Increased T-bet levels and senescence in CD8 T cells from aged individuals as possible mechanisms for decreased influenza-specific functionality.
Keywords: CD57, PD-1, KLRG-1, Eomes, CD107, exhaustion
Abstract
Aged individuals have increased morbidity and mortality following influenza and other viral infections, despite previous exposure or vaccination. Mouse and human studies suggest increased senescence and/or exhaustion of influenza virus-specific CD8 T cells with advanced age. However, neither the relationship between senescence and exhaustion nor the underlying transcriptional pathways leading to decreased function of influenza virus-specific cellular immunity in elderly humans are well-defined. Here, we demonstrate that increased percentages of CD8 T cells from aged individuals express CD57 and KLRG1, along with PD-1 and other inhibitory receptors, markers of senescence, or exhaustion, respectively. Expression of T-box transcription factors, T-bet and Eomes, were also increased in CD8 T cells from aged subjects and correlated closely with expression of CD57 and KLRG1. Influenza virus-specific CD8 T cells from aged individuals exhibited decreased functionality with corresponding increases in CD57, KLRG1, and T-bet, a molecular regulator of terminal differentiation. However, in contrast to total CD8 T cells, influenza virus-specific CD8 T cells had altered expression of inhibitory receptors, including lower PD-1, in aged compared with young subjects. Thus, our data suggest a prominent role for senescence and/or terminal differentiation for influenza virus-specific CD8 T cells in elderly subjects.
Introduction
Aging is associated with defective immune responses, including increased severity of many infections and decreased vaccine-induced immunity. Despite a lifetime of repetitive exposure to vaccinations or infections with influenza virus, increased morbidity and mortality occur in the elderly following infection with this virus [1–3]. Although yearly influenza virus vaccines are based on neutralizing antibody, T cell responses are critical for clearing virus once infection occurs [4]. CD8 T cells are capable of responding to conserved viral epitopes and may contribute to protection against heterosubtypic influenza strains [5, 6]. Thus, it is perhaps counterintuitive that elderly humans, having accumulated influenza virus-specific CD8 T cell responses over time, are not better protected. One possible explanation is that CD8 T cells in the elderly are functionally impaired. Indeed, CD8 T cells from aged individuals have reduced cytokine production [7], granzyme B production [8], and cytolytic potential [9], as well as skewed proportions of effector/memory populations [10] and poor vaccine-induced responses [11]. Therefore, deficits in CD8 T cell function in aged individuals may account for impaired protection during influenza virus infection. These findings have been recapitulated and extended in mice [12]; however, it is still unclear what factors lead to poorly protective, virus-specific CD8 T cells during aging, especially in humans.
Senescence is a major feature of both cellular and organismal aging when cells reach a developmental or replicative endpoint. Senescent T cells have been described in studies of persisting viral infections, such as CMV and HIV, where T cells may be subjected to repetitive stimulation or inflammation [13–15]. Senescence has also been associated with decreased or ineffective CD8 T cell responses in aged individuals or mice [11, 12, 16]. Several phenotypic markers have been associated with senescence in CD8 T cells. For example, several groups have previously demonstrated loss of costimulatory molecules, such as CD27 and CD28, and an associated reduction in replicative capacity in human CD8 T cells [17]. CD57 and KLRG1 have been used to define populations of senescent effector CD8 T cells in mice and humans [15, 18, 19]. In addition KLRG1+ cells have shortened telomeres, indicative of extensive, proliferative history and deteriorating genomic integrity [20]. Although senescent CD8 T cells typically have preserved effector function (i.e., the ability to produce cytokines and even kill target cells), lack of proliferative potential impairs their ability to mount robust immune responses and expand in number upon reinfection [19, 21, 22]. During HIV infection, senescent CD57+ CD8 T cells produce IFN-γ but proliferate poorly and are more sensitive to activation-induced cell death [15]. Thus, the accumulation of senescent CD8 T cells during aging could lead to deterioration of protective immune responses.
Exhaustion is another mechanism by which CD8 T cells fail to elicit an effective response to infection. Exhaustion was demonstrated originally during chronic lymphocytic choriomeningitis virus infection in mice as an alternate fate of T cell differentiation [23, 24]. CD8 T cell exhaustion is associated with loss of CD8 T cell function, coexpression of inhibitory receptors, dependence on antigen for survival, and poor protective immunity and differs from CD8 T cell memory in requirements for maintenance [25]. Sustained, elevated expression of PD-1 is a hallmark of CD8 T cell exhaustion [26]. Other recent studies have found an increase in PD-1 on T cells during aging, suggesting a potential link between aging and exhaustion of CD8 T cells [27–29]. Moreover, recent studies in mice indicate that the transcriptional profiles of CD8 T cells from old mice contain signatures of exhaustion and senescence [30]. Whereas some observations indicate coexpression of markers of senescence and exhaustion [31], others suggest lack of concordence between these two processes [32]. For example, Petrovas et al. [32] showed distict differences between PD-1+ and CD57+ HIV-specific CD8 T cells, including greater sensitivity to ex vivo spontaneous and Fas-mediated apoptosis for PD-1+ versus CD57+ cells. The relationship between senescence and exhaustion of CD8 T cells and the impact on antiviral immunity in the elderly remain poorly understood [33].
The T-box transcription factors, T-bet and Eomes, play crucial roles in determining differential fate of CD8 T cells reponding to infection and have been implicated in regulating optimal memory, terminal differentiation, or exhaustion [19, 34, 35]. In CD8 T cells, T-bet is up-regulated upon activation and is associated with induction of effector functions, including cytotoxicity [36, 37]. T-bet expression is high in effector CD8 T cells but then declines to a moderate level as resting memory CD8 T cells are formed [19]. During the effector phase, T-bet expression is associated with the terminal differentiation of the KLRG1+ subset of effector CD8 T cells [19]. Furthermore, T-bet has been shown to repress PD-1 expression and is required for persistence of antigen-specific CD8 T cells during chronic antigen exposure [35]. In contrast, following acute infection, Eomes expression is higher in long-term, stable, self-renewing memory CD8 T cells [38]. Thus, Eomes and T-bet appear to regulate effector and memory CD8 T cell differentiation, with T-bet promoting terminal differentiation and senescence while repressing severe exhaustion.
To begin to investigate senescence and exhaustion of CD8 T cells in elderly humans, we analyzed the expression of PD-1 and other inhibitory receptors, CD57, and KLRG1 in CD8 T cell subsets from healthy young and aged subjects. Markers of exhaustion and senescence are increased on total CD8 T cells from aged individuals. Influenza virus-specific CD8 T cells in elderly subjects were moderately less functional and had a substantial increase in expression of markers of senescence, but not PD-1, compared with young individuals. The transcription factor T-bet was also increased in these influenza virus-specific CD8 T cells from aged individuals, and higher T-bet was associated with increased coexpression of CD57 and KLRG1, but not PD-1. Thus, cellular senescence appears to be a major feature of influenza virus-specific CD8 T cells in the elderly, and the transcription factor T-bet is likely involved in the underlying molecular regulation of this terminally differentiated state. These findings have implications for strategies designed to improve anti-influenza virus immunity in the elderly.
