Abstract
Purpose of review
Dipeptidyl peptidase 4 (DPP4, CD26] is a protease that cleaves selected amino acids at the N-terminal penultimate position and has the potential to alter the protein function. The regulation and roles of DPP4 activity are not well understood; therefore, the purpose of this review is to discuss the recent literature regarding DPP4 regulation, as well as the variety of molecules it may affect, and their potential clinical applications.
Recent findings
Recent insight into the number of proteins that have DPP4 sites, and how DPP4 truncation may alter hematopoiesis based on the protein full length vs. truncated state, has shown that DPP4 truncation of colony-stimulating factors (CSFs) alters their function and that the activity of these CSFs can be enhanced when DPP4 activity is inhibited. DPP4 inhibition has recently been used in a clinical trial to attempt to enhance the engraftment of cord blood cells, and an endogenous DPP4 inhibitor tissue factor pathway inhibitor has been discovered, increasing our understanding of the potential importance of DPP4.
Summary
DPP4 plays a role in regulating the activity of CSFs and other cytokines involved in hematopoiesis. This information may be useful for enhancing hematopoietic cell transplantation, blood cell recovery after stress, and for understanding the physiology and pathophysiology of blood and other cell systems.
Keywords: CD26, cord blood transplant, dipeptidyl peptidase 4, hematopoiesis
INTRODUCTION
Hematopoiesis is a highly regulated process involving sell-renewal and proliferation of hematopoietic stem cells (HSCs), as well as differentiation of HSCs into the various cell lineages that form the blood and immune system. Under the control of specific cytokines or growth factors, such as interleukins and colony-stimulating factors (CSFs), multipotent HSCs are capable of differentiating into hematopoietic progenitor cells (HPCs) and mature blood cells [1–3]. As our knowledge of the regulation and functions of HSCs/HPCs rapidly accelerates, a better understanding of how modifications of cytokines, chemokines, and other growth factors [3] may alter HSCs/HPCs homeostasis should provide us with innovative approaches to manipulate these proteins, and cells, for both basic research information and clinical utility.
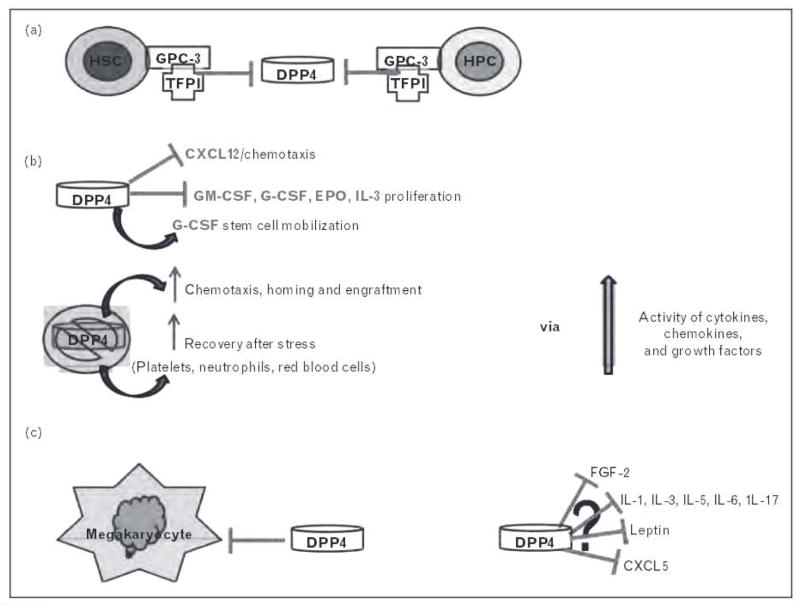
One modification that has recently been shown to be important in the regulation of some hematopoietically active factors is N-terminal truncation of selected proteins by dipeptidyl peptidase 4 (DPP4, CD26). DPP4 was discovered in 1966 and is a 110-kDa member of the prolyl oligopeptidase family [4]. It functions as a cell surface serine protease, selectively cleaving the N-terminal penultimate proline or alanine amino acids, though kinetic studies of its catalytic activity suggest that other amino acids in the second position may be able to be cleaved as well. Currently, the regulation of DPP4 is unclear, but new literature outlined in Fig. 1a shows that the tissue factor pathway inhibitor (TFPI) acts as a biological inhibitor of DPP4 via glypican-3 (GPC3), a heparan sulfate proteoglycan present on murine and human HSCs/HPCs. Treatment of HSCs/HPCs with TFPI in vitro resulted in increased chemotaxis toward CXCL12 and increased transplant homing and engraftment [5▪▪]. Bone marrow cells from GPC3 knockout mice showed higher DPP4 activity, increased HSC/HPC proliferation, along with lower HSC maintenance, which resulted in increased circulating HSCs/HPCs and decreased bone marrow HSCs [5▪▪].
FIGURE 1.
Dipeptidyl peptidase 4 (DPP4) can modulate a variety of factors that alter stem and progenitor cell maturation and function. (a) Tissue factor protein inhibitor (TFPI), primarily from endothelial cells, acts as a biological inhibitor of DPP4 via glypican-3 (GPC3). HSC, hematopoietic stem cell; HPC, hematopoietic progenitor cell, (b) Overview of factors shown to be influenced by activity or inhibition of DPP4. (c) DPP4 acts as a negative regulator of megakaryocytes (Lin CD41 +CD61 +] as well as megakaryocyte progenitor cells (Lin CD41 +CD61 +c-kit+Sca-1). (d) Proteins with putative DPP4 truncation sites whose function may be altered by truncation.
DPP4 is found as both a type II cell surface protein as well as a soluble form that lacks the intracellular and transmembrane domains [6]. The soluble form of DPP4 is found in body fluids such as serum/plasma, cerebrospinal fluid, synovial fluid, and semen [7,8]. The transmembrane form of the protein is relatively ubiquitously expressed in many tissues including, but not limited to, the bone marrow, the venular end of blood vessels, the lung, spleen, pancreas, kidney, liver, intestines, and many types of epithelial cells [9,10]. Within blood cells, DPP4 is expressed on HSCs/HPCs as well as other more mature cells and is expressed at high levels on memory T cells [3], The enzymatic activity of DPP4 has been shown to be important in steady-state regulation of cellular functions, as well as modulating, and being modulated in, certain disease states and cell types [11–14]. The importance of DPP4 in specific cell types as well as physiological and pathophysiological settings, and most recently in hematology, is just starting to be fully realized and will be discussed in this review.
DIPEPTIDYL PEPTIDASE 4 IN HEMATOPOIESIS AND CORD BLOOD TRANSPLANTATION
Foundational studies from our laboratory, shown in Fig. 1b, demonstrated the role of DPP4 in the regulation of CXCL12 [stromal cell-derived factor 1 (SDF-1)], stem cell mobilization with granulocyte-colony stimulating factor (G-CSF), as well as the ability of DPP4 to modulate stem cell homing and engraftment [15–20]. Recently, the effect of DPP4 on the hematopoietic system has been more widely investigated. Megakaryocytes influence quiescence of HSCs directly through its receptor c-Mpl or indirectly by secreting many cytokines that affect the proliferation and survival of HSCs. Megakaryocyte development was investigated in DPP4−/− mice. Although there is no major effect of DPP4 knockout on peripheral blood counts in DPP4−/− mice, interestingly, the number of megakaryocytes (Lin−CD41+CD61+) as well as megakaryocyte progenitor cells (Lin−CD41+CD61+c-kit+Sca-1−) were increased in the isolated bone marrow from DPP4−/− mice (Fig. 1c). These findings were further supported by the results suggesting that absence of negative regulation through DPP4 cleavage may be beneficial for immature megakaryocyte progenitors, although the mechanism remains to be determined [21]. DPP4 is expressed on mononuclear blood cells (MNCs) from cord blood, bone marrow, and mobilized peripheral blood, and inhibition of endogenous DPP4 using diprotin A (a tri-peptide inhibitor of DPP4 activity) enhanced the migration of MNCs to CXCL12 from all three cell sources [22▪]. The modulation of the CXCL12/CXCR4 axis via DPP4 for recruitment and homing of bone-marrow-derived stem cells is not limited to HSCs and hematopoietic cell transplantation. DPP4 has been shown to be necessary for G-CSF-induced mobilization [16,17,23], and inhibition of DPP4 has been shown to increase the transplant efficiency [15,19,24] and homing of stem cells to bone marrow regardless of how the stem cells were mobilized [22▪].
A recent study reported on the efficacy of diprotin A in stimulating muscle cell engraftment by modulation of the CXCL12/CXCR4 axis [25]. Targeting the CXCL12 axis through DPP4 inhibition also benefits ischemia–reperfusion injury in mouse lung transplantation [26] and homing of stem cells to the ischemic heart [27]. Recent studies from our laboratory [28▪▪] have shown that DPP4 is able to cleave human and mouse growth factors, and that DPP4 acts a regulatory component in hematopoiesis (Fig. 1b). Granulocyte macrophage-colony stimulating factor (GM-CSF), G-CSF, CXCL12, erythropoietin (EPO), and IL-3 were shown to have an N-terminal site for DPP4 cleavage which resulted in a protein that was not fully functional but was able to block the function of the full-length protein, similar to a dominant negative effect. Truncated GM-CSF was shown to bind the GM-CSF receptor (GM-CSFR) with higher affinity than full-length GM-CSF, but had blunted ability to produce proliferative signaling, potentially because of either preventing hexamer to dodecamer formation of the GM-CSFR complex needed for full GM-CSF activity or the truncated GM-CSF being unable to fully signal through the dodecamer GM-CSFR complex. Inhibition of DPP4 with sitagliptin (Januvia), or the use of DPP4−/− mice, resulted in increased recovery of blood neutrophils, platelets, and HPCs after the treatment of mice with chemotherapy or radiation, and increased engraftment after HSC transplant was also seen if DPP4 was inactive or inhibited [28▪].
Although DPP4 inhibition via sitagliptin is clinically used for the treatment of type 2 diabetes, it has only recently been utilized in a clinical hematopoietic setting. Delayed engraftment is one of the major cavets associated with cord blood transplantation because of diminished HSC/HPC numbers; therefore, sitagliptin was recently utilized to possibly enhance the engraftment in single-unit cord blood transplantations in adults with hematopoietic malignancies [29▪]. This was the first clinical trial to evaluate the safety and efficacy of DPP4 inhibition on engraftment following cord blood transplantation. Most patients in this trial had 4 out of 6 HLA-matched grafts and active marrow disease at the time of transplantation. Inhibition of DPP4 exhibited promising engraftment kinetics, including a median time to neutrophil engraftment of 21 days and 94% (95% confidence interval 84–100%) engraftment at 50 days in patients receiving red cell depleted grafts. This study suggests that with further optimization of inhibition of DPP4 activity with sitagliptin, which was not optimal in this study, the inhibition of DPP4 may be a promising treatment to decrease the time to engraftment after cord blood transplantation, even though more work is required to definitively prove that sitagliptin enhances the time to neutrophil engraftment. Another study observing the expression of DPP4 on human mobilized peripheral blood stem cells harvested from cancer patients or allogeneic-related donors suggested that DPP4 expression significantly correlated with while blood cell engraftment and could serve as an early predictor of engraftment [30]. This highlights the importance of understanding how expression vs. activity of DPP4 may be predictive of engraftment and could potentially be altered with different tissue sources of cells used for transplantation. The studies presented above suggest that although more investigation into the regulation and potential clinical applications of DPP4 and its inhibition are needed, modulating DPP4 activity may serve as a novel therapeutic tool for transplantation. This highlights the importance of determining the proteins that have DPP4 truncation sites and establishing how their function may be changed, and potentially alter hematopoiesis, after protein truncation.
POTENTIAL REGULATORY ROLE FOR DIPEPTIDYL PEPTIDASE 4 THROUGH THE MICROENVIRONMENT
By searching through the NCBI database and Uniprot, our laboratory has identified a large number of proteins that have putative truncation sites for DPP4 (Ou, O’Leary, Broxmeyer unpublished observation), including chemokines/cytokines, growth factors, and additional modulatory factors involved in hematopoiesis and the regulation of other tissues and cells. Many papers have been published this year that focus on proteins that are expressed by the microenvironment or HSCs/HSPCs that regulate hematopoiesis, thus leading us to contemplate whether the factors being investigated are in their full length or truncated form and how this might alter the findings and interpretations of such studies.
For example, a recent publication suggested that parathyroid hormone (PTH) stimulated IL-6 protein levels in primary osteoblast cultures in vitro, and bone marrow in vivo, and subsequently IL-6 induced transforming growth factor beta (TGF-β) release from osteoblasts, resulting in increased expansion of myeloid cells in the bone marrow microenvironment [31]. This is interesting as human and mouse PTH and TGF-β do not have a DPP4 truncation site, but IL-6 does, suggesting the possibility that alterations in DPP4 levels could influence IL-6 activity and subsequently alter the induction of TGF-β. A recent study also showed that stimulation of osteoblasts or endothelial cells with fibroblast growth factor-2 (FGF-2; both murine and human forms have putative DPP4 truncation sites) resulted in regulation of the chemokine CXCL5 (which has a murine DPP4 site), which induced the migration of HSCs [CD34( /low)LSK cells], suggesting that these factors may be involved in HSC mobilization from the osteoblast niche in bone marrow to peripheral blood [32], FGF-2 treatment in vitro has been shown to promote HSC/HPC expansion, and recently in-vivo treatment with FGF-2 showed the expansion of stromal cells. These cell populations included perivascular Nestin (+) supportive stromal cells and expansion of a heterogeneous population of undifferentiated HSCs/HPCs, which the authors suggested may facilitate the expansion of these cells by increasing stem cell factor and reducing CXCL12 via mir-31 upregulation [33]. DPP4 has the potential to play multiple roles here as CXCL12 has shown functional alterations [18,19,28▪▪] when truncated by DPP4 and FGF-2 has a putative DDP4 truncation site.
POTENTIAL REGULATORY ROLES FOR DIPEPTIDYL PEPTIDASE 4 IN INFLAMMATION AND DISEASE
Proinflammatory cytokines such as IL-1 (human and murine), IL-5 (human), IL-6 (human and murine), IL-13 (human), and IL-17 (mouse/human), which have putative DPP4 truncation sites, do not only affect the local microenvironment of HSCs, but also have a direct role in HSC/HPC activity. A recent study suggested that IL-17 affects erythropoiesis by promoting the development of primitive erythroid precursors, BFU-E, but suppressing the growth of later-stage erythroid progenitors, CFU-E, and the effects might be through MAPK signaling pathways [34]. Another study has demonstrated that IL-33 stimulates minimal eosinophil hematopoiesis through direct interactions with mouse bone marrow progenitors ex vivo, whereas it antagonizes IL-5-mediated eosinophil differentiation from committed bone marrow cells. The antagonism of IL-5 was mediated in part by GM-CSF [35], both of which have a DPP4 truncation site. Taken together, manipulation of these and other cytokines, via DPP4 inhibition, may have potential effects on inflammatory processes as well as hematopoietic cell development.
In addition to hematopoiesis in normal physiological states, pathophysiological conditions such as obesity that alter hematopoiesis at baseline could be influenced by DPP4 modification of proteins that have truncation sites. For example, recent studies using high-fat diet (HFD) models have shown an increase in leptin (which has a putative DPP4 site in both its human and mouse forms) as well as an increase in myelopoiesis and lymphopoiesis [36]. Specifically, they found a continued escalation in the numbers of cells found in bone marrow and thymus of HFD mice from day 90 to day 180, The numbers of monocytes, granulocytes, erythrocytes, and mixed progenitor lineages remained normal in the marrow, but the percentage of lymphocytes increased [36]. However, DPP4 may be more relevant during stress compared to homeostatic conditions, as we have shown for hematopoiesis [28▪▪]. Another HFD model recently suggested that HFD rats had increased IL-1, IL-6, and TNF-α production by bone marrow mesenchymal stem cells [37]. IL-1 and IL-6, which have DPP4 truncation sites for human, mouse, and rat (as well as TNF-α which does not have a DPP4 site), are commonly increased in inflammation and have been implicated in the negative regulation of lymphopoiesis, making it tempting to speculate that alterations in DPP4 activity may play a role in the microenvironmental regulation of hematopoiesis at multiple levels.
CONCLUSION
We have discussed the aspects of the recently published work on DPP4 and its recently published, and potential, involvement in hematopoiesis, affecting HSCs/HPCs as well as the supportive cells of the bone marrow microenvironment. A large number of factors with putative DPP4 truncation sites have been identified (of which a very limited list of proteins are shown in Fig. 1d) which present an important and emerging facet of the potential roles for DPP4 in cellular regulation through modification of certain proteins. Because of the complexity of DPP4, the overall effects may be altered by localized or tissue-specific expression of DPP4/CD26 and chemokines/cytokines/growth factors, and the presence of a disease state or modified physiological homeostasis. Biochemical and biological (in vitro and in vivo) studies are needed to verify, for each protein identified as having a putative DPP4 truncation site, whether the putative truncation sites are real, especially with regard to differing alanine/proline vs. other penultimate amino acids at the N-terminus of each molecule. Most importantly, it must be determined whether the truncated form has modified activity (up or down), compared to its full-length form, and whether the truncated form can modulate the activity of the full length form, and if so, how. Another point to note is that the relevance of DPP4 and the applicability of rodent models in translating to human disease states need to be carefully evaluated. Not all responses are the same in the mouse/rat as in the human and we have found that not all human proteins that have the truncation site also have the site in mouse, and vice versa, further highlighting the importance of discriminating between putative and validated DPP4 truncation sites.
KEY POINTS.
DPP4 can cleave hematopoietic factors and modulate their function.
Inhibition of DPP4 may increase cord blood transplantation efficiency.
Many proteins have putative DPP4 truncation sites that are thus far unstudied with regard to functional alterations.
Acknowledgments
H.A.O. is supported by a postdoctoral stipend from NIH T32 DK07519 to H.E.B. The laboratory work of these investigators is supported by the Public Health Service Grants from the NIH to H.E.B.: R01 HL056416, R01 HL67384, R01 HL112669, and P01 DK090948.
Footnotes
Conflicts of interest
H.E.B. is on the MSAB of Corduse, a cord blood banking company. The other authors have no financial interests to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as.
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 394–395).
- 1.Shaheen M, Broxmeyer HE. Hematopoietic cytokines and growth factors. In: Broxmeyer HE, editor. Cord blood biology, transplantation, banking, and regulation. Bethesda, MD: AABB Press; 2011. pp. 35–74. [Google Scholar]
- 2.Shaheen M, Broxmeyer HE. Principles of cytokine signalling. In: Hoffman R, Benz EJ Jr, Silberstein LE, et al., editors. Hematology: basic principles and practice. 6. Chapter 14. Elsevier Churchill Livingstone; Philadelphia: 2012. pp. 136–146. [Google Scholar]
- 3.Campbell TB, Broxmeyer HE. CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front Biosci. 2008;13:1795–1805. doi: 10.2741/2800. [DOI] [PubMed] [Google Scholar]
- 4.Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie. 1966;7:197–201. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Khurana S, Margamuljana L, Joseph C, et al. Glypican-3 mediated inhibition of CD26 by TFPI: a novel mechanism in hematopoietic stem cell homing and maintenance. Blood. 2013 doi: 10.1182/blood-2012-09-456715. Epub ahead of print. This is the first study to show a biological inhibitor of DPP4 that specifically has an impact on hematopoietic stem and progenitor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 7.Solau-Gervais E, Zerimech F, Lemaire R, et al. Cysteine and serine proteases of synovial tissue in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2007;36:373–377. doi: 10.1080/03009740701340172. [DOI] [PubMed] [Google Scholar]
- 8.Lambeir AM, Diaz Pereira JF, Chacon P, et al. A prediction of DPP IV/CD26 domain structure from a physicochemical investigation of dipeptidyl peptidase IV (CD26) from human seminal plasma. Biochim Biophys Acta. 1997;1340:215–226. doi: 10.1016/s0167-4838(97)00045-9. [DOI] [PubMed] [Google Scholar]
- 9.Bonig H, Priestley GV, Oehler V, Papayannopoulou T. Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC. Exp Hematol. 2007;35:326–334. doi: 10.1016/j.exphem.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grim M, Carlson BM. Alkaline phosphatase and dipeptidylpeptidase IV staining of tissue components of skeletal muscle: a comparative study. J Histochem Cytochem. 1990;38:1907–1912. doi: 10.1177/38.12.1701462. [DOI] [PubMed] [Google Scholar]
- 11.Shigeta T, Aoyama M, Bando YK, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. doi: 10.1161/CIRCULATIONAHA.112.096479. [DOI] [PubMed] [Google Scholar]
- 12.Moran GW, O’Neill C, Padfield P, McLaughlin JT. Dipeptidyl peptidase-4 expression is reduced in Crohn’s disease. Regul Pept. 2012;177:40–45. doi: 10.1016/j.regpep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, Seigel B, Flecken T, et al. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26) J Immunol. 2012;188:5438–5447. doi: 10.4049/jimmunol.1103801. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki M, Kato M, Tanaka K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in nonalcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 15.Campbell TB, Hangoc G, Liu Y, et al. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 16.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 17.Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF induced progenitor cell mobilization as determined by CD26−/− mice. Exp Hematol. 2003;31:1126–1134. doi: 10.1016/j.exphem.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/DPP4 dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 19.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Hangoc G, Bian H, et al. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 21.Kidd S, Bueso-Ramos C, Jagan S, et al. In vivo expansion or the megakaryocyte progenitor cell population in adult CD26-deficient mice. Exp Hematol. 2011;39:580.e1–590.e1. doi: 10.1016/j.exphem.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 22▪.Christopherson KW, 2nd, Frank RR, Jagan S, et al. CD26 protease inhibition improves functional response of unfractionated cord blood, bone marrow, and mobilized peripheral blood cells to CXCL12/SDF-1. Exp Hematol. 2012;40:945–952. doi: 10.1016/j.exphem.2012.07.009. This study shows that inhibition of DPP4 is functionally relevant regardless of the origin, or mobilization, of stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganessi LA, Walker AL, Tan LL, et al. Effective mobilization of hematopoietic progenitor cells in G-CSF mobilization defective CD26−/− mice through AMD3100-induced disruption of the CXCL12–CXCR4 axis. Exp Hematol. 2011;39:384–390. doi: 10.1016/j.exphem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo E, Paganessi LA, Alikhan WA, et al. Loss of CD26 protease activity in recipient mice during hematopoietic stem cell transplantation results in improved transplant efficiency. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03826.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker MH, Loretz C, Tyler AE, et al. Inhibition of CD26/DPP-IV enhances donor muscle cell engraftment and stimulates sustained donor cell proliferation. Skelet Muscle. 2012;2:4. doi: 10.1186/2044-5040-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jungraithmayr W, De Meester I, Matheeussen V, et al. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg. 2012;41:1166–1173. doi: 10.1093/ejcts/ezr180. [DOI] [PubMed] [Google Scholar]
- 27.Huber BC, Brunner S, Segeth A, et al. Parathyroid hormone is a DPP-IV inhibitor and increases SDF-1-driven homing of CXCR4(+) stem cells into the ischaemic heart. Cardiovasc Res. 2011;90:529–537. doi: 10.1093/cvr/cvr014. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–1796. doi: 10.1038/nm.2991. This study is the first to show functional alterations of colony-stimulating factors (CSFs) because of DPP4 truncation and the enhancement of the activity of these CSFs when DPP4 activity is inhibited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013 doi: 10.1089/scd.2012.0636. Epub ahead of print. This study is the first clinical trial evaluating the potential of DPP4 inhibition to increase the engraftment of patients receiving cord blood transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhash K, Khattry N, Bakshi A, et al. CD26 expression in donor stem cell harvest and its correlation with engraftment in human haematopoietic stem cell transplantation: potential predictor of early engraftment. Ann Oncol. 2010;21:582–588. doi: 10.1093/annonc/mdp342. [DOI] [PubMed] [Google Scholar]
- 31.Cho SW, Pirih FQ, Koh AJ, et al. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. J Biol Chem. 2013;288:6814–6825. doi: 10.1074/jbc.M112.393363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon KA, Cho HS, Shin HI, Cho JY. Differential regulation of CXCL5 by FGF2 in osteoblastic and endothelial niche cells supports hematopoietic stem cell migration. Stem Cells Dev. 2012;21:3391–3402. doi: 10.1089/scd.2012.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itkin T, Ludin A, Gradus B, et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood. 2012;120:1843–1855. doi: 10.1182/blood-2011-11-394692. [DOI] [PubMed] [Google Scholar]
- 34.Krstic A, Kocic J, Ilic V, et al. Effects of IL-17 on erythroid progenitors growth: involvement of MAPKs and GATA transcription factors. J Biol Regul Homeost Agents. 2012;26:641–652. [PubMed] [Google Scholar]
- 35.Dyer KD, Percopo CM, Rosenberg HF. IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent eosinophil hematopoiesis ex vivo. Immunol Lett. 2013;150:41–47. doi: 10.1016/j.imlet.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci USA. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortez M, Carmo LS, Rogero MM, et al. A high-fat diet increases IL-1, IL-6, and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]