Abstract
Analyses of the Drosophila hematopoietic system are becoming more and more prevalent as developmental and functional parallels with vertebrate blood cells become more evident. Investigative work on the fly blood system has, out of necessity, led to the identification of new molecular markers for blood cell types and lineages and to the refinement of useful molecular genetic tools and analytical methods. This review briefly describes the Drosophila hematopoietic system at different developmental stages, summarizes the major useful cell markers and tools for each stage, and provides basic protocols for practical analysis of circulating blood cells and of the lymph gland, the larval hematopoietic organ.
Introduction
Blood cells have been studied in invertebrate animal models for more than one hundred years, although how these cells relate to vertebrate blood cells in function and development has only recently begun at the molecular genetic level. This work is highlighted by studies in the fruit fly Drosophila melanogaster, an established premier model system for genetic studies in other contexts. The earliest studies of blood cells (known as hemocytes) in Drosophila date back to the late 1800s, however significant progress in this area did not begin in earnest until the 1950s when Rizki and Rizki began their studies of blood cell types and associated functions, particularly in the areas of innate immune responses and self-/non-self-recognition, and established the nomenclature for the Drosophila blood cell system that is still in use today [1, 2]. Furthermore, their observational work led to the earliest suggestion that the various Drosophila blood cell types arise from a common precursor cell type, an idea similar to that which would be established in the mouse within the next few years [3].
Since that time, with the advent of molecular genetics, quite a bit has been discovered about how Drosophila blood cells are specified and develop and how this relates to blood-forming processes in vertebrate systems. Much of this knowledge has been acquired with the identification and use of many new cellular markers, genetic tools, and analytical methods. This review will briefly describe the key elements of the Drosophila hematopoietic system and highlight useful reagents and approaches that are currently available.
The Drosophila hematopoietic system
Mature Drosophila blood cells are found as at least three distinct types known as plasmatocytes, crystal cells and lamellocytes. The plasmatocyte behaves as a macrophage-like cell and is the predominant cell type comprising more than 90% of the hemocyte repertoire [1, 4]. Crystal cells, named for their cytoplasmic paracrystalline protein inclusions, represent approximately 5% of hemocytes in circulation while lamellocytes are rarely observed. Lamellocytes can be induced to differentiate, however, in the context of various conditions including immune challenge by parasitic wasps [5-7]. Several additional cell types have been described based on morphological features or as precursor populations lacking differentiation markers [2, 8-10], but their identification as independent cell types will require the discovery of new and unique markers.
A major function of hemocytes is in the provision of cellular innate immune responses, which is achieved primarily through the phagocytic clearance of microbial pathogens or the encapsulation of larger parasites. Additionally, plasmatocytes are known to promote the humoral immune response by secreting cytokine-like proteins and antimicrobial peptides [11-15]. Accordingly, flies with impaired plasmatocyte function are more susceptible than wild-type flies to microbial infection and are less effective in the potentiation of humoral immune responses [16-19]. Hemocytes also have roles during development where they secrete and remodel extracellular matrix components as well as remove cellular debris by phagocytosis [20-23]. These roles are highlighted by their requirement in the embryonic nervous system, the development of which is severely disrupted in the absence of plasmatocyte function [24].
As professional phagocytes, plasmatocytes use a large repertoire of receptors to identify and engulf different targets. Receptors recognizing dying cells include Croquemort (Crq), a CD36 family member [25], PSR, a phosphatidyl serine receptor [26], and Draper, which is homologous to the CED-1 phagocytosis receptor in C. elegans [27, 28]. Numerous receptors that bind microbial pathogens are also known and include the peptidoglycan recognition protein PGRP-LC and the scavenger receptor D-SR-CI [29-31]. Additionally, the receptors Eater, Nimrod (NimC1, also known as the P1 antigen) and Dscam have been found to be important in bacterial clearance. Eater and Nimrod both contain multiple EGF-like motifs, called NIM repeats, which, in the case of Eater, have been shown to bind bacteria and mediate an array of intermolecular interactions [32, 33]. Nimrod belongs to a family of ten related proteins (grouped into three classes, NimA-NimC), the majority of which are expressed by hemocytes [33]. Interestingly, the NimB proteins appear to function as opsonins because they enter the secretory pathway but lack transmembrane and cytosolic domains. Several isoforms of Dscam have also been identified in the hemolyph suggesting that it may also function to opsonize microbes [34]. The extracellular domain of Dscam receptors is composed of repeating immunoglobulin-like domains, a subset of which are highly variable due to alternative splicing [35]. Though microarray analysis has shown that more than 18,000 alternatively-spliced isoforms are expressed by hemocytes, just five isoforms comprise 80-90% of Dscam mRNAs in these cells, suggesting a blood-specific role for these particular Dscam types [34].