MATERIALS AND METHODS
Human subjects
Study subjects were recruited and consented at the Duke Geriatric Evaluation and Treatment Clinic at Duke University Medical Center (Durham, NC, USA), in accordance with the Institutional Review Boards of both Duke University and the University of Pennsylvania (Philadelphia, PA, USA). Subjects were young individuals between 21 and 45 years of age or aged individuals, 65 years of age or older. All subjects were free from other ailments and not currently being treated for other conditions. Two hundred seven young and 405 aged subjects were screened for T cell responses to overlapping peptides of the influenza A NP and matrix protein upon the initial visit. Subjects were then HLA-typed to identify HLA-restricted responses for further studies (Table 1). Subjects with positive influenza responses and common HLAs were then tested for HLA-restricted responses, and CD8 T cell phenotypes were examined. After the intial screening for influenza virus and CMV-specific responses and inhibitory receptor expression using fresh PBMCs (see Figs. 1, 5B and D, and 6D), all subsequent experiments were performed using frozen subject samples with HLA-restricted peptides.
Table 1. Enrollment Numbers for Human Subjects.
| Age | Number enrolled screening (25 ml bleeds) | Female | Male | White | Black | Hispanic | Filipino | Asian | Native American |
|---|---|---|---|---|---|---|---|---|---|
| 65 and over | 405 | 203 | 202 | 394 | 8 | 1 | 2 | ||
| 21–40 | 207 | 143 | 64 | 117 | 72 | 5 | 6 | 4 | |
| Totals | 612 | 346 | 266 | 511 | 80 | 6 | 6 | 6 |
Enrollment numbers for subjects selected based on HLA expression and virus-specific responses separated by age and race.
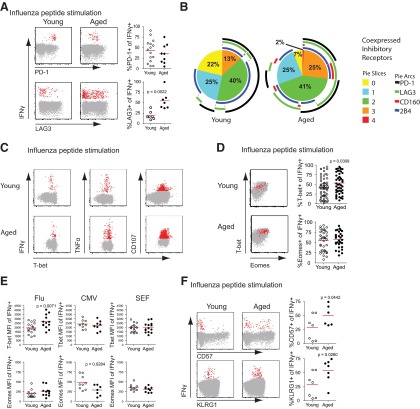
Figure 1. Increased inhibitory receptor expression on CD8 T cells from aged subjects.
(A) CD8 T cells, gated on total lymphocytes, singlets, and CD3+CD14−CD16−CD19− LIVE/DEAD Aqua−, show expression of inhibitory receptors PD-1 (n=58 young and 54 aged), LAG3 (n=38 young and 45 aged), CD160 (n=27 young and 30 aged), and 2B4 (n=43 young and 48 aged), with representative histograms and graphs comparing percent positive in young and aged subjects (Mann-Whitney) for each marker. Solid lines in histograms show expression levels on total CD8 T cells from aged (blue) subjects, compared with young subjects (red), compared with naïve CD8 T cells (black). (B) Graph showing an increase in inhibitory receptor PD-1 expression over the differentiation state of CD8 T cells in aged and young subjects (Mann-Whitney; n=66 young and 32 aged). (C) Differential distribution of CD8 T cell maturation states in representative young and aged subjects gating on naïve (upper-right quadrant) and non-naïve cells for each representative. (D) Expression levels of inhibitory receptors on non-naïve CD8 T cells of young and aged subjects (Mann-Whitney), PD-1 (n=69 young and 34 aged), LAG3 (n=26 young and 22 aged), CD160 (n=29 young and 19 aged), and 2B4 (n=67 young and 34 aged). (E) SPICE anaylsis of inhibitory receptor coexpression on non-naive CD8 T cells from young and aged subjects with mean percentage (n=15 young and 9 aged). Pie-section colors correspond to number of inhibitory receptors expressed on a cell (yellow for 0, light blue for 1, etc.). Arcs around the pie signify which inhibitory receptor(s) are expressed (i.e., black for PD-1, green for LAG3, etc.).
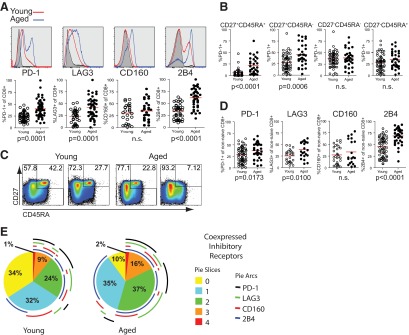
Figure 5. Antigen-specific CD8+ T cell responses differ between young and aged subjects.
Representative responses from young and aged individuals making IFN-γ and TNF-α (A) in response to influenza or CMV peptides or superantigen SEF compared with no stimulation controls. (B) Pooled responses coexpressing IFN-γ and TNF-α in response to influenza, CMV, or SEF stimulation (Mann-Whitney; flu, n=54 young and 44 aged; CMV, n=12 young and 21 aged; SEF, n=102 young and 73 aged). (C) Coexpression analysis of multiple CD8+ T cell functions, IFN-γ, TNF-α, granzyme B, MIP-1β, and degranulation (i.e., CD107) in young and aged subjects in response to influenza virus peptides. (D) Relative expression of CD107 or CD107, IFN-γ, and TNF-α in CD8 T cells to influenza peptides or SEF (Mann-Whitney; flu, n=6 young and 8 aged; SEF, n=8 young and 8 aged for SPICE analysis).
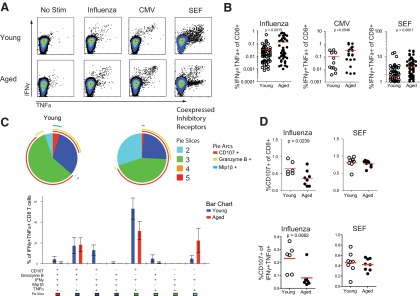
Figure 6. T-bet and Eomes expression on functional CD8 T cells in young and aged individuals.
(A) Expression of inhinbitory receptors PD-1 and LAG3 in representative young and aged subjects (Mann-Whitney; PD-1, n=15 young and 9 aged; LAG3, n=7 young and aged). (B) Coexpression of inhibitory receptors on influenza virus-specific CD8 T cells using SPICE analysis. (C) Coexpression of T-bet with IFN-γ, TNF-α, or CD107 as effector functions of CD8 T cells in young and aged individuals in response to influenza virus peptides. (D) Overlay of T-bet and Eomes expression of influenza-specific (red) and total CD8 T cells (gray) with relative expression of T-bet and Eomes in influenza-specific CD8 T cells from young and aged subjects (Mann-Whitney; T-bet, n=61 young and 48 aged; Eomes, n=36 young and 30 aged). (E) Pooled data showing T-bet and Eomes levels (MFI) in CD8 T cells producing IFN-γ in response to influenza (Mann-Whitney; n=17 young and 13 aged T-bet; n=13 young and 11 aged Eomes), CMV peptides (n=9 young and 8 aged T-bet; n=9 young and 7 aged Eomes), or SEF superantigen (n=16 young and 14 aged T-bet; n=10 young and 9 aged Eomes). (F) CD8 T cell function relative to CD57 and KLRG1 expression in CD8 T cells from young and aged subjects responding to influenza peptides. Staining of CD57 and KLRG1 on cells making IFN-γ and relative percentages of CD57 (n=7 young and 6 aged) or KLRG1 (n=7 young and 7 aged) expression on the same cells (Mann-Whitney).
Peptide and superantigen stimulation for intracellular cytokine production
Directly isolated PBMCs or thawed PBMCs were washed in prewarmed RPMI 1640 containing 10% FCS and rested overnight at 37°C before stimulation. Cells were then stimulated with appropriate HLA-restricted peptide, peptide pools, or superantigen SEF in the presence of Brefeldin A and anti-CD107a FITC (BD Biosciences, San Jose, CA, USA) for 5 h at 37°C before staining.
Flow cytometry
Immediately after peptide or SEF stimulation, PBMCs were stained for surface markers and intracellular cytokines. The following antibody conjugations were used: CD4-PE-Cy5.5 and Aqua LIVE/DEAD (Invitrogen, Carlsbad, CA, USA); CD57-PE, CD14-APC-H7, CD16-APC-H7, and CD19-APC-H7 (BD Biosciences); IFN-γ Alexa Fluor 700, IL-2 Brilliant Violet 421, PD-1-PE and PE-Cy7, T-bet Pacific Blue, TNF-α-PE-Cy7, and CD27-PerCP/Cy5.5 (BioLegend, San Diego, CA, USA); Eomes Alexa Fluor 647, CD8 eFluor 650NC, CD27-PE-Cy5, and CD45RA eFluor 605NC (eBioscience, San Diego, CA, USA); CD244-PE-Cy5 (Beckman Coulter, Brea, CA, USA); LAG-3 Biotin (Enzo Life Sciences, Farmingdale, NY, USA); and CD3 Qdot 585, CD57 Qdot 565, CD160 [39] FITC, KLRG1 FITC, and Alexa Fluor 700 (conjugated in-house). Intracellular cytokines and transcription factors were stained using the BD Cytofix/Cytoperm kit (BD Biosciences). Samples were acquired on a BD Biosciences LSR II and analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Fluorescence-minus-one controls were performed in initial studies to define positive versus negative staining and determine where marker cutoffs should be set. All experiments on thawed samples were performed using young and aged samples stained simultaneously to control for staining variability between groups.
Statistics
Statistical analysis was done using Excel (Microsoft, Redmond, WA, USA) and Prism software (GraphPad, La Jolla, CA, USA). Statistical significance was determined using the Mann-Whitney test for nonparametric, pairwise comparisons in all figures. The Holm-Bonferroni method was used to correct P values where multiple comparisons were done (see Fig. 4).
Figure 4. CD57 and PD-1 mark unique populations of CD8 T cells having different associations with T-bet and Eomes.
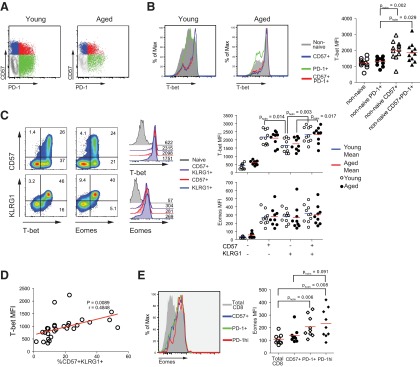
(A) CD57 and PD-1 staining on non-naïve (non CD27+CD45RA+) from young (left) and aged (right) subjects shows representative expression on non-naïve CD8 T cells with (B) histograms comparing representative expression levels (left) and pooled MFI data (right; Mann-Whitney with Holm-Bonferoni) of T-bet in young or aged, non-naïve CD8 T cells (gray-filled histogram), CD57+ CD8 T cells (blue) versus PD-1+ CD8 T cells (green), or cells coexpressing CD57 and PD-1 (red; n=11). (C) Representative FACS plots showing that T-bet and Eomes are expressed on CD8+ T cells relative to CD57 or KLRG1. Relative expression of T-bet or Eomes by CD8 T cells with single or dual expression of CD57 and KLRG1 shown as overlayed histograms with respective MFI. T-bet or Eomes expression relative to expression of CD57 and/or KLRG1 (MFI) (Mann-Whitney with Holm-Bonferoni; n=10 young and 9 aged). (D) Correlation of CD57+KLRG1+ CD8 T cells with T-bet MFI (n=28). (E) Histograms showing Eomes staining in total CD8 T cells (gray), CD57+ CD8 T cells (blue), PD-1+ CD8 T cells (green), or PD-1hi CD8 T cells (red) with pooled data at right (Mann-Whitney with Holm-Bonferoni; n=9 for all groups).
RESULTS
Increased expression of inhibitory receptors and senescence markers on CD8 T cells from aged subjects
To examine the pathways associated with exhaustion and senescence in CD8 T cells from aged individuals, we used multiparameter flow cytometry to analyze the expression of inhibitory receptors (PD-1, LAG3, CD160, and 2B4), as well as senescence markers (CD57 and KLRG1) on CD8 T cells from young and aged individuals. The percentage of CD8 T cells expressing inhibitory receptor on their surface (with the exception of CD160) was increased on total CD8 T cells from aged compared with young subjects (Fig. 1A). Significance was determined using Mann-Whitney tests for nonparametric data. Similar results were observed when the analysis was restricted to subjects with known CMV responses or comparing subjects younger than age 30 with those older than age 75 (Supplemental Fig. 1A and B). To examine whether fewer naïve cells in the elderly could explain these differences, we analyzed the expression of these markers on CD8 T cells defined as naïve, central memory, effector memory, and effector subsets using expression of CD45RA and CD27 (Fig. 1B). The percentage of PD-1-expressing cells was increased on the CD27+CD45RA− subsets in aged subjects compared with young subjects but was not different on the CD27−CD45RA− or CD27−CD45RA+ subsets. However, PD-1 was also expressed by a greater percentage of the potentially naïve CD27+CD45RA+ subset in the elderly. It was possible that this latter observation reflected non-naïve T cells in the CD27+CD45RA+ subset in older subjects. Indeed, additional analysis of the CD27+CD45RA+ subset using CD127 and CD11a to help define naïve T cells rigorously (i.e., CD27+CD45RA+CD127+CD11a−) demonstrated substantial non-naïve contamination in the CD27+CD45RA+ subset in the elderly (Supplemental Fig. 1C) [9]. The vast majority of PD-1 expression in the CD27+CD45RA+ fraction from the elderly was lost when the CD27+CD45RA+ subset was further gated on CD11a− and CD127+ cells. Moreover, these data indicate an even more dramatic loss of phenotypically defined, naïve CD8 T cells in the elderly compared with what was suggested by CD27 and CD45RA expression alone. Although this decrease in CD27+CD45RA+ naïve T cells may play a significant role in decreased T cell function in aged individuals, we focused subsequent analyses on phenotypically defined memory CD8 T cells, as these have been shown to contribute to heterosubtypic immunity [40, 41]. Because of the complications of the non-naïve CD11a+CD127− cells in the CD27+CD45RA+ gate and the difficulty fitting these additional naïve markers into all flow panels, we have not performed additional detailed analysis of these CD27+CD45RA+CD11a+CD127− cells here. Rather, we focused on the non-CD27+CD45RA+ subset of CD8 T cells from young versus aged subjects to normalize comparisons of phenotypically defined memory/non-naïve CD8 T cells. Thus, to control for the potential differences in the proportion of naïve (defined as CD8+CD27+CD45RA+ for this and future analyses) T cells in young versus elderly subjects, we re-examined expression of inhibitory receptors after gating out the CD27+CD45RA+ cells (Fig. 1C; data not shown). In general, inhibitory receptors were expressed on a greater percentage of CD8 T cells in the non-naïve compartment compared with total CD8 T cells in the young and elderly cohorts (Fig. 1B and D). However, examining only non-naïve CD8 T cells indicated that expression of PD-1, LAG3, and 2B4 remained elevated in the aged subjects compared with young (Fig. 1D). In addition, non-naïve CD8 T cells from aged individuals also coexpressed more inhibitory receptors at the same time compared with non-naïve CD8 T cells from young individuals (Fig. 1E). These data demonstrate an increase in markers associated with exhaustion in aged humans and indicate that coexpression of multiple inhibitory receptors is increased on CD8 T cells from aged subjects compared with young subjects.
We next examined the expression of markers of senescence, CD57 and KLRG1, on total CD8 T cells from aged and young subjects. Consistent with previous reports [11, 20, 21, 42–44], the percentage of CD57- and KLRG1-expressing CD8 T cells was increased in aged subjects (Fig. 2A). Analysis of CD57 and KLRG1 expression showed that CD57 and KLRG1 were highly expressed among CD27− CD8 T cells (Fig. 2B) and although some KLRG1 was expressed on CD27+ CD8 T cells, nearly all CD57+KLRG1+ CD8 T cells were CD27− (Fig. 2B and C). Thus, it is not surprising that CD57 and KLRG1 were not differentially expressed in young and aged subjects when CD27+CD45RA+ naïve CD8 T cells were gated out (Fig. 2D). Whereas the increase in CD57+ and KLRG1+ CD8 T cells from aged subjects appears to be attributed to the increased proportion of non-naïve CD8 T cells, increased percentages of CD8 T cells expressing one inhibitory receptor, as well as coexpression of some inhibitory receptors, remain, even when analyzing antigen-experienced CD8 T cell populations. This distinction between markers associated with exhaustion and senescence may therefore be important among non-naïve CD8 T cells subsets.
Figure 2. Increased in markers of senescence on CD8 T cells from aged subjects associated with differentiation.
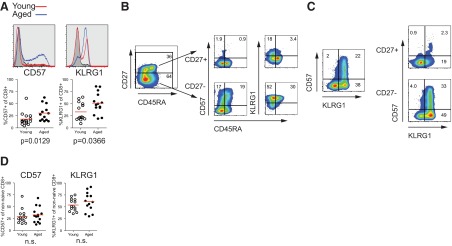
(A) CD8 T cell expression of senescence markers CD57 and KLRG1 with representative histograms and graphs comparing percent positive for each marker (Mann-Whitney). Lines in histograms show expression levels on total CD8 T cells from aged (blue) subjects, compared with young subjects (red), compared with naïve CD8 T cells (black; n=14 young and aged). (B) Expression of CD57 and KLRG1 relative to CD27 and CD45RA. (C) Coexpression of CD57 and KLRG1 on total CD8 T cells or CD27+ versus CD27− CD8 T cells. (D) Expression levels of CD57 and KLRG1 on non-naïve CD8 T cells of young and aged subjects (Mann-Whitney; n=14 young and aged).
T-bet and Eomes expression is increased in CD8 T cells from elderly humans
The T-box transcription factors T-bet and Eomes are associated with effector and memory CD8 T cell differentiation, and these factors may also influence terminal differentiation/senescence and/or exhaustion [45]. To begin to understand how the T-box transcription factors T-bet and Eomes are associated with age-related changes in CD8 T cells in humans, we examined the expression pattern of these two transcription factors by flow cytometry. T-bet and Eomes mRNA expression is low in naïve CD8 T cells in young subjects [34], and the antibodies used to detect T-bet and Eomes in humans show little if any, detectable staining in phenotypically defined, naïve CD8 T cells from young subjects (Fig. 3A). T-bet and Eomes expression were increased, however, in total CD8 T cells from aged versus young subjects. These differences were observed for the percent of T-bet+ or Eomes+ CD8 T cells, as well as the amount of these transcription factors expressed per cell (MFI; Fig. 3A and B). As naïve CD8 T cells are over-represented in the CD8 T cell subset from young individuals, we again examined T-bet and Eomes expression in phenotypically defined subsets of CD8 T cells from young and aged. Indeed, T-bet and Eomes were expressed in a larger percentage of phenotypically defined, non-naïve (all non-CD27+CD45RA+) CD8 T cells in aged compared with young subjects (Fig. 3C). When we further examined the differentiation state of CD8 T cells, expression of T-bet and Eomes was increased in the phenotypically defined “central memory” (CD27+CD45RA−; Eomes only), “effector memory” (CD27−CD45RA−; T-bet only), and “effector/effector memory RA” (CD27−CD45RA+; both) subsets (Fig. 3D). These data demonstrate that T-bet and Eomes are increased in non-naïve CD8 T cells from aged subjects.
Figure 3. Increased expression of T-box transcription factors T-bet and Eomes in CD8+ T cells from aged individuals.
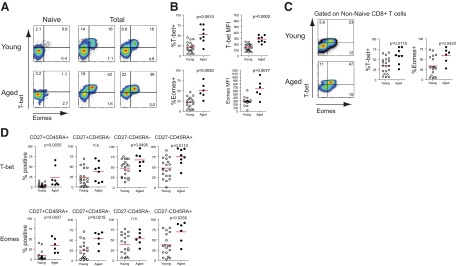
(A) Representative FACS plots showing T-bet and Eomes expression on naïve (CD27+CD45RA+) and total CD8+ T cells from young and aged subjects. (B) Pooled numbers showing percentage and MFI of T-bet and Eomes expression on total CD8+ T cells from young and aged individuals. Each dot signifies one individual, with the mean for each group marked by the red lines (Mann-Whitney; T-bet percent and T-bet MFI, n=20 young and 8 aged; Eomes percent, n=18 young and 6 aged; and Eomes MFI, n=17 young and 6 aged). (C) T-bet and Eomes expression on only non-naïve (non-CD27+CD45RA+) CD8+ cells from both young and aged subjects as representative FACS plot and percent expression for all subjects (Mann-Whitney; T-bet percent, n=20 young and 8 aged; Eomes percent, n=18 young and 6 aged). (D) T-bet and Eomes expression in each differentiation subset of young or aged CD8+ T cells from naïve to most terminally differentiated (Mann-Whitney; T-bet percent, n=20 young and 8 aged; Eomes percent, n=18 young and 6 aged).
Phenotypically defined “exhausted” and “senescent” CD8 T cells are largely separate populations
As T-bet and Eomes regulate terminal differentiation and senescence of effector versus memory CD8 T cells in animal models [34], we hypothesized an association between these transcription factors and T cell fate in humans. T-bet is known to have an inverse correlation with PD-1 in mice [35], and as PD-1, CD57, T-bet, and Eomes all showed increased expression with age (see Figs. 1–3), we sought to define the relationship between the expression of these molecules in humans. Flow cytometric analysis of PD-1 and CD57 expression on non-naïve CD8 T cells (non-CD27+CD45RA+) from young or aged subjects revealed distinct populations of CD57- and PD-1-expressing cells (Fig. 4A). Examining T-bet in PD-1+ or CD57+ CD8 T cells showed increased MFI of T-bet expression in CD57+ cells but not in PD-1+ CD8 T cells compared with the total population of non-naïve T cells (Fig. 4B). T-bet expression in PD-1+ cells was similar to that of total non-naïve CD8 T cells in both young and aged subjects (Fig. 4B). CD57+ cells expressed a significantly increased MFI of T-bet compared with the PD-1+ CD8 T cell subset (Fig. 4B), and P values were corrected using the Holm-Bonferroni method to control for multiple comparisons. Notably, the CD57+PD-1+ subpopulation expressed less PD-1 (MFI) than the PD-1hi CD8 T cells and had a similar T-bet profile to the CD57+PD-1− subset (Fig. 4B; data not shown).
Next, we further investigated the relationship among T-bet, CD57, and KLRG1. CD57 and KLRG1 were coexpressed with T-bet and Eomes in CD8 T cells (Fig. 4C). CD57+ and KLRG1+ CD8 T cells expressed significantly increased amounts of T-bet and Eomes/cell compared with naïve CD8 T cells (Fig. 4C). Moreover, CD57-expressing CD8 T cells, with or without coexpression of KLRG1, had the highest expression of T-bet (Fig. 4C). T-bet expression also showed a direct correlation with the percentage of CD57+KLRG1+ CD8 T cells (P=0.0089; r=0.4848; Fig. 4D). Eomes expression, on the other hand, was increased in PD-1+ or PD-1hi cells compared with total CD8 T cells or CD57+ CD8 T cells (Fig. 4E). Whereas this association of Eomes with PD-1 expression was surprising, given the association of Eomes with central memory CD8 T cells in mice [34], Eomes mRNA is also highly expressed in exhausted CD8 T cells in mice [46, 47]. Thus, the transcription factors T-bet and Eomes appear to be differentially expressed in CD57+ or PD-1+ CD8 T cells, respectively. High expression of T-bet, which can promote terminal differentiation in mice, was associated with the expression of the senescence and terminal differentiation markers CD57 and KLRG1, but not PD-1, in aged humans.
Function of virus-specific CD8 T cells differs in young and aged subjects
We next investigated whether virus-specific CD8 T cells differed in young versus elderly subjects. We first examined the responses to influenza virus using individually defined, HLA-restricted CD8 T cell epitopes, largely derived from influenza NP and matrix proteins. In aged subjects, there was an increase in the frequency of influenza virus NP and matrix-specific CD8 T cells, as determined by IFN-γ and TNF-α production after peptide stimulation (Fig. 5A and B and Supplemental Fig. 2). Although this study was not designed to quantitatively compare responses in young and aged individuals, this observation is consistent with accumulated responses to previous influenza virus exposure over time (Fig. 5B). This difference from previous studies [11, 48] may be a result of the fact that we examined responses to conserved NP and matrix peptides rather than stimulation with whole virus. We also observed increased frequencies of CD8 T cells specific for CMV in aged subjects (Fig. 5B and Supplemental Fig. 2), in agreement with previous reports [13, 49]. In addition, elderly subjects had a higher percentage of IFN-γ producing CD8 T cells following stimulation with the superantigen SEF (Fig. 5B and Supplemental Fig. 2), which may reflect differences in relative numbers of non-naïve CD8 T cell subsets between young and aged subjects.
To define how virus-specific CD8 T cells in the elderly compared with the young qualitatively, we used multiparameter flow cytometry and simultaneously measured multiple functional parameters. This approach has been used to assess the polyfunctionality of virus-specific CD8 T cells in other settings [50] and provides information on the quality of an antigen-specific T cell population. Although there were quantitatively more CD8 T cells that produced IFN-γ (Supplemental Fig. 2A and B) or coproduced IFN-γ and TNF-α in aged subjects (Fig. 5A and B), an analysis of polyfunctionality assessing CD107a-based degranulation and IFN-γ, IL-2, and TNF-α production revealed that virus-specific CD8 T cells were overall less polyfunctional compared with influenza virus-specific CD8 T cells from young subjects. This difference was mainly a result of a deficit in the ability to degranulate (i.e., up-regulate CD107 on the cell surface), while also producing IFN-γ and TNF-α (light-blue pie piece) in response to influenza antigens (Supplemental Fig. 2C). This difference was not observed in CMV-specific CD8 T cells nor those responding to SEF, which were equally polyfunctional in young and elderly individuals (Supplemental Fig. 2C). To examine these functional differences in more detail, we extended this analysis to include coproduction of granzyme B, MIP-1β, in addition to IFN-γ, TNF-α, production, and degranulation (i.e., CD107), following influenza virus peptide stimulation. When restricting the analysis to CD8 T cells producing IFN-γ and TNF-α in response to influenza virus peptides, the overall ability to coproduce multiple cytokines, release cytotoxic granules, and/or express granzyme B was decreased in the elderly compared with young subjects (Fig. 5C). The analysis of CD107a degranulation and expression of IFN-γ, granzyme B, MIP-1β, and TNF-α in response to influenza peptides revealed a relative increase in cells performing three or four functions simultaneously (green or blue pie sections, respectively) in young subjects compared with aged subjects (Fig. 5C). Conversly, in the elderly, an increased proportion of influenza virus-specific CD8 T cells was able to produce IFN-γ and TNF-α in the absence of the other functions assessed (Fig. 5C). An analysis of only CD107-based degranulation confirmed a decrease in the relative proportion of influenza-specific CD8 T cells that up-regulated CD107 in response to influenza virus peptides in aged subjects (Fig. 5D). This difference was confined to influenza virus-specific CD8 T cells, as cells responding to SEF stimulation did not show relative differences in degranulation (Fig. 5D). These data suggest that CD8 T cell responses to influenza virus are different in the elderly versus young and that there may be a shift toward less optimal polyfunctionality with advanced age.
Increased senescence and terminal differentiation of influenza virus-specific CD8 T cells from elderly subjects
Finally, we examined how markers of senescence and exhaustion related to the expression of T-bet and Eomes in influenza virus-specific CD8 T cells from young versus elderly humans. Examination of inhibitory receptor expression on influenza virus-specific CD8 T cells from aged and young subjects revealed a distinct difference from that observed on total CD8 T cells. Although a lower percentage of influenza virus-specific CD8 T cells from aged subjects expressed PD-1, more cells expressed LAG3, similar to bulk CD8 T cell populations (Figs. 1A and D and 6A and B). Also, whereas the percentage of influenza virus-specific CD8 T cells in aged subjects coexpressing inhibitory receptors remained higher, the patterns of coexpression of those receptors differed from that seen on total CD8 T cells (Figs. 1E and 6B). Many influenza virus-specific CD8 T cells producing IFN-γ expressed high levels of T-bet and/or Eomes (Fig. 6C and D), and T-bet and Eomes were largely coexpressed in the IFN-γ+ CD8 T cells (Fig. 6D). Whereas the percentage of Eomes+ and MFI of this protein in influenza virus-specific CD8 T cells was not different in the two age groups, aged subjects had a significantly increased proportion of influenza virus-specific CD8 T cells expressing increased amounts of T-bet (Fig. 6D and E). In contrast to influenza virus-specific CD8 T cells, CD8 T cells specific for CMV or those responding to SEF had no differences in T-bet expression between young and aged subjects (Fig. 6E). Interestingly, Eomes expression was statistically decreased in CD8 T cells from aged subjects responding to CMV (Fig. 6E). Finally, CD8 T cells from aged subjects making IFN-γ in response to influenza virus peptides had increased percentages of CD57+ and KLRG1+ CD8 T cells compared with CD8 T cells from young subjects, suggesting a potential connection between T-bet and terminal differentiation/senescence in influenza-specific CD8 T cells in the elderly (Fig. 6F). Again, CD8 T cells responding to CMV did not show age-related differences in CD57 or KLRG1 expression (data not shown). Thus, the association of inhibitory receptor expression on CD8 T cells responding to influenza virus in aged subjects was distinct from the total pool of CD8 T cells and included lower PD-1 expression. In addition, increased expression of markers associated with terminal differentiation and senescence corresponded with up-regulation of the transcription factor T-bet in aged individuals.
DISCUSSION
Mechanisms of decreased CD8 T cell function with age are poorly understood. Here, we demonstrate differences in expression of inhibitory receptors versus markers of senescence on total and influenza virus-specific CD8 T cells in aged versus young individuals. We also define a novel relationship between T-box transcription factors T-bet and Eomes and terminal differentiation/senescence in CD8 T cells from aged human subjects. Both CD57 and KLRG1 have been independently associated with CD8 T cell dysfunction and aging [20, 44]. Our data confirm and extend these observations by showing age-dependent differences in expression of the transcription factor T-bet associated with high KLRG1 and CD57 expression. Although KLRG1 and CD57 are increased with age and correlate with effector memory and effector memory RA subsets [20], several groups have associated CD57 [15, 51] and KLRG1 [18, 52] with loss of function. Although our results are correlative, they agree with these previous data and show an association between expression of these markers and the transcription factor T-bet. Accumulation of influenza virus-specific CD8 T cells expressing CD57, KLRG1, and T-bet occurs with increasing age and is accompanied by decreased overall polyfunctionality of these cells. Whereas alterations in the patterns of inhibitory receptor expression also occur, expression of the major inhibitory receptor PD-1 was reduced on influenza virus-specific CD8 T cells from elderly versus young subjects. Taken together, these data illustrate a possible mechanism for decreased protective capacity of influenza virus-specific CD8 T cells during aging. Furthermore, understanding the specific transcriptional regulation of exhaustion versus senescence in human CD8 T cells may provide opportunities to improve vaccine and immunomodulatory responsiveness.
CD8 T cell effector functions decrease with age [2] and/or chronic infection [44, 53]. It is unclear, however, if a lifetime of repetitive exposure to influenza virus (and possible vaccination) has a significant impact on CD8 T cell differentiation and function. Repetitive or intermittent exposure to influenza virus might be one difference between CD8 T cells from young and elderly humans. Although repetitive infection may cause a preferential expansion of non-naïve CD8 T cells observed in aged subjects, repetitive, intermittent, acute infections typically have not been associated with loss of T cell function. For example, multiple sequential acute infections in mouse models do indeed skew CD8 T cells toward effector memory phenotype cells [54–56], but protective immunity to acute infections was preserved in young animals [56]. Previous studies of antiviral immunity in old mice have typically used single-infection approaches, although some recent data suggest differences with latent infections [57]. It is possible that repetitive infection with influenza virus or chronic stimulation of T cells responding to CMV or HIV could result in distinct patterns of senescence and/or exhaustion [7, 15]. HIV or CMV infection can lead to premature “aging” of the immune system, resulting in increased senescent and/or exhausted CD8 T cells and decreased function [30, 32, 49, 58]. This decrease in function is thought to be a result of chronic antigenic stimulation and agrees with mouse models of chronic infection [46]. On the other hand, there are also clear effects of advanced age on T cell quality, independent of infection, such as decreased, naïve T cell populations, narrowed TCR repertoire, decreased memory turnover, and skewed differentiation [10, 13]. We have confirmed observed decreases in naive CD8 T cells in aged subjects using CD27 and CD45RA staining. With the use of additional markers to define naïve T cells (CD11a and CD127), we found an even greater reduction in naïve T cells in the elderly than expected, based on CD27 and CD45RA staining. These observations reinforce the importance of understanding how a lack of new responses generated by naïve CD8 T cells in aged individuals might impact immune protection against influenza and other infections. It will be important to examine how these changes, coupled with different types of repetitive antigen re-exposure, might result in unique, virus-specific CD8 T cell differentiation states in elderly humans.
Age-related CD8 T cell dysfunction and the effects on vaccine-induced responses have only recently begun to be dissected [13, 16]. An increase in senescent T cells specific for influenza virus has been associated with decreased vaccine responsiveness [11], but possible causes for these differences are poorly understood. Our findings demonstrate that influenza-specific CD8 T cells in aged individuals expressed higher levels of CD57 and/or KLRG1. These increases in markers of terminal differentiation were associated with increased T-bet expression. This pattern of differentiation was not observed for influenza-specific CD8 T cells in young subjects. However the more senescent pattern of differentiation in the elderly is consistent with the notion of accumulated, repetitive stimulation [54, 56] or stimulation in the presence of increased inflammation [19]. Future studies will be necessary to distinguish the relative contribution of repetitive exposure to influenza virus antigens versus the increased inflammation associated with aging to this differentiation program.
Influenza virus-specific CD8 T cells in elderly humans were slightly less functional than their counterparts in young subjects. This modest dysfunction was somewhat surprising and contrasted what was observed for the CMV-specific CD8 T cells that were equally functional in these young versus aged cohorts. The decrease in polyfunctionality for the influenza virus-specific CD8 T cells in the aged cohort was associated with higher expression of markers of senescence (e.g., CD57 and KLRG1) and elevated T-bet. However, there was no increase in PD-1, an inhibitory receptor that can, at least during chronic infections, inhibit functionality. Although PD-1 does not seem to factor into dysfunction of influenza virus-specific CD8 T cells in aged subjects, other inhibitory receptors, such as LAG3, are elevated. These observations were unexpected, as senescent or terminally differentiated CD8 T cells, such as KLRG1+ effector CD8 T cells in mice, retain strong functionality. Whereas T-bet can repress IL-2 production [37], the reduced polyfunctionality in this human cohort was not a result of loss of IL-2 production. Repetitive influenza virus infection over a short time-frame in mice can lead to CD8 T cell dysfunction and a type of exhaustion [59]. However, in this setting in mice, PD-1 was up-regulated [59], unlike what we have observed in humans in this cohort. One potential model to explain these data is that whereas PD-1 expression appears central to the expression pattern of inhibitory receptors in many chronic infections, other receptors, such as LAG3, might take the place of PD-1 in certain settings. The precise role of LAG3 in elderly subjects, however, remains to be determined. Alternatively, whereas high T-bet expression is important to coordinate appropriate effector CD8 T cell responses during acute infections and perhaps during low-grade, persisting infections, such as CMV, prolonged high expression of T-bet following cleared infections, such as influenza virus, might be detrimental to the overall quality of the memory CD8 T cell pool. Support for such a model comes from studies in mice that demonstrate a negative impact on long-term CD8 T cell memory when T-bet expression is artificially elevated [19]. Thus, during human aging, T-bet might act as a rheostat that integrates repetitive stimulation history and perhaps signals from inflammation to shape memory CD8 T cell quality. Future studies will be necessary to test these models and to determine whether altering T-bet expression or manipulating LAG3 function could improve the quality of the influenza virus-specific CD8 T cell population. Although manipulating either of these molecules or other inhibitory receptors is beyond the scope of this study, future animal studies and in vitro-blocking experiments may reveal whether recovery of more optimal T cell function is possible.
Protection against influenza virus infection is a major goal of vaccination programs in aging humans. Despite repeated exposure to influenza virus and prophylactic vaccination, the elderly are more susceptible to morbidity and mortality as a result of influenza virus infection compared with younger adults [1, 3]. Waning effectiveness of CD8 T cell responses in aged individuals is thought to contribute to this loss of protection in this population [8]. Although sterilizing antibody responses are capable of efficient protection against influenza virus infection, neutralizing antibody responses rarely crossreact with new, emerging or pandemic influenza virus strains. This lack of cross-protection and ineffectiveness of vaccine-induced responses in susceptible populations reveal a clear need for a better understanding of antiviral protection. CD8 T cell responses can provide broader protection from divergent influenza virus strains by recognizing conserved viral epitopes [6]. Differentiation and expansion of CD8 T cells are important for antiviral responses, but identifying the specific CD8 T cell subsets responsible for protection or lack thereof in aging humans as well as the mechanisms and factors contributing to their development still remains unclear. Our data correlate transcriptional regulators that integrate signals and drive CD8 T cell differentiation and dysfunction, underscoring the important role transcription factors play in CD8 T cell responses [46, 60]. The understanding and manipulation of transcriptional regulators of CD8 T cell subsets may therefore lead to better responses and protection by more functional CD8 T cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by U. S. National Institutes of Health grants P01-A1078897 and U19-AI082630 to G.J.F. and E.J.W. and U. S. grant BAA 266200500030C to G.J.F., K.E.S., and E.J.W.
The authors thank the members of the Wherry lab for discussions and critical reading of the manuscript and Sarah J. Ratcliffe for statistical analysis.
SEE CORRESPONDING EDITORIAL ON PAGE 819
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- Eomes
- eomesodermin
- KLRG1
- killer cell lectin-like receptor G1
- LAG3
- lymphocyte-activation gene 3
- MFI
- mean fluorescence intensity
- NP
- nucleoprotein
- PD-1
- programmed death 1
- SEF
- Staphylococcal enterotoxin F
- SPICE
- Simplified Presentation of Incredibly Complex Evaluations
AUTHORSHIP
D.V.D., S.A.D., K.E.S., and E.J.W. designed the experiments. D.V.D., K.D.M., A.M.P., and S.A.D. performed the experiments and collected data. G.J.F. and H.P. contributed critical reagents. D.V.D. analyzed the data. D.V.D. and E.J.W. wrote the manuscript.
DISCLOSURES
E.J.W. and G.J.F. disclose patents related to the PD-1 pathway.
REFERENCES
- 1. Centers for Disease Control and Prevention (2010) Estimates of deaths associated with seasonal influenza—United States, 1976–2007. Morbidity and Mortality Weekly Report (MMWR) 59, 1057–1062 [PubMed] [Google Scholar]
- 2. Fulton R. B., Varga S. M. (2009) Effects of aging on the adaptive immune response to respiratory virus infections. Aging Health 5, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson W. W., Shay D. K., Weintraub E., Brammer L., Cox N., Anderson L. J., Fukuda K. (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 [DOI] [PubMed] [Google Scholar]
- 4. Topham D. J., Tripp R. A., Doherty P. C. (1997) CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159, 5197–5200 [PubMed] [Google Scholar]
- 5. Guo H., Santiago F., Lambert K., Takimoto T., Topham D. J. (2011) T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J. Virol. 85, 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen J. P., Doherty P. C., Branum K. C., Riberdy J. M. (2000) Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J. Virol. 74, 11690–11696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan N., Hislop A., Gudgeon N., Cobbold M., Khanna R., Nayak L., Rickinson A. B., Moss P. A. (2004) Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173, 7481–7489 [DOI] [PubMed] [Google Scholar]
- 8. McElhaney J. E., Ewen C., Zhou X., Kane K. P., Xie D., Hager W. D., Barry M. B., Kleppinger A., Wang Y., Bleackley R. C. (2009) Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 27, 2418–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou X., McElhaney J. E. (2011) Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine 29, 2169–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koch S., Larbi A., Derhovanessian E., Ozcelik D., Naumova E., Pawelec G. (2008) Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immunity Ageing 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagar L. E., Gentleman B., Pircher H., McElhaney J. E., Watts T. H. (2011) Influenza-specific T cells from older people are enriched in the late effector subset and their presence inversely correlates with vaccine response. PLoS ONE 6, e23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Decman V., Laidlaw B. J., Dimenna L. J., Abdulla S., Mozdzanowska K., Erikson J., Ertl H. C., Wherry E. J. (2010) Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J. Immunol. 184, 5151–5159 [DOI] [PubMed] [Google Scholar]
- 13. Akbar A. N., Fletcher J. M. (2005) Memory T cell homeostasis and senescence during aging. Curr. Opin. Immunol. 17, 480–485 [DOI] [PubMed] [Google Scholar]
- 14. Hoji A., Connolly N. C., Buchanan W. G., Rinaldo C. R., Jr., (2007) CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin. Vaccine Immunol. 14, 74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brenchley J. M., Karandikar N. J., Betts M. R., Ambrozak D. R., Hill B. J., Crotty L. E., Casazza J. P., Kuruppu J., Migueles S. A., Connors M., Roederer M., Douek D. C., Koup R. A. (2003) Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 [DOI] [PubMed] [Google Scholar]
- 16. McElhaney J. E., Effros R. B. (2009) Immunosenescence: what does it mean to health outcomes in older adults? Curr. Opin. Immunol. 21, 418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Effros R. B., Boucher N., Porter V., Zhu X., Spaulding C., Walford R. L., Kronenberg M., Cohen D., Schachter F. (1994) Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp. Gerontol. 29, 601–609 [DOI] [PubMed] [Google Scholar]
- 18. Voehringer D., Koschella M., Pircher H. (2002) Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100, 3698–3702 [DOI] [PubMed] [Google Scholar]
- 19. Joshi N. S., Cui W., Chandele A., Lee H. K., Urso D. R., Hagman J., Gapin L., Kaech S. M. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henson S. M., Akbar A. N. (2009) KLRG1—more than a marker for T cell senescence. Age (Dordr) 31, 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinrichs C. S., Borman Z. A., Gattinoni L., Yu Z., Burns W. R., Huang J., Klebanoff C. A., Johnson L. A., Kerkar S. P., Yang S., Muranski P., Palmer D. C., Scott C. D., Morgan R. A., Robbins P. F., Rosenberg S. A., Restifo N. P. (2011) Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 117, 808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaech S. M., Tan J. T., Wherry E. J., Konieczny B. T., Surh C. D., Ahmed R. (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 23. Gallimore A., Glithero A., Godkin A., Tissot A. C., Pluckthun A., Elliott T., Hengartner H., Zinkernagel R. (1998) Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zajac A. J., Blattman J. N., Murali-Krishna K., Sourdive D. J., Suresh M., Altman J. D., Ahmed R. (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wherry E. J. (2011) T cell exhaustion. Nat. Immunol. 12, 492–499 [DOI] [PubMed] [Google Scholar]
- 26. Barber D. L., Wherry E. J., Masopust D., Zhu B., Allison J. P., Sharpe A. H., Freeman G. J., Ahmed R. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 [DOI] [PubMed] [Google Scholar]
- 27. Clambey E. T., White J., Kappler J. W., Marrack P. (2008) Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc. Natl. Acad. Sci. USA 105, 12997–13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henson S. M., Macaulay R., Franzese O., Akbar A. N. (2012) Reversal of functional defects in highly differentiated young and old CD8 T cells by PDL blockade. Immunology 135, 355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lages C. S., Lewkowich I., Sproles A., Wills-Karp M., Chougnet C. (2010) Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell 9, 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Decman V., Laidlaw B. J., Doering T. A., Leng J., Ertl H. C., Goldstein D. R., Wherry E. J. (2012) Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 188, 1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bengsch B., Seigel B., Ruhl M., Timm J., Kuntz M., Blum H. E., Pircher H., Thimme R. (2010) Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6, e1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrovas C., Chaon B., Ambrozak D. R., Price D. A., Melenhorst J. J., Hill B. J., Geldmacher C., Casazza J. P., Chattopadhyay P. K., Roederer M., Douek D. C., Mueller Y. M., Jacobson J. M., Kulkarni V., Felber B. K., Pavlakis G. N., Katsikis P. D., Koup R. A. (2009) Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J. Immunol. 183, 1120–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akbar A. N., Henson S. M. (2011) Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 11, 289–295 [DOI] [PubMed] [Google Scholar]
- 34. Intlekofer A. M., Takemoto N., Wherry E. J., Longworth S. A., Northrup J. T., Palanivel V. R., Mullen A. C., Gasink C. R., Kaech S. M., Miller J. D., Gapin L., Ryan K., Russ A. P., Lindsten T., Orange J. S., Goldrath A. W., Ahmed R., Reiner S. L. (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 [DOI] [PubMed] [Google Scholar]
- 35. Kao C., Oestreich K. J., Paley M. A., Crawford A., Angelosanto J. M., Ali M. A., Intlekofer A. M., Boss J. M., Reiner S. L., Weinmann A. S., Wherry E. J. (2011) Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pearce E. L., Mullen A. C., Martins G. A., Krawczyk C. M., Hutchins A. S., Zediak V. P., Banica M., DiCioccio C. B., Gross D. A., Mao C. A., Shen H., Cereb N., Yang S. Y., Lindsten T., Rossant J., Hunter C. A., Reiner S. L. (2003) Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 [DOI] [PubMed] [Google Scholar]
- 37. Szabo S. J., Sullivan B. M., Stemmann C., Satoskar A. R., Sleckman B. P., Glimcher L. H. (2002) Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 295, 338–342 [DOI] [PubMed] [Google Scholar]
- 38. Intlekofer A. M., Takemoto N., Kao C., Banerjee A., Schambach F., Northrop J. K., Shen H., Wherry E. J., Reiner S. L. (2007) Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204, 2015–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cai G., Anumanthan A., Brown J. A., Greenfield E. A., Zhu B., Freeman G. J. (2008) CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 9, 176–185 [DOI] [PubMed] [Google Scholar]
- 40. Epstein S. L., Kong W. P., Misplon J. A., Lo C. Y., Tumpey T. M., Xu L., Nabel G. J. (2005) Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 23, 5404–5410 [DOI] [PubMed] [Google Scholar]
- 41. McMichael A. J., Gotch F., Cullen P., Askonas B., Webster R. G. (1981) The human cytotoxic T cell response to influenza A vaccination. Clin. Exp. Immunol. 43, 276–284 [PMC free article] [PubMed] [Google Scholar]
- 42. Bandres E., Merino J., Vazquez B., Inoges S., Moreno C., Subira M. L., Sanchez-Ibarrola A. (2000) The increase of IFN-γ production through aging correlates with the expanded CD8(+high)CD28(−)CD57(+) subpopulation. Clin. Immunol. 96, 230–235 [DOI] [PubMed] [Google Scholar]
- 43. Di Mitri D., Azevedo R. I., Henson S. M., Libri V., Riddell N. E., Macaulay R., Kipling D., Soares M. V., Battistini L., Akbar A. N. (2011) Reversible senescence in human CD4+CD45RA+CD27− memory T cells. J. Immunol. 187, 2093–2100 [DOI] [PubMed] [Google Scholar]
- 44. Appay V., Fastenackels S., Katlama C., Ait-Mohand H., Schneider L., Guihot A., Keller M., Grubeck-Loebenstein B., Simon A., Lambotte O., Hunt P. W., Deeks S. G., Costagliola D., Autran B., Sauce D. (2011) Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 25, 1813–1822 [DOI] [PubMed] [Google Scholar]
- 45. Rutishauser R. L., Kaech S. M. (2010) Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol. Rev. 235, 219–233 [DOI] [PubMed] [Google Scholar]
- 46. Wherry E. J., Ha S. J., Kaech S. M., Haining W. N., Sarkar S., Kalia V., Subramaniam S., Blattman J. N., Barber D. L., Ahmed R. (2007) Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 [DOI] [PubMed] [Google Scholar]
- 47. Paley M. A., Kroy D. C., Odorizzi P. M., Johnnidis J. B., Dolfi D. V., Barnett B. E., Bikoff E. K., Robertson E. J., Lauer G. M., Reiner S. L., Wherry E. J. (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie D., McElhaney J. E. (2007) Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mech. Ageing Dev. 128, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pawelec G., Akbar A., Caruso C., Solana R., Grubeck-Loebenstein B., Wikby A. (2005) Human immunosenescence: is it infectious? Immunol. Rev. 205, 257–268 [DOI] [PubMed] [Google Scholar]
- 50. Betts M. R., Nason M. C., West S. M., De Rosa S. C., Migueles S. A., Abraham J., Lederman M. M., Benito J. M., Goepfert P. A., Connors M., Roederer M., Koup R. A. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Focosi D., Bestagno M., Burrone O., Petrini M. (2010) CD57+ T lymphocytes and functional immune deficiency. J. Leukoc. Biol. 87, 107–116 [DOI] [PubMed] [Google Scholar]
- 52. Henson S. M., Franzese O., Macaulay R., Libri V., Azevedo R. I., Kiani-Alikhan S., Plunkett F. J., Masters J. E., Jackson S., Griffiths S. J., Pircher H-P., Soares M. V. D., Akbar A. N. (2009) KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113, 6619–6628 [DOI] [PubMed] [Google Scholar]
- 53. Gamberg J., Pardoe I., Bowmer M. I., Howley C., Grant M. (2004) Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol. Cell Biol. 82, 38–46 [DOI] [PubMed] [Google Scholar]
- 54. Vezys V., Yates A., Casey K. A., Lanier G., Ahmed R., Antia R., Masopust D. (2009) Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457, 196–199 [DOI] [PubMed] [Google Scholar]
- 55. Wirth T. C., Xue H. H., Rai D., Sabel J. T., Bair T., Harty J. T., Badovinac V. P. (2010) Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity 33, 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nolz J. C., Harty J. T. (2011) Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity 34, 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lang A., Nikolich-Zugich J. (2011) Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J. Immunol. 187, 3759–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Van de Berg P. J., Griffiths S. J., Yong S. L., Macaulay R., Bemelman F. J., Jackson S., Henson S. M., ten Berge I. J., Akbar A. N., van Lier R. A. (2010) Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J. Immunol. 184, 3417–3423 [DOI] [PubMed] [Google Scholar]
- 59. Bucks C. M., Norton J. A., Boesteanu A. C., Mueller Y. M., Katsikis P. D. (2009) Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J. Immunol. 182, 6697–6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaech S. M., Hemby S., Kersh E., Ahmed R. (2002) Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.