 | 29.6± 4.4 | |||
Values are shown as mean± SD. CPR was performed for 6 min with either SNPeCPR or S-CPR. All pressures in mmHg, all flows in mL/min. SBP: systolic blood pressure; DBP: diastolic blood pressure; RA: right atrial pressure; CPP: coronary perfusion pressure; CBF: carotid blood flow.
means statistically significant difference with p < 0.05 compared to S-CPR.
3.2. Return of spontaneous circulation and 24-h survival
In the S-CPR group, 9/11 animals achieved ROSC, and 5/11 animals survived to 24 h. In the SNPeCPR group, 10/11 animals had ROSC and survived to 24 h (p = 0.6 for ROSC and p = 0.03 for 24-h survival) (Table 1). The S-CPR group received epinephrine per protocol and 2/11 pigs treated with SNPeCPR required one dose of epinephrine for successful defibrillation.
3.3. Neurological function at 24 hours
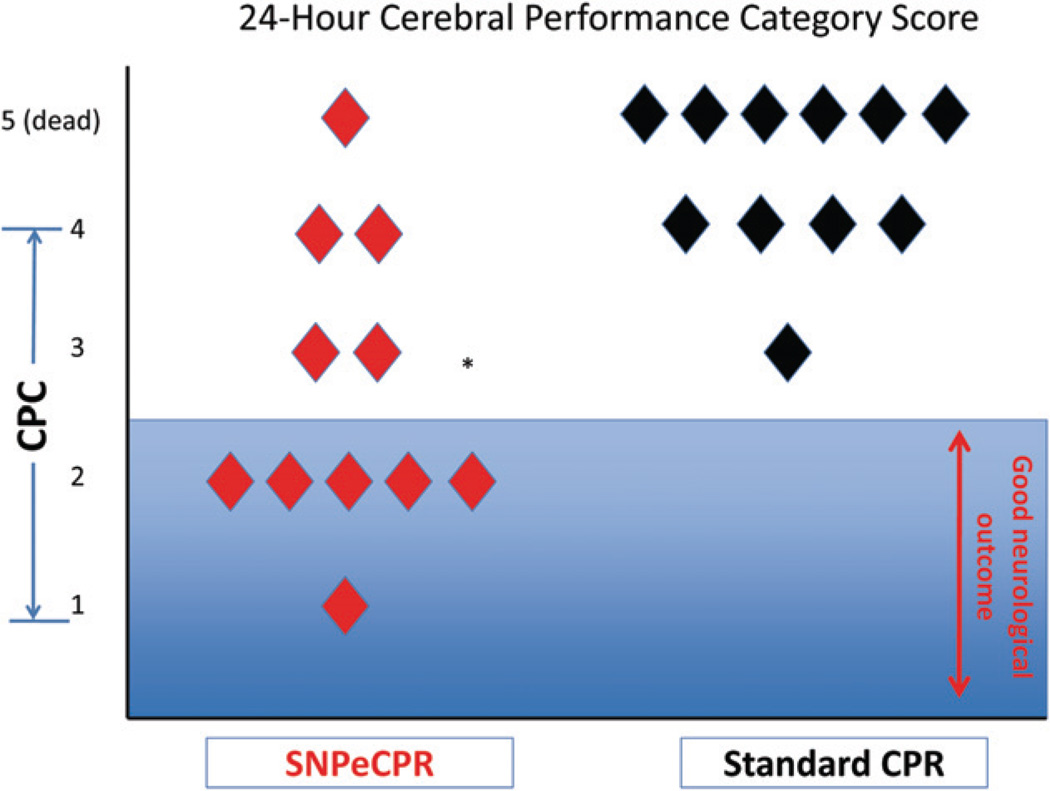
Neurologic outcomes 24-h after ROSC, as determined by the Cerebral Performance Category Score, were significantly improved as reflected in the lower scores in the SNPeCPR group compared to the S-CPR group (CPC: 2.5 ± 1 vs. 3.8 ± 0.4, p = 0.004). A dichotomous evaluation of good versus poor outcomes demonstrated that SNPeCPR had 6/11 animals with good outcomes versus 0/11 S-CPR (p < 0.001) (Fig. 2).