While plasmatocytes primarily function in phagocytic clearance, the main function of crystal cells is in the process of melanization, the darkening and hardening of tissue due to the local deposition of melanin. Melanization commonly occurs during immune responses in the context of barrier formation around pathogens too large to remove by phagocytosis, as well as during the process of wound healing, particularly when the cuticular epithelia has been breached. Phenoloxidase (PO) enzymes mediate the oxidation of phenols into quinones that then polymerize into melanin. Tightly-regulated serine protease cascades convert Prophenoloxidase (PPO) zymogens into the active PO form [36-39]. Experimental evidence indicates that the large, cytoplasmic inclusions that crystal cells exhibit (and from which they derive their name) are composed primarily of PPO [2, 5, 10, 40]. Furthermore, Drosophila mutant lines that lack crystal cells also lack PO activity in the circulating hemolymph, identifying crystal cells as the primary source of this activity [40-43]. Genetic analysis suggests that crystal cell rupture is the mechanism by which PO activity is delivered to the hemolymph, a process mediated by JNK signaling and the TNF homolog Eiger [41]. Despite the distribution of PPO throughout the hemolymph, PO activity is tightly regulated and spatially restricted, such as at sites of coagulation and clot formation [44].
Lamellocytes are the most morphologically distinct blood cell type, being large (15-40 μm across), disc-shaped cells. These cells function during the encapsulation response, where a cellular barrier, which includes plasmatocytes and crystal cells [45], forms around foreign objects that cannot be removed by phagocytosis. Normally, very few lamellocytes are observed [46]; however numerous lamellocytes can be induced to differentiate in response to signals that include parasitic wasp infestation, injection of foreign objects into the hemocoel, and sterile wounding [5, 6, 13, 47].
With regard to understanding innate immune mechanisms, Drosophila has proven to be an exceptional model that has provided key insights into systems, such as Toll-like receptor signaling, with relevance to human biology [48]. The majority of this work in flies has focused on understanding the humoral response to microbial pathogens, however the role of hemocytes in the immune context is beginning to be explored in more detail. Methodologies and protocols describing microbial challenge and the monitoring immune signaling have been previously published by Lemaitre and colleagues [49], (Editor: please add current reference if Lemaitre contributes to this issue). A detailed protocol demonstrating wasp paristization and associated analytical methods has also recently been made available [50].
Much like in vertebrate systems, hematopoietic development in Drosophila is biphasic with regard to timing and location [8, 51, 52]. Drosophila blood cells are first specified in the head mesoderm and migrate throughout the developing embryo [53]. At later stages of embryogenesis, a second group of hematopoietic precursors are specified within the cardiogenic mesoderm (thoracic segments 1-3) and form the lymph gland, a specialized organ that supports the hematopoietic process throughout subsequent larval development [54, 55]. At the end of the larval stage, the lymph gland breaks down and releases blood cells into circulation [56, 57]. Several reports have also suggested that hematopoietic cell populations exist as sessile cells in association with the internal larval body wall and/or the dorsal vessel (heart), however a better understanding of the blood-forming potential of these cells will require further exploration [58-60]. It has been demonstrated that circulating blood cells derived from both the embryonic head mesoderm and the lymph gland persist into the adult stage [56], however a major unanswered question is whether any adult blood cells arise from de novo hematopoiesis during the pupal or adult stages.
