Abstract
Changes in vascular endothelial growth factor (VEGF) in pulmonary vessels have been described in congenital diaphragmatic hernia (CDH) and may contribute to the development of pulmonary hypoplasia and hypertension; however, how the expression of VEGF receptors changes during fetal lung development in CDH is not understood. The aim of this study was to compare morphological evolution with expression of VEGF receptors, VEGFR1 (Flt-1) and VEGFR2 (Flk-1), in pseudoglandular, canalicular, and saccular stages of lung development in normal rat fetuses and in fetuses with CDH. Pregnant rats were divided into four groups (n=20 fetuses each) of four different gestational days (GD) 18.5, 19.5, 20.5, 21.5: external control (EC), exposed to olive oil (OO), exposed to 100 mg nitrofen, by gavage, without CDH (N-), and exposed to nitrofen with CDH (CDH) on GD 9.5 (term=22 days). The morphological variables studied were: body weight (BW), total lung weight (TLW), left lung weight, TLW/BW ratio, total lung volume, and left lung volume. The histometric variables studied were: left lung parenchymal area density and left lung parenchymal volume. VEGFR1 and VEGFR2 expression were determined by Western blotting. The data were analyzed using analysis of variance with the Tukey-Kramer post hoc test. CDH frequency was 37% (80/216). All the morphological and histometric variables were reduced in the N- and CDH groups compared with the controls, and reductions were more pronounced in the CDH group (P<0.05) and more evident on GD 20.5 and GD 21.5. Similar results were observed for VEGFR1 and VEGFR2 expression. We conclude that N- and CDH fetuses showed primary pulmonary hypoplasia, with a decrease in VEGFR1 and VEGFR2 expression.
Keywords: Congenital diaphragmatic hernia, VEGFR, Rat model, Nitrofen, Lung development
Introduction
Congenital diaphragmatic hernia (CDH) occurs in approximately 1 in 2500 live births and still causes high neonatal mortality as a consequence of pulmonary hypoplasia and persistent pulmonary hypertension (1). Vascular endothelial growth factor (VEGF) and its receptors, VEGFR1 (Flt-1) and VEGFR2 (Flk-1), participate in angiogenesis by stimulating proliferation of new vessels and by offering a background for airway branching (2,3).
VEGFR2 signaling produces various cellular responses, including mitogenic and survival signals for endothelial cells and their precursors (4,5), therefore silencing the VEGFR2 gene reduces or suppresses of vasculogenesis in rats (6,7). VEGFR1 participates in the development of blood vessels by negatively modulating endothelial cell division through a restriction of VEGFR2 signaling (8,9). Knockout mice for the VEGFR1 gene did not survive to the end of pregnancy due to hypertrophy and disorganization of the vascular bed (10).
The changes in pulmonary vessels and serum levels of VEGF described in CDH likely contribute to the development of pulmonary hypoplasia and hypertension (11). VEGF participates in pulmonary development and surfactant production and induces conversion of glycogen into surfactant in type II pneumocytes (12); however, there are a limited number of studies on VEGF receptors. Changes in the levels of VEGF and its receptors cause a disturbance in angiogenesis, leading to a detrimental remodeling of small arteries. Studies of the expression of VEGF in CDH have produced conflicting results.
One study showed that VEGF increased in the nitrofen-induced CDH rat model with pulmonary hypertension; however, several other studies showed that VEGF decreased in the same model (13,14). Considering that not only VEGF but also the VEGF receptors may be involved in the development of pulmonary hypertension, our aim was to examine the expression of these receptors in the pseudoglandular, canalicular, and saccular stages of lung development of normal rat fetuses and of fetuses with CDH.
Material and Methods
Experimental protocols were reviewed and approved by the Animal Experimentation Committee of the Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (FMRP-USP; #043/2011).
Sprague-Dawley rats (±250 g) were mated during the night cycle and females were checked on the following day for a sperm-positive vaginal smear. The day of plugging was defined as gestational day 0 (term=22 days). A total of 52 pregnant rats were studied in four groups: 10 external control (EC), 10 exposed to olive oil (OO), and 32 rats exposed to nitrofen that did not develop CDH (N-) or that did develop CDH (CDH).
Nitrofen administration
To induce CDH, timed-pregnant rats were given 100 mg nitrofen (2,4-dichloro-4-nitrodiphenyl ether, Maybridge¯; UK) dissolved in 1 mL olive oil on gestational day (GD) 9.5, by gavage. The placebo group received 1 mL olive oil without nitrofen at the same gestational age (OO group). This dose of nitrofen has been shown to cause left-sided CDH in 24% of the litter (15).
Model and experimental groups
All four groups of fetuses (EC, OO, N-, and CDH) were harvested at 18.5, 19.5, 20.5, or 21.5 GD (20 fetuses per group for each gestational day, giving a total of 80 fetuses in each group). The pregnant female rats were anesthetized on the specific day of gestation using 50 mg/mL ketamine (175 mg/kg; Ketamina¯, Pfizer do Brasil Ltda., Brazil) in conjunction with 10 mg/mL xylazine (2.5 mg/kg; Rompum¯, Bayer do Brasil Ltda., Brazil), and the fetuses were collected through a median maternal laparotomy and hysterotomy.
Morphological measurements
The fetuses were weighed [body weight (BW)] and dissected. Their left lung weight (LLW) and total lung weight (TLW) were measured, and the lung-to-body weight ratio (TLW/BW) was determined as were the total lung volume [V(lu)], left lung volume [V(llu)], left lung parenchymal area density [AA(pa/llu)], and left lung parenchymal volume [V(pal)].
Histological analysis
The left lungs were fixed, washed in phosphate buffer, and processed following a protocol for paraffin embedding. The histological samples were sectioned (5 µm thick), and sections were deparaffinized and stained with hematoxylin-eosin, and examined by light microscopy.
Obtaining images for analysis
Images from the histological sections of the left lung were obtained at 200× magnification. We analyzed 50 images per animal, from five animals per group in each endpoint, totaling 200 images for each group. For stereological analysis, we used a grid with 100 points generated by the Image-Pro Plus software, version 4.1.0.0 (Media Cybernetics, USA).
Stereological calculations
Lung volume [V(lu)]
The total V(lu) was calculated using Archimedes' principle. The volume of the lung was determined from the volume of water displaced when it was suspended by a wire in a container of water (and not touching the walls or bottom of the container), as described by Scherle (16) and Howard and Reed (17).
Volume of parenchyma [V(pa)]
The density of the parenchymal area [AA(pa/lu)] was estimated by dividing the number of points that fall in the pa, excluding large bronchi and large blood vessels, by the number of points that fall within the lung as a whole (lu), excluding the airspace, at 200× magnification [AA(pa/lu)=pa/lu]. The volume of the left lung parenchyma [V(pal)] was obtained by multiplying the left lung volume [V(llu)] by the AA(pa/llu), according to Papadakis et al. (18) [V(pal)=V(llu) ×AA(pa/llu)], utilizing the Image Pro Plus 6.0 software (Media Cybernetics).
Western blotting
Whole lungs from 6 animals per group were homogenized in 1 mL extraction buffer containing 100 mM Tris, pH 7.4, 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride, 0.1 mg/mL aprotinin, and 1% Triton-X 100 at 4°C with a tissue homogenizer (Tecnal, Brazil) operated at maximum speed for 30 s. The extracts were centrifuged at 9000 g at 4°C in a Mikro 200R centrifuge (Hettich, Germany) for 30 min to remove insoluble material, and the supernatants of these tissues were used for protein quantification using the Bradford method. Then, 90 µg protein was denatured, run on SDS-PAGE, and transferred to nitrocellulose membranes in two stages for 1 h each at 120 V. The membranes were blocked for 1 h in 5% milk in 0.01 M PBS, pH 7.4, and incubated with anti-VEGFR1 (sc-316, Santa Cruz Biotechnology, USA) or anti-VEGFR2 (sc-6251, Santa Cruz Biotechnology) diluted 1:200 in 5% milk in 0.01 M PBS, pH 7.4, for 2 h at room temperature. They were then incubated with biotin-conjugated anti-rabbit IgG (1:10,000 in 1% BSA) for 2 h at room temperature for VEGFR1 and with biotin-conjugated anti-mouse IgG (1:5000 in 1% BSA) for 2 h for VEGFR2. Finally, the membranes were probed with the Supersignal Chemiluminescence kit (Pierce, USA), exposed to radiographic films for 7 min (Kodak, USA), and developed.
Statistical analysis
Morphological data were compared by analysis of variance and by the Tukey-Kramer post hoc test using the Statistical Analysis System for Windows (SAS 8.01, SAS Institute, USA). Statistical significance was considered to be P<0.05.
Results
Morphological
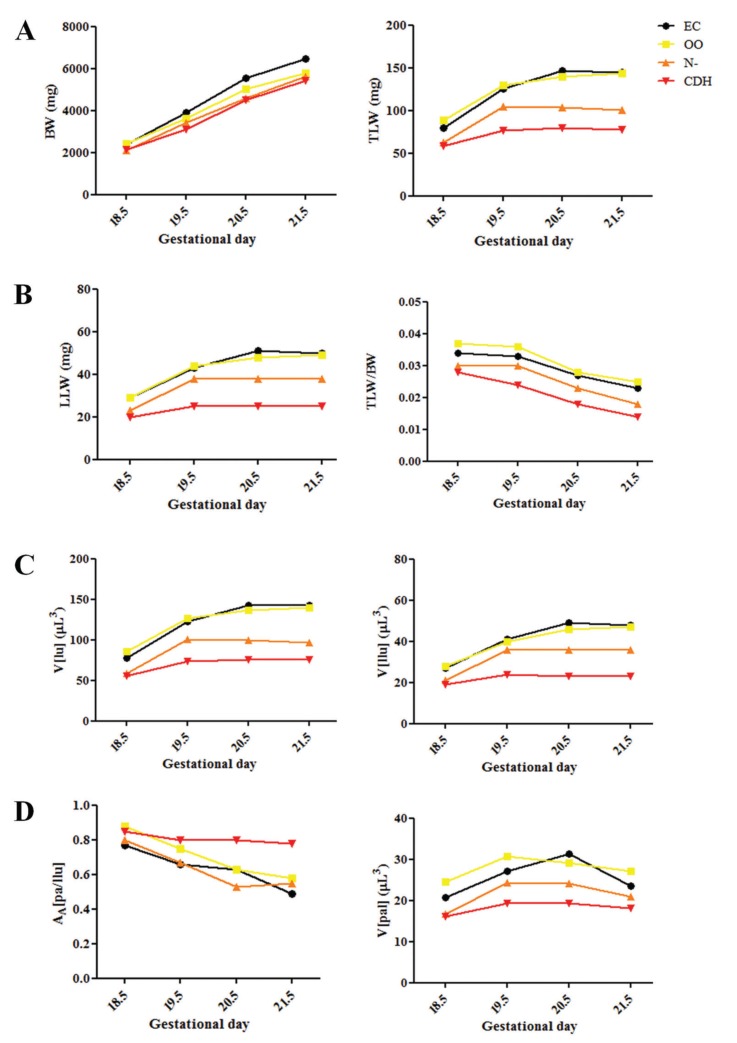
Examination of the nitrofen-exposed fetuses showed that 37% (80/216) had CDH. All the morphological and histometric variables showed reductions in the N- and CDH groups, compared with the controls (EC and OO groups), which were more pronounced in those with CDH). All fetuses exposed to nitrofen (N- and CDH groups) had decreased BW, TLW, LLW, TLW/BW, V(lu), V(llu), AA(pa/llu), and V(pal) compared with non-exposed fetuses (EC and OO groups, P<0.05). Fetuses with CDH showed decreased TLW, LLW, TLW/BW, V(lu), V(llu), AA(pa/llu), and V(pal) compared with N- fetuses at GD 19.5, 20.5, and 21.5 (P<0.001), but there was no difference at GD 18.5 (see Table 1 and Figure 1).

Figure 1. Evolution at 18.5, 19.5, 20.5, and 21.5 gestational days of: A, body weight (BW) and total lung weight (TLW); B, left lung weight (LLW) and TLW/BW ratio; C, total lung volume [V(lu)] and left lung volume [V(llu)]; D, left lung parenchymal area density [AA(pa/llu)] and left lung parenchyma volume [V(pal)]. EC: external control; OO: exposed to olive oil; N-: exposed to nitrofen without CDH; CDH: exposed to nitrofen with CDH.
Histology
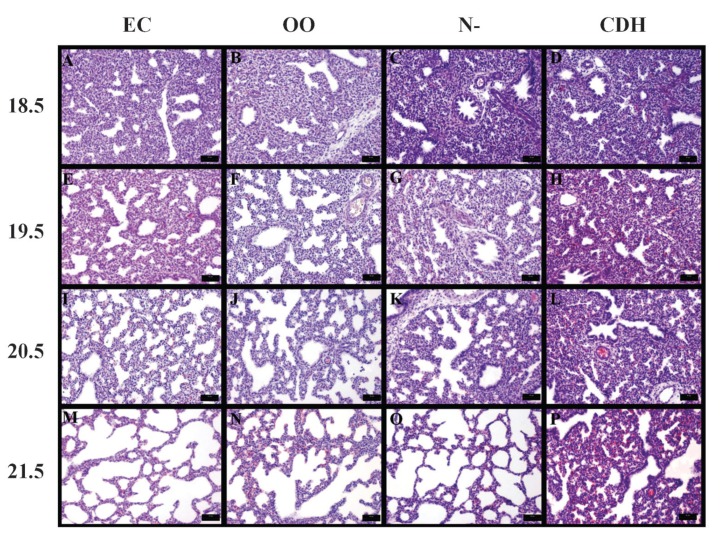
On GD 18.5, 19.5, 20.5, and 21.5, the development of an airspace in the EC, OO, and N- groups was observed. However, the opposite occurred in the CDH group, with decreased airspace and pulmonary hypoplasia increasingly evident at the later gestational ages (Figure 2).
Figure 2. Photomicrographs of fetal lungs from the external control (EC), exposed to olive oil (OO), exposed to nitrofen without CDH (N-), and exposed to nitrofen with congenital diaphragmatic hernia (CDH) groups at gestational days 18.5, 19.5, 20.5, and 21.5. There was a decrease in the airspace in panels H, L, and P, demonstrating pulmonary hypoplasia in the CDH group at later gestational ages. H&E staining, bars: 50 µm.
Western blotting
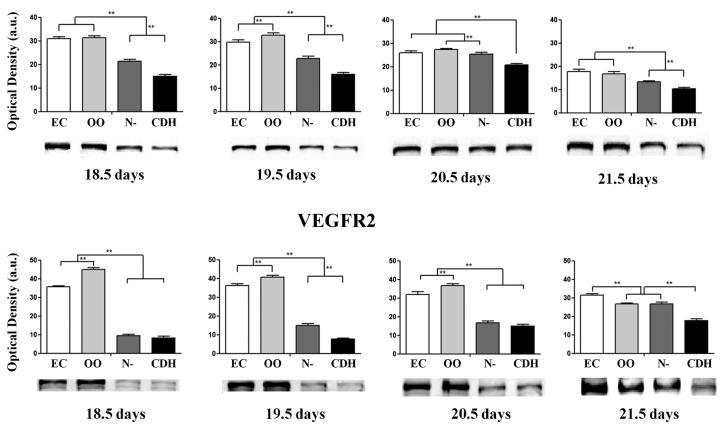
Our results demonstrated that the protein expression levels of VEGFR1 and VEGFR2 in these four development phases were lower in the CDH group than in the N-, EC, and OO groups (P<0.001; see Figure 3).
Figure 3. Evolution of vascular endothelial growth factor receptors (VEGFR1 and VEGFR2) at 18.5, 19.5, 20.5, and 21.5 days of gestation. EC: external control; OO: exposed to olive oil; N-: exposed to nitrofen without CDH; CDH: exposed to nitrofen with CDH. **P<0.001 (analysis of variance with Tukey-Kramer post hoc test).
Discussion
The rat model of CDH pioneered by Iritani (19) allowed reproduction of all components of the malformation by administration of nitrofen (2,4-dichloro-4′-nitrodiphenyl ether) to pregnant rats during the appropriate embryologic window. The nature of lung hypoplasia associated with CDH has been investigated in this model, and a dual-hit mechanism involving primary maldevelopment and secondary compressive changes is currently accepted (20-22).
Although we did not address the early (embryonic) stage of lung development, our results are consistent with those of Kluth et al. (15) in terms of the proportion of CDH in exposed fetuses (37 vs 24%) and with those of Pringle (23) in terms of the timing of the pseudoglandular (GD 18.5), canalicular (GD 18.5, 19.5 and 20.5), and saccular (GD 20.5 and 21.5) stages of lung development.
We found that BW, TLW, LLW, V(lu), and V(llu) increased progressively during fetal development, whereas TLW/BW decreased in all groups, particularly in those with CDH (P<0.001), in agreement with previous reports (22). Recently, we published two studies that showed similar results in BW, TLW and LLW, but we have not calculated the lung volume at 21.5 days (24,25). The V(pal) in fetuses exposed to nitrofen was smaller than for those in the EC and OO groups, indicating that the primary lung injury caused by nitrofen occurred early, evidenced by similar V(pal) in N- and CDH groups. The V(llu) and the LLW were also similar in the two groups exposed to nitrofen, possibly due to impaired airspace development because of the intrathoracic presence of abdominal organs. This is more evident in fetuses with CDH in comparison to all other groups (P<0.05).
We observed that the EC and OO groups were very similar when comparing variables during the same gestational period. We believe that small differences between these groups may be related to the stress of gavaging olive oil to the pregnant rats, or to the nutritional supply of extra fat because the olive oil is not part of normal diet of these animals. Moreover, none of the authors who used the nitrofen model have shown a comparison with an EC group, perhaps because they believe that the OO group is the only real control.
VEGF is a key stimulator of angiogenesis that acts to induce mitosis and migration of vascular endothelial cells. In addition to promoting angiogenesis and neovascularization, it is also a survival factor for newly formed blood vessels (26-28). The vessels' receptors, VEGFR1 (Flt-1) and VEGFR2 (Flk-1) in rats and KDR in humans, have tyrosine kinase activity and function in the opening of Ca2+ channels located in the membranes of endothelial cells (29-31).
Among the various effects of VEGF in vascular and epithelial cells, the temporal-spatial expression of VEGF has been suggested to be important, not only for the formation of capillaries but also for controlling bronchial and alveolar growth (32-34). Changes in VEGF and its receptors cause a disturbance of angiogenesis, leading to a detrimental remodeling of small arteries. Tenbrinck et al. (35) showed that, in CDH, the arterioles on the level of respiratory bronchioles and larger airways had an increased wall thickness (muscularization) that extends into the peripheral pulmonary artery.
The level of VEGF is increased in primary pulmonary hypertension of the newborn (PPHN), but this increase appears to result from hypoxia in lung tissue (due to pulmonary hypertension and lower blood flow) rather than hypertension being due to increased VEGF levels. Thus, the VEGF increase is a consequence of the pulmonary hypertension and not the cause. In this process, a derangement in angiogenesis occurs and the resulting increase in VEGF might compensate for this problem and produce more blood vessels and blood flow, but this mechanism worsens the situation because the vessels are formed in a disorganized fashion (36). Although the mechanism of PPHN is not the same because in CDH the hypertension is secondary to anatomical changes in the pulmonary vasculature before birth, the reduced flow could lead to a final picture similar to PPHN.
VEGF increased in CDH in just one study (13), and others showed that VEGF decreased in the nitrofen model (14). There is no consensus as to whether VEGF increases or decreases in this model. Shehata et al. (37) found increased VEGF expression in the lungs of newborns with CDH, who died of severe pulmonary hypertension. Oue et al. (38) reported that VEGF increased, but it could not be excluded that this increase resulted from nitrofen's own toxic action. Okazaki et al. (11) found that VEGF expression was reduced in CDH and suggested that the mechanisms involved in its receptors could contribute to increase pulmonary hypertension in the disease. Chang et al. (14) found decreased VEGF, and Hara et al. (39) found, in fetal rats, that VEGF increased in lung tissue after performing a tracheal occlusion. We previously reported that VEGF expression decreased in CDH, but after performing a tracheal occlusion with the use of corticosteroids, VEGF expression increased (24).
Similar to our previous results for VEGF (38), the levels of VEGFR1 and VEGFR2 in pseudoglandular, canalicular, and saccular phases of lung development decreased in the nitrofen group relative to the EC and OO groups, with an even lower expression in CDH fetuses (P<0.001).
VEGFR2 is expressed in the early stages of lung development, whereas VEGFR1 expression increases in the later stages of development. Thus, it is believed that VEGFR2 affects the activation of angiogenesis, while VEGFR1 functions in the maturation and integrity of the vessel wall. Due to its high affinity for VEGF, but lower tyrosine kinase activity, VEGFR1 has been implicated in the sequestration of VEGF, decreasing its availability to bind VEGFR2, thus acting in the resolution of the angiogenesis phase (8,40). There is a correlation between high levels of VEGF and fetal lung maturation. Hypoxia of lung tissues activates the HIF-2α gene, inducing the production of VEGF that appears to act on type II pneumocytes through tyrosine kinase receptors (Flt-1 and Flk-1) and increases the production of surfactant.
VEGF-deficient mice exposed to intrauterine anti-VEGF receptor (Flk-1) antibodies present respiratory distress syndrome, due to surfactant deficiency. Intrauterine application or intratracheal postnatal instillation of VEGF stimulates surfactant production and may protect against respiratory distress syndrome (12). We found that there is negative modulation in the evolution of the expression of VEGF receptors, and it is noted that as VEGFR1 tends to decrease during these stages, VEGFR2 tends to increase. This result is always more evident on CDH fetuses, as previously reported (8,9).
This decrease in both receptors may be explained by the lung immaturity that results in CDH and also because of a toxic component of the herbicide that might interfere in the model, as both VEGF receptors decreased regardless of pulmonary hypoplasia being primary or secondary. Although a toxic component in the nitrofen group may exist, the CDH lungs had lower concentrations of VEGF receptors than all other groups. We believe that there is a synergy between increased VEGF and VEGFR1 and VEGFR2 in CDH, which may have clinical implications in compensating for the existing lung hypoplasia with CDH.
Therefore, the decrease in VEGFR1 and VEGFR2 in the CDH rat model may suggest that these proteins are involved in the genesis of vascular growth and may correlate with pulmonary hypoplasia. This information may lead to new studies involving the mechanism of lung angiogenesis in CDH and possible treatment of pulmonary hypertension in the neonatal period.
Acknowledgments
Research supported by FAPESP (#2008/50347-9, #2011/00794-1, and Scholarships #2008/52772-9 and #2011/12587-0).
Footnotes
First published online January 17, 2014.
References
- 1.Golombek SG. The history of congenital diaphragmatic hernia from 1850s to the present. J Perinatol. 2002;22:242–246. doi: 10.1038/sj.jp.7210701. [DOI] [PubMed] [Google Scholar]
- 2.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288:L167–L178. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- 3.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–673. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 4.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 5.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 6.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/S0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 8.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.V99.7.2397. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164:1531–1535. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T, Sharma HS, Aikawa M, Yamataka A, Nagai R, Miyano T, et al. Pulmonary expression of vascular endothelial growth factor and myosin isoforms in rats with congenital diaphragmatic hernia. J Pediatr Surg. 1997;32:391–394. doi: 10.1016/S0022-3468(97)90588-1. [DOI] [PubMed] [Google Scholar]
- 12.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm1102-1329b. [DOI] [PubMed] [Google Scholar]
- 13.Oue T, Shima H, Taira Y, Puri P. Administration of antenatal glucocorticoids upregulates peptide growth factor gene expression in nitrofen-induced congenital diaphragmatic hernia in rats. J Pediatr Surg. 2000;35:109–112. doi: 10.1016/S0022-3468(00)80025-1. [DOI] [PubMed] [Google Scholar]
- 14.Chang R, Andreoli S, Ng YS, Truong T, Smith SR, Wilson J, et al. VEGF expression is downregulated in nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:825–828. doi: 10.1016/j.jpedsurg.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kluth D, Kangah R, Reich P, Tenbrinck R, Tibboel D, Lambrecht W. Nitrofen-induced diaphragmatic hernias in rats: an animal model. J Pediatr Surg. 1990;25:850–854. doi: 10.1016/0022-3468(90)90190-K. [DOI] [PubMed] [Google Scholar]
- 16.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 17.Howard CV, Reed MG. Unbiased stereology. 1st edn. New York: Springer-Verlag New York Inc; 1998. in association with BIOS Scientific Publishers Ltd. [Google Scholar]
- 18.Papadakis K, De Paepe ME, Tackett LD, Piasecki GJ, Luks FI. Temporary tracheal occlusion causes catch-up lung maturation in a fetal model of diaphragmatic hernia. J Pediatr Surg. 1998;33:1030–1037. doi: 10.1016/S0022-3468(98)90526-7. [DOI] [PubMed] [Google Scholar]
- 19.Iritani I. Experimental study on embryogenesis of congenital diaphragmatic hernia. Anat Embryol. 1984;169:133–139. doi: 10.1007/BF00303142. [DOI] [PubMed] [Google Scholar]
- 20.Tenbrinck R, Tibboel D, Gaillard JL, Kluth D, Bos AP, Lachmann B, et al. Experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg. 1990;25:426–429. doi: 10.1016/0022-3468(90)90386-N. [DOI] [PubMed] [Google Scholar]
- 21.Keijzer R, Puri P. Congenital diaphragmatic hernia. Semin Pediatr Surg. 2010;19:180–185. doi: 10.1053/j.sempedsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Alfanso LF, Arnaiz A, Alvarez FJ, Qi B, Diez-Pardo JA, Soler A, et al. Lung hypoplasia and surfactant system immaturity induced in the fetal rat by prenatal exposure to nitrofen. Biol Neonate. 1996;69:94–100. doi: 10.1159/000244283. [DOI] [PubMed] [Google Scholar]
- 23.Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol. 1986;29:502–513. doi: 10.1097/00003081-198609000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt AF, Goncalves FL, Nassr AC, Pereira LA, Farmer D, Sbragia L. Antenatal steroid and tracheal occlusion restore vascular endothelial growth factor receptors in congenital diaphragmatic hernia rat model. Am J Obstet Gynecol. 2010;203:18–20. doi: 10.1016/j.ajog.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt AF, Goncalves FL, Regis AC, Gallindo RM, Sbragia L. Prenatal retinoic acid improves lung vascularization and VEGF expression in CDH rat. Am J Obstet Gynecol. 2012;207: doi: 10.1016/j.ajog.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 27.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 28.Larrivee B, Karsan A. Signaling pathways induced by vascular endothelial growth factor (review) Int J Mol Med. 2000;5:447–456. doi: 10.3892/ijmm.5.5.447. [DOI] [PubMed] [Google Scholar]
- 29.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 30.Quinn TP, Peters KG, de Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewett PW, Murray JC. Coexpression of flt-1, flt-4 and KDR in freshly isolated and cultured human endothelial cells. Biochem Biophys Res Commun. 1996;221:697–702. doi: 10.1006/bbrc.1996.0659. [DOI] [PubMed] [Google Scholar]
- 32.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 33.Hislop AA. Airway and blood vessel interaction during lung development. J Anat. 2002;201:325–334. doi: 10.1046/j.1469-7580.2002.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Shiraishi I, Dai P, Hamaoka K, Takamatsu T. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, Flk-1 and Flt-1. Anat Rec. 2007;290:958–973. doi: 10.1002/ar.20564. [DOI] [PubMed] [Google Scholar]
- 35.Tenbrinck R, Gaillard JL, Tibboel D, Kluth D, Lachmann B, Molenaar JC. Pulmonary vascular abnormalities in experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg. 1992;27:862–865. doi: 10.1016/0022-3468(92)90385-K. [DOI] [PubMed] [Google Scholar]
- 36.Mata-Greenwood E, Meyrick B, Soifer SJ, Fineman JR, Black SM. Expression of VEGF and its receptors Flt-1 and Flk-1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003;285:L222–L231. doi: 10.1152/ajplung.00388.2002. [DOI] [PubMed] [Google Scholar]
- 37.Shehata SM, Mooi WJ, Okazaki T, El-Banna I, Sharma HS, Tibboel D. Enhanced expression of vascular endothelial growth factor in lungs of newborn infants with congenital diaphragmatic hernia and pulmonary hypertension. Thorax. 1999;54:427–431. doi: 10.1136/thx.54.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oue T, Yoneda A, Shima H, Taira Y, Puri P. Increased vascular endothelial growth factor peptide and gene expression in hypoplastic lung in nitrofen induced congenital diaphragmatic hernia in rats. Pediatr Surg Int. 2002;18:221–226. doi: 10.1007/s003830100625. [DOI] [PubMed] [Google Scholar]
- 39.Hara A, Chapin CJ, Ertsey R, Kitterman JA. Changes in fetal lung distension alter expression of vascular endothelial growth factor and its isoforms in developing rat lung. Pediatr Res. 2005;58:30–37. doi: 10.1203/01.PDR.0000163614.20031.C5. [DOI] [PubMed] [Google Scholar]
- 40.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]