Figure 2.
24-hours neurological assessment. SNPeCPR significantly improved neurological function compared to S-CPR. Good neurological function (CPC of 1 & 2) was only present in the SNPeCPR group. CPC: cerebral performance category score (1 = normal, 4 = coma); SNPeCPR: sodium nitroprusside-enhanced CPR; S-CPR: standard CPR.
* Means statistical significance with a p < 0.05.
3.4. Left ventricular function
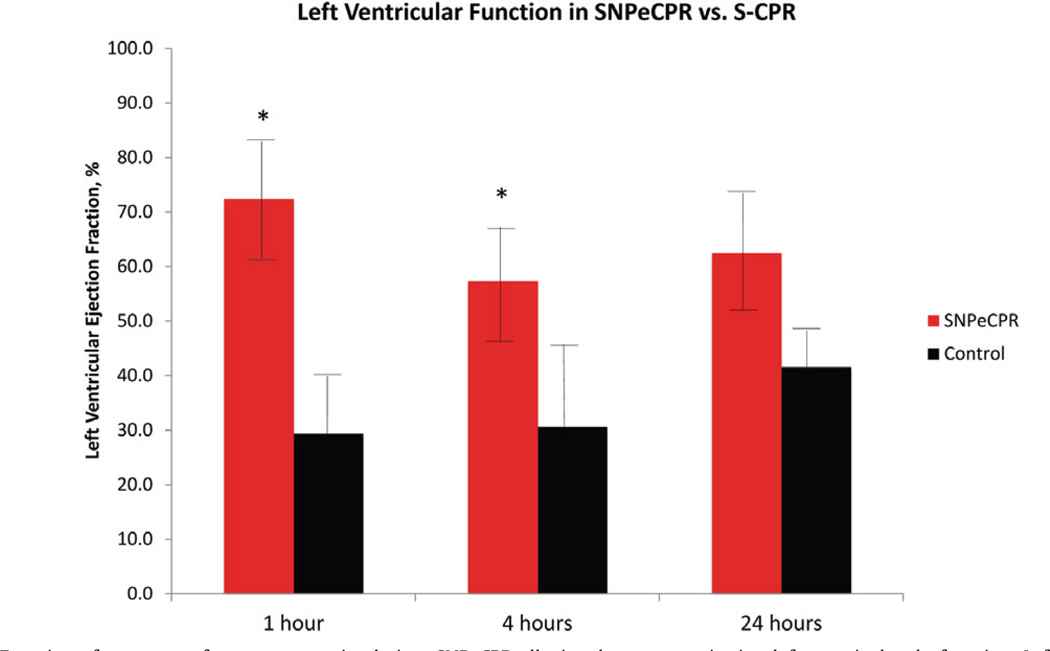
Echocardiographic evaluation at 1 h revealed that SNPeCPR animals had a significantly higher left ventricular EF than those animals treated with S-CPR (72 ± 11% vs. 32 ± 18%, p < 0.001). The effect was maintained at 4 h (53 ± 18% vs. 35 ± 11%, p = 0.001) and persisted up to 24 h (62.5 ± 10.4 vs 41.5 ± 5.4, p = NS) (Fig. 3, Table 2)
Figure 3.
Left Ventricular Function after return of spontaneous circulation. SNPeCPR alleviated post resuscitation left ventricular dysfunction. Left ventricular ejection fraction (LVEF) was normal over the 24 h of observation in the SNPeCPR and severely depressed in the S-CPR. LVEF was calculated using Simpson’s method of volumetric analysis.
* means statistically significant difference with p < 0.05 compared to S-CPR.
Table 2.
Echocardiographic measurements
| IVSd | IVSs | LVd | LVs | FS | LVEF | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| SNPeCPR | 1.0± 0.2 | 1.4± 0.2 | 3.9± 0.4 | 2.6± 0.3 | 33.4± 3.1 | 62± 3 |
| S-CPR | 1.0± 0.2 | 1.3± 0.2 | 4.0± 0.3 | 2.7± 0.3 | 35± 3.3 | 65± 4 |
| 1 hour | ||||||
| SNPeCPR | 1.4± 0.1 | 1.9± 0.4 | 3.1± 0.2 | 1.8± 0.5* | 43.7± 14* | 72± 11* |
| S-CPR | 1.2± 0.3 | 1.5± 0.6 | 3.6± 0.6 | 3.1± 0.8 | 15.7± 8 | 29± 10 |
| 4 hours | ||||||
| SNPeCPR | 1.2± 0.3 | 1.4± 0.1 | 3.9± 0.6 | 2.8± 0.8 | 32.8± 8* | 56± 10* |
| S-CPR | 1.1± 0.1 | 1.4± 0.1 | 4.0± 0.2 | 3.3± 0.3 | 17.7± 3 | 30± 15 |
| 24 hours | ||||||
| SNPeCPR | 0.9± 0.2 | 1.3± 0.1 | 3.8± 1.1 | 2.5± 0.7 | 35± 6* | 62± 10* |
| S-CPR | 1.1± 0.2 | 1.5± 0.4 | 2.9± 0.7 | 2.1± 0.6 | 25.7± 3 | 41± 5 |
Mean± SD. IVSd: interventricular septum at end-diastole (cm); IVSs: interventricular septum at end-systole (cm); LVd: left ventricular diameter at end-diastole (cm); LVs: left ventricular diameter at end-systole (cm); FS: fractional shortening (%); LVEF: left ventricular ejection fraction (%).
means statistically significant difference with p < 0.05 compared to S-CPR.
3.5. Blood gases
There were no significant differences in blood gas values at baseline between groups. During CPR there were no significant differences in blood gases except for a significantly lower arterial pH in the SNPeCPR group. ETCO2 was significantly higher in the SNPeCPR group during CPR (35 ± 2 vs. 21 ± 2) at 5 min of CPR (p < 0.05); Thirty min post ROSC there was no difference in acid–base balance between groups (Table 3).
Table 3.
Arterial blood gasses during CPR and after ROSC.
| Arterial Blood Gases | Baseline | 5 min CPR | 30 min ROSC |
|---|---|---|---|
| SNPeCPR | |||
| pH | 7.43± 0.01 | 7.19± 0.02* | 7.27± 0.04 |
| pCO2 | 43± 1 | 48± 3 | 40± 2 |
| pO2 | 319± 64 | 181± 15 | 267± 70 |
| HCO3 | 28.4± 0.5 | 17.5± 0.8* | 17.1± 0.8 |
| SaO2 (%) | 99± 1 | 100 | 100± 0 |
| ETCO2 | 41± 1 | 35± 2* | 39± 3 |
| S-CPR | |||
| pH | 7.42± 0.02 | 7.26± 0.02 | 7.24± 0.02 |
| pCO2 | 45± 1 | 38± 1 | 39± 2 |
| pO2 | 329± 64 | 185± 37 | 252± 58 |
| HCO3 | 29.3± 0.6 | 15.9± 0.9 | 18.1± 0.7 |
| SaO2 (%) | 99± 1 | 100 | 100± 0 |
| ETCO2 | 41± 1 | 21± 2 | 38± 1 |
Mean± SD. Arterial blood gas measurements at baseline, during CPR, and after ROSC. Partial pressures in torr. SaO2: percent oxygen saturation; HCO3: bicarbonate; ETCO2: end-tidal CO2.
means statistically significant difference with p < 0.05 compared to S-CPR.
4. Discussion
Results from this investigation demonstrate that SNPeCPR improves 24-h survival rates with favorable neurological function and prevents post resuscitation cardiac dysfunction in a model of prolonged untreated cardiac arrest. These favorable outcomes were associated with neurological recovery after 15 min of untreated arrest without hypothermia and with a clinically feasible non-invasive method of CPR. With this novel approach to CPR, neurological function was restored after a period of prolonged ischemia beyond what was previously considered possible. These findings support the hypothesis that long-term survival with favorable neurological function is possible after cardiac arrest if pharmacological and mechanical interventions are combined to optimize perfusion to the heart and brain without inducing further ischemic insult.
The lack of left ventricular dysfunction in the SNPeCPR-treated animals was striking in this study and suggests that ischemia-induced myocardial contracture and dysfunction can be prevented. These findings contrast sharply with the post-resuscitation left ventricular function that is typically observed beginning immediately after successful ROSC following S-CPR and lasting several days or more in animals and patients.14,15 The mechanism of this left ventricular dysfunction is poorly understood. Several proposed mechanisms have been studied and range from ischemiareperfusion injury secondary to low flow during CPR,16,17 low flow after ROSC,18,19 global myocardial stunning,20 or microvascular obstruction.21 During resuscitation, SNPeCPR provides significantly higher forward flow as documented by the 5-fold increase in carotid blood flow and a >65% increase in ETCO2. We speculate that the increase in flow with SNPeCPR ameliorates reperfusion injury and results in better post-ROSC left ventricular function.
This flow hypothesis is substantiated by multiple observations. First, Halperin et al. have shown that low flow reperfusion has very poor outcomes.22 Second, active compression–decompression CPR with generation of negative intrathoracic pressure, a hemodynamically superior CPR method, has been shown to provide 3–4 times more blood flow to the heart and brain and to provide superior hemodynamics compared to S-CPR in multiple animal and human trials.23–25 Further, the fundamental mechanism of action of SNP is to release nitric oxide (NO) and thereby cause vasodilation and increased perfusion. It is reasonable, therefore, to hypothesize that benefits of SNPeCPR could be explained by the favorable hemodynamic profile which promotes forward blood flow.
Studies have demonstrated that NO donation improved coronary flow following cardioplegic arrest and independently reduced left ventricular work in a dose-dependent fashion.26 We hypothesize that prolonged cardiac arrest promotes a low flow microcirculatory state at the initiation of CPR, similar to that observed in acute myocardial infarction, after which SNP has been shown to reduce infarct size.27,28 Although we cannot be certain about the mechanism by which SNP is protective of myocardial function following cardiopulmonary arrest, it is clear from this investigation that despite prolonged cardiac arrest, SNPeCPR has the ability to preserve left ventricular function when compared to S-CPR and effectively prevent post-resuscitation left ventricular dysfunction in the majority of animals.
In our study there was no significant difference in ROSC between the two groups. Our reported control animal (S-CPR) ROSC rate after 15 min of untreated VF is probably the highest reported in the literature today and therefore strengthens the validity of our results. In contrast to our previously published outcomes, in this study we used epinephrine within the first min and all pigs in the S-CPR group received two doses before the first shock. This approach was meant to maximize resuscitation rates in the S-CPR group because when epinephrine was given only at min 5 of CPR, ROSC rates in our laboratory were poor and we would be unable to compare neurological function between groups.11 In contrast to ROSC rates, there were significant differences in 24-h survival rates. We speculate that SNPeCPR provides neuroprotection, but the protective mechanisms other than the increase in circulation remain unknown.
The recovery of neurologic function remains a significant barrier to patients who present with sudden cardiac death. The only therapies over many years which have been identified to improve neurologic recovery have been early defibrillation,29–31 therapeutic hypothermia32,33 and more recently ACD CPR + ITD.6 Studies have demonstrated the effect of NO donation in smaller animals have a positive impact on neurologic outcomes.34 One study by Shaffner et al. performed in a similar model, concluded that cerebral blood flow could not be restored in order to regenerate ATP after 12 min of untreated arrest due to severe cerebral edema and increased vascular resistance.35 A study by Hogler s et al. demonstrated that, in the few animals resuscitated after 13 min of untreated VF, there were significant ischemic brain lesions documented by MRI and pathohistological assessment.7 The current study demonstrates that full neurological recovery is possible despite prolonged cardiac arrest with a non-invasive CPR method.
This study has several limitations. First, the biochemical mechanisms through which SNPeCPR exerts its effects were not studied. Second, despite similarities between the porcine and human hemodynamics and response to CPR, it is difficult to extrapolate these findings to a human population. Third, since SNPeCPR implements several different interventions at once, it is difficult to determine which intervention is incrementally responsible for the observed outcomes. However, another study performed by our group demonstrated incremental benefit to each mechanical component of SNPeCPR by examining them independently.36 The role of SNP in addition to the mechanical CPR platform of SNPeCPR, has also been established and has been shown to improve neurological intact survival.10 Finally, in this model the coronary arterial bed was patent, and we are not able to extrapolate these results to an ischemic model of cardiac arrest.
5. Conclusion
After 15 min of untreated VF, SNPeCPR significantly improved 24-h survival rates, neurological function and prevented post-resuscitation left ventricular dysfunction when compared to S-CPR.
Acknowledgments
The study was funded by an Institutional, Division of Cardiology grant at the University of Minnesota and R01 HL108926-01 NIH grant to Dr. Yannopoulos.
Footnotes
Conflict of interest
Demetris Yannopoulos, MD, is the Medical Director of the Minnesota Resuscitation Consortium, a state wide initiative to improve survival in the state of MN from cardiac arrest. This initiative is sponsored by the Medtronic Foundation and is part of the HeartRescue Program. There are no conflicts related to this investigation.
Tom P. Aufderheide, MD, has board membership for Take Heart America and Citizen CPR Foundation, has consulted for JoLife Medical and Medtronic Foundation, and has received grants/ grants pending from the NHLBI Immediate Trial, NHLBI Resuscitation Outcomes Consortium, NINDS Neurological Emergency Treatment Trials Network, and NHLBI Medical College of Wisconsin K12 Research Career Development.
Keith Lurie, MD, is the inventor of ACD+ITD CPR used in this study and has formed a company that promotes its sales (ACSI).
The remaining authors have no conflict of interest to report.
References
- 1.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 2.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik LS. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 3.Cummin RO. From concept to standard-of-care? Review of the clinical experience with automated external defibrillators. Ann Emerg Med. 1989;18:1269–1275. doi: 10.1016/s0196-0644(89)80257-4. [DOI] [PubMed] [Google Scholar]
- 4.Janata A, Weihs W, Vet M, et al. Cold aortic flush and chest compressions enable good neurologic outcome after 15 min of ventricular fibrillation in cardiac arrest in pigs. Crit Care Med. 2010;38:1637–1643. doi: 10.1097/CCM.0b013e3181e78b9a. [DOI] [PubMed] [Google Scholar]
- 5.Högler S, Sterz F, Sipos W, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81:1577–1583. doi: 10.1016/j.resuscitation.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression–decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-ofhospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolcke BB, Mauer DK, Schoefmann MF, et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression–decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108:2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 8.Niemann JT, Rosborough JP, Ung S, Criley JM. Hemodynamic effects of continuous abdominal binding during cardiac arrest and resuscitation. Am J Cardiol. 1984;53:269–274. doi: 10.1016/0002-9149(84)90438-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhou M, Ran Q, Liu Y, Li Y, Liu T, Shen H. Effects of sustained abdominal aorta compression on coronary perfusion pressures and restoration of spontaneous circulation during cardiopulmonary resuscitation in swine. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Yannopoulos D, Matsuura T, Schultz J, Rudser K, Halperin HR, Lurie KG. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz J, Segal N, Caldwell E, et al. Sodium nitroprusside enhanced CPR improves resuscitation rates after prolonged untreated cardiac arrest in two porcine models. Crit Care Med. 2011 doi: 10.1097/CCM.0b013e31822668ba. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marino BS, Yannopoulos D, Sigurdsson G, et al. Spontaneous breathing through an inspiratory impedance threshold device augments cardiac index and stroke volume index in a pediatric porcine model of hemorrhagic hypovolemia. Crit Care Med. 2004;32:S398–S405. doi: 10.1097/01.ccm.0000139950.39972.68. [DOI] [PubMed] [Google Scholar]
- 13.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez MM, Berg RA, Nadkarni VM, et al. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117:1864–1872. doi: 10.1161/CIRCULATIONAHA.107.740167. [DOI] [PubMed] [Google Scholar]
- 15.Kern K, Zuercher M, Cragun D, et al. Myocardial microcirculatory dysfunction after prolonged ventricular fibrillation and resuscitation. Crit Care Med. 2008;36:S418–S421. doi: 10.1097/ccm.0b013e31818a82e8. [DOI] [PubMed] [Google Scholar]
- 16.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–3093. doi: 10.1161/01.cir.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 17.Kern K, Helwig R, Rhee K, Berg R. Myocardial dysfunction after resuscitation after cardiac arrest: an example of global myocardial stunning. j Am Coll Card. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Idris H, Roberts L, Caruso L, et al. Oxidant injury occurs early after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med. 2005;33:2043–2048. doi: 10.1097/01.ccm.0000174104.50799.bd. [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Weil M, Sun S, et al. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation. 1997;96:683–688. doi: 10.1161/01.cir.96.2.683. [DOI] [PubMed] [Google Scholar]
- 20.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 21.Ferenc M, Neumann F. Efficacy of primary PCI: the microvessel perspective. Eur Heart J. 2005;7(Suppl):14–19. [Google Scholar]
- 22.Halperin HR, Lee K, Zviman M, et al. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med. 2010;28:195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression–decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 24.Aufderheide TP, Frascone RJ, Wayne MA, et al. Comparative effectiveness of an impedance threshold device and active compression decompression CPR versus standard CPR for treatment of out-of-hospital cardiac arrest. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voelckel WG, Lurie KG, Sweeney M, et al. Effects of active compression–decompression cardiopulmonary resuscitation with the inspiratory threshold valve in a young porcine model of cardiac arrest. Pediat Res. 2002;51:523–527. doi: 10.1203/00006450-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Ali IS, Gandhi M, Finegan BA, Koshal A, Clanachan AS. Cardioprotection by activation of NO/cGMP pathway after cardioplegic arrest and 8-hour storage. Ann Thorac Surg. 1998;65:1303–1309. doi: 10.1016/s0003-4975(98)00182-9. [DOI] [PubMed] [Google Scholar]
- 27.Tesic MB, Stankovic G, Vukcevic V, Ostojic MC. The use of intracoronary sodium nitroprusside to treat no-reflow after primary percutaneous coronary intervention in acute myocardial infarction. Herz. 2010;35:114–118. doi: 10.1007/s00059-010-3243-4. [DOI] [PubMed] [Google Scholar]
- 28.Paulus WJ, Vantrimpont PJ, Shah AM. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994;89:2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- 29.Nichol G, Stiell IG. The impact of first-responder defibrillation. JAMA. 1994;271:504–505. doi: 10.1001/jama.271.7.504b. [DOI] [PubMed] [Google Scholar]
- 30.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-ofhospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343:1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 32.Group THaCAS. Mild therapeutic hypothermia to improve neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 33.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 34.Salom J, Orti M, Centeno J, Torregrosa G, Alborch E. Reduction of infract size by the NO donors sodium nitroprusside and permine/NO after transient focal cerebral ischemia in rats. Brain Res. 2000;865:149–156. doi: 10.1016/s0006-8993(00)02095-3. [DOI] [PubMed] [Google Scholar]
- 35.Shaffner DH, Eleff SM, Brambrink AM, et al. Effect of arrest time and cerebral perfusion pressure during cardiopulmonary resuscitation on cerebral blood flow, metabolism, adenosine triphosphate recovery, and pH in dogs. Crit Care Med. 1999;27:1335–1342. doi: 10.1097/00003246-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Schultz J, Kolbeck J, Segal N, McKnite S, Caldwell E, Yannopoulos D. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.07.038. accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]