Analysis of embryonic hemocytes
Molecular genetic analysis of embryonic blood cells has made use of standard in situ hybridization and immunostaining methods in combination with specific markers. The number of genes with expression patterns overlapping with embryonic hemocytes has grown in the last several years, however with regard to early developmental analysis of blood cells derived from the head mesoderm, several markers stand out: serpent (srp), glial cells missing (gcm), lozenge (lz), Prophenoloxidase A1 (ProPOA1), collagen (Cg25c), and Peroxidasin (Pxn; see Table 1). The onset of Srp (a GATA transcriptional regulator) expression in the head mesoderm defines blood cell identity, while the expression of Gcm and Lz (a Runx family transcriptional regulator) mark the plasmatocyte and crystal cell lineages, respectively [8, 52]. Maturing macrophages express collagen and Pxn, while ProPOA1 is a marker of mature crystal cells. The lamellocyte cell type has not been reported in the embryo. Each of the described markers has been analyzed by in situ hybridization and/or antibody staining, and some excellent examples describing their specific use, either alone or in combination, can be found in Waltzer et al., (2003) [61] and Milchanowski et al., (2004) [62], among others.
Table 1.
Established genetic markers for the embryonic hematopoietic system
| Hematopoietic Cells | Marker | Genetic Reporters | Antibody* | Cell Type | References |
|---|---|---|---|---|---|
| embryonic circulating cells | srp | srp-gal4 srp-HEMO-gal4 srpD-gal4 | P | Pan | Milchanowski et al., 2004 [62] Bruckner et al., 2004 [108] Crozatier et al., 2004 [63] |
| gcm | gcm-lacZ gcm-gal4 | P | PL | Bernardoni et al., 1997 [109] Cho et al., 2002 [110] |
|
| Iz | lz-gal4 | M | CC | Lebestky et al., 2000 [52] | |
| crq | crq-gal4 | P | PL | Franc et al., 1996 [25]; Olofsson and Page, 2005 [111] | |
| proPO-A1 | P | CC | Rizki et al., 1985 [40]; Waltzer et al., 2002 [112] | ||
| CgC25 | Cg-gal4 | PL | Milchanowski et al., 2004 [62]; our observation | ||
| Pxn | Pxn-gal4 | P | PL | Nelson et al., 1994 [94]; Stramer et al., 2005 [68] | |
| embryonic lymph gland | srp | P | Pan | Jung et al., 2005 [54] | |
| odd | odd-gal4 odd-lacZ | P | Pan | Ward and Skeath, 2000 [113]; Jung et al., 2005 [54] | |
| Hand | Hand-gal4 Hand-GFP Hand-lacZ | Pan | Han et al., 2005 [114]; Evans et al., 2009 [115] | ||
| Antp | Antp-gal4 | M | PSC | Mandal et al., 2007 [64] | |
| hth | P | Pan, non-PSC | Mandal et al., 2007 [64] | ||
| col | col-gal4 | Pan, PSC | Crozatier et al., 2004 [63]; Krzemien et al., 2007 [78] | ||
| Dot | Dot-gal4 | Pan, PSC | Kimbrell et al., 2002 [116]; Jung et al., 2005 [54] |
P = polyclonal; M = monoclonal
Embryonic lymph gland cells express srp, similar to head mesoderm blood cells, however the lymph gland lineage also expresses odd skipped (odd) [54, 63]. Because of the existence of Odd antibody and the reporters odd-lacZ and odd-gal4, odd expression has been a marker of choice for identifying lymph gland cells in late-stage embryos. Expression of the homeotic gene Antennapedia (Antp) also defines the earliest subdivision (stage 11) of the lymph gland by specifying Posterior Signaling Center (PSC) cells, which behave as niche-like cells at later developmental stages [64]. The EBF factor Collier (Col, also known as Knot) is also an important regulator and marker of the PSC (stage 11 onward) [63]. Collier functions downstream of Antp in the PSC and is important for its maintenance during larval development [64]. In col mutant animals, the PSC is lost, which causes lymph gland progenitors to differentiate prematurely [63]. Additional embryonic lymph gland markers are described in Table 1.
Drosophila embryos have also been ideal for research groups interested in live imaging of blood cells. Because of their small size, relative transparency, and the vast genetic tools that are available, live imaging of embryonic hemocytes has been used to address several basic developmental and cell biological questions, such as cell migration mechanics and chemotaxis [65-68]. Because of the versatility of this system, relatively recent detailed protocols describing live imaging and in vivo tracking of embryonic hemocytes have been published [69, 70].
Analysis of larval hemocytes
Over the last several years, analysis of Drosophila hematopoiesis has primarily focused on the larval stages. Recently, there has been growing interest in characterizing larval circulating cells in both functional and hematopoietic contexts, and several protocols for in vitro, ex vivo, and in vivo hemocyte analysis have been described [58, 70-76]. A basic protocol for the collection and preparation of circulating larval hemocytes for analysis is described in Box 1. Most larval analyses, however, have examined blood development in the lymph gland, which has proven to be a useful system for understanding mechanisms of progenitor cell maintenance and differentiation [8, 77]. Although the lymph gland is specified in the embryo, its growth and differentiation into mature blood cells occurs during the larval stages. Owing to a lack of good molecular markers, early work was limited mainly to observation. However, many new markers and tools have been identified (see Table 2) that, in combination with the application of advanced microscopic techniques, have greatly enhanced the current understanding of how hematopoiesis occurs in the lymph gland. In particular, these advances demonstrated that the primary lobes of the lymph gland can be subdivided into distinct cellular populations that are spatially organized [54]. The periphery of each primary lobe contains maturing blood cells was named the Cortical Zone (CZ), whereas the juxtaposed medial region contains blood progenitor cells and was termed the Medullary Zone (MZ). The posterior tip of each primary lobe harbors the Posterior Signaling Center (PSC), several dozen specialized lymph gland cells that do not behave as blood cells but rather form a niche-like group that supports hematopoietic development [64, 78, 79].
Table 2.
Established genetic markers for the larval lymph gland
| Hematopoietic Cells | Marker | Genetic Reporters | Antibody* | Mature cell type | References |
|---|---|---|---|---|---|
| Pan | srp | P | Lebestky et al., 2000 [52] | ||
| odd | odd-lacZ | P | Jung et al., 2005 [54] | ||
| He | He-gal4 | M | Kurucz et al., 2003 [117] | ||
| Posterior Signaling Center (PSC) | Antp | Antp-gal4 | M | Mandal et al., 2007 [64] | |
| Ser |
Ser9.5-lacZ
Ser9.6-gal4 |
P | Lebestky et al., 2000 [52] Jung et al., 2005 [54] |
||
| hh | hh-GFP | P | Mandal et al., 2007 [64]; Tokusumi et al., 2009 [81] | ||
| col | col-gal4 | Crozatier et al., 2004 [63]; Krzemien et al., 2007 [78] | |||
| Pvf1 | P | Mondal et al., 2011 [84] | |||
| Progenitors/Medullary Zone | dome |
dome-gal4
dome-gal4; ELAV-gal80 dome-MESO-lacZ dome-MESO-EGFP dome-MESO-EBFP2 |
Jung et al., 2005 [54] Krzemien et al., 2007 [78] Mondal et al., 2011 [84] this work |
||
| DE-Cad | M | Jung et al., 2005 [54] | |||
| upd3 | upd3-gal4 | Jung et al., 2005 [54] | |||
| ptc | M | Mandal et al., 2007 [64] | |||
| wg | M | Sinenko et al., 2009 [85] | |||
| ci | M | Mandal et al., 2007 [64] | |||
| ROShigh | Owusu-Ansah and Banerjee, 2009 [89] | ||||
| bam | M | Tokusumi et al., 2011 [88] | |||
| p-CamK-II | P | Mondal et al., 2011 [84] | |||
| p-Akt | P | Shim et al., 2012 [87] | |||
| P | Shim et al., 2013 [118] | ||||
| GABA-Rhigh | P | Shim et al., 2013 [118] | |||
| TepIV |
TepIV-gal4
TepIV-gal4; ELAV-gal80 |
Irving et al., 2005 [101]; Krzemien et al., 2007 [78] Avet-Rochex et al., 2010 [86] |
|||
| Pvrlow | P | Jung et al., 2005 [54]; Mondal et al., 2011 [84] | |||
| Differentiating cells/Cortical Zone | NimC1 (P1) | M | PL | Kurucz et al., 2007 [33]; Kurucz et al., 2007 [96] | |
| Hml |
Hml-gal4
Hml-DsRed |
PL | Sinenko et al., 2004 [119]; Makhijani et al., 2011 [58] | ||
| eater |
eater-gal4
eater-GFP |
PL | Tokusumi et al., 2009 [81] | ||
| Pvrhigh | P | Jung et al., 2005 [54]; Mondal et al., 2011 [84] | |||
| Pxn | Pxn-gal4 | P | PL | Jung et al., 2005 [54]; Stofanko et al., 2008 [60] | |
| Collagen (gal4) | Cg-gal4 | M | PL | Jung et al., 2005 [54] | |
| GABA-Rlow | P | Shim et al., 2013 [118] | |||
| Iz | Iz-gal4 | M | CC | Lebestky et al., 2000 [52]; Jung et al., 2005 [54] | |
| proPO-A1 |
Bc1
Bc-GFP, -BFP, -RFP |
P | CC | Rizki et al., 1980 [120]; Jung et al., 2005 [54]; Tokusumi et al., 2009 [81] | |
| Sima | P | CC | Muhkerjee et al., 2011 [121] | ||
| ItgaPS4 | P | LM | Crozatier et al., 2004 [63]; Irving et al., 2005 [101] | ||
| msn |
msn-lacZ
msn-GFP, msn-RFP |
LM | Braun et al., 1997 [98]; Tokusumi et al., 2009 [99] | ||
| atilla (L1) | atilla-GFP | M | LM | Honti et al., 2009 [102] | |
| mys | M | LM | Irving et al., 2005 [101] |
P = polyclonal; M = monoclonal
Among lymph gland markers there are several that stand out as being particularly useful for genetic analysis (see Table 2 for these and others). The first lymph gland zone to be discovered was the PSC through the expression of Serrate (Ser9.5-lacZ), encoding a Notch receptor ligand. The Serrate enhancer, which is active in the PSC from the second instar onward, was subsequently utilized to generate a Gal4 driver line utilizing a fluorescent reporter (UAS-GFP) and as a genetic tool to manipulate PSC cells [54, 79]. Later research demonstrated that Antennapedia, as described previously, is expressed in and specifies PSC cells [64]. Antennapedia expression in the PSC is maintained throughout lymph gland development, making it a marker and driver of choice for PSC-related experiments (both Antennapedia-gal4 and a monoclonal antibody are available, see Table 2). Similarly useful reagents are antibodies against Collier as well as the col-gal4 reporter line, both of which mark PSC cells throughout larval stages [78, 80]. PSC cells also express and secrete Hedgehog protein, which is sensed by medullary zone progenitor cells and is important for their long-term maintenance within the lymph gland [64]. A direct hedgehog-GFP reporter is available that allows for direct visualization of PSC cells [81], and can be placed in the context of other Gal4 drivers for analysis of non-autonomous PSC regulation (something not possible when using Antennapedia-gal4 as a reporter, for example).
The medullary and cortical zones were identified simultaneously through their mutually exclusive expression of progenitor and mature cell markers, respectively. The classic medullary zone marker is domeless-gal4 (domeless encodes a receptor that activates the JAK/STAT pathway), which was first isolated as an enhancer trap [54, 82]. Subsequently the domeless enhancer responsible for medullary zone expression was identified and has been used as a Gal4-independent lacZ (domeless-MESO-lacZ) reporter [78, 83]. Recent analysis demonstrating lymph gland regulation by the central nervous system (CNS) found that domeless-gal4 is also expressed by various neurons in the brain. To circumvent CNS effects when using domeless-gal4 to manipulate medullary zone blood progenitors, ELAV-gal80 has been placed in combination with domeless-gal4 [84]. The ELAV enhancer is neuron-specific and expresses high levels of Gal80, a natural protein inhibitor of Gal4, thereby mitigating any brain effects due to domeless-gal4 expression. Additional useful genetic markers for lymph gland medullary zone progenitors include E-cadherin [54], Cubitous interruptus [64], Wingless [85], TepIV-gal4 [86], phospho-Akt (p-Akt) [87], and Bag of marbles [88] (see Table 2).
Previous analysis of mitochondrial function in the lymph gland demonstrated that blood progenitor cells exhibit relatively high levels of reactive oxygen species (ROS), and that these ROS establish a critical signaling threshold for proper progenitor maintenance and differentiation [89]. Since then, fluorescent staining for ROS levels has become a standard analytic procedure for assessing how genetic change or immune challenge affects ROS within lymph glands and circulating cells. Many oxidation-sensitive dyes are available, however dihydroethidium (DHE) has been the marker of choice because of its high specificity for superoxide radicals and its ability to freely permeate cell membranes. Upon oxidation, fluorescent DHE metabolites are well retained by cells and tolerate mild fixation [90], which may be critical for simultaneous analysis of other markers such as GFP. Brief protocols for DHE analysis in both lymph glands and circulating blood cells are included here (see Boxes 5 and 6), with a more detailed protocol available as an online resource [91].
Differentiating cells of the lymph gland cortical zone express several useful markers, most notably Hemolectin-gal4 and the P1 antigen, which are both blood specific [54]. The Hemolectin-gal4 driver is one of the earliest known markers defining the onset of progenitor differentiation within the lymph gland at the mid-second instar, and stays on in mature cells [54, 92]. Expression of Peroxidasin (both the protein and the Peroxidasin-gal4 reporter) [93, 94] is also an early marker of cortical zone formation [54], however it is also expressed at various levels in other tissues such as the fat body and brain. The Collagen-gal4 driver [95] is slightly later than Peroxidasin expression in the cortical zone [54], but also has strong expression in the fat body and, at lower levels, in various cell types [95]. The P1 antigen is specific to mature plasmatocytes [33, 96] and, by comparison, is considered to be a relatively late marker [54]. The P1 antigen was identified as one of several proteins interacting with monoclonal antibodies derived from Drosophila blood cell preparations [96]. The P1 antigen was subsequently identified as the protein product of the Nimrod C1 gene (NimC1), described previously [33]. While the anti-P1 monoclonal antibody remains among the first in the choice of tools to be used for blood analysis given its specific expression in late plasmatocytes, ease of use, and widespread availability, it should be noted that several commonly used Drosophila parental stocks are P1-negative [97]. This recessive expression polymorphism can lead to serious problems with experimental interpretation of progeny phenotypes, making it imperative to analyze P1-expression in parental strains. The P1-negative genetic background of any important experimental stock can easily be corrected through standard chromosome mechanics [97].
Crystal cells in the cortical zone can be identified by expression of the determinant gene lozenge (both the protein and the lozenge-gal4 reporter) and mature marker Prophenoloxidase A1 (ProPOA1; protein). The gene encoding ProPOA1 is the Black cells (Bc) gene, and the crystal cell enhancer has been used to generate several different fluorescent, Gal4-independent Bc-expression reporters [81]. Lamellocytes also now have several useful markers available. A classic marker of lamellocyte differentiation is a misshapen (a JNK activator)-lacZ reporter gene (msn-lacZ) [98], initially isolated as an enhancer trap. The msn lamellocyte enhancer has subsequently been identified and used to generate several useful Gal4-independent fluorescent reporter lines [99]. Useful lamellocyte protein markers, for which antibodies are available, include L1 (identified along with P1 described above) [96], Filamin-240 [100], α-PS4 integrin [78], and Myospheroid βPS-integrin) [101]. A GFP enhancer trap of the L1 gene (atilla) is also available [102].
Development of new tools for lymph gland analysis
Although several new markers for lymph gland analysis have been identified, their relative utility varies. As described, some markers are antibody based (many of which are not monoclonal) and their availability and quality can vary widely. Genetic reporters such as Hml-gal4, while extremely useful, can be difficult for experiments in which it is necessary to discern whether Gal4 is a cell identity marker (through UAS-GFP expression, for example) or is a tool to drive other UAS-transgenes (eg., dsRNA) for genetic analysis; simultaneous use can make phenotypic interpretations difficult. Another key issue common to the use of Gal4-based drivers in Drosophila is that it is nearly impossible to perform and/or interpret results from experiments that combine multiple Gal4 reporters into the same background, as expression patterns will combine. In short, continued progress in characterizing the fly hematopoietic system will require the implementation of new or modified genetic technologies that can complement existing tools. In the past few years, several groups have expanded the Drosophila molecular genetic tool kit through the generation of new bipartite gene expression systems [103], such as the LexA-lexAop [104] and QF-QUAS systems [105]. Once adapted for use in the hematopoietic system (through the creation of appropriate driver lines such as Hml-QF or dome-QF), it will be possible to use such lines in combination with the Gal4/UAS system to enhance and refine analyses.
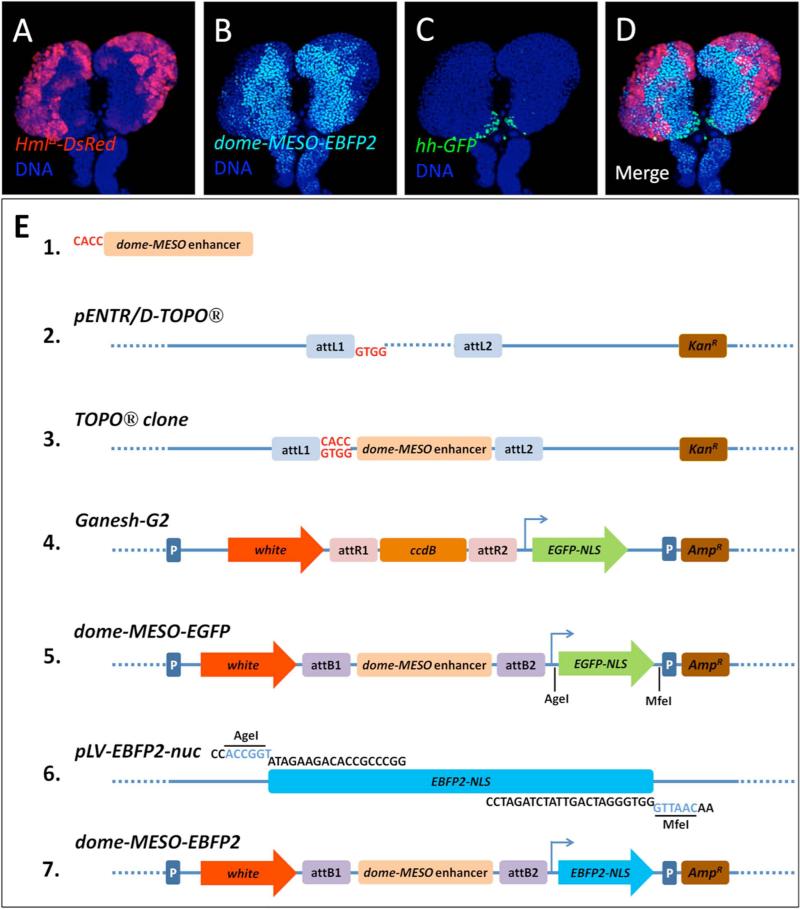
An alternative approach taken by our lab to overcome current limitations and to facilitate genetic analysis in the lymph gland has been to generate a new line of flies that uses multiple Gal4-independent fluorescent protein reporters to simultaneously monitor different lymph gland cell populations. To generate this new hematopoietic tool, we first re-tasked the dome-MESO progenitor cell enhancer [83] to drive expression of enhanced blue fluorescent protein 2 (EBFP2, Ex/Em: 383/448 nm) [106]. This transgene was then recombined with differentiating-cell marker Hemolectin-DsRed [58] and the PSC/niche cell marker hedgehog-GFP [81] onto the X chromosome. As can be seen in Figure 1, each reporter clearly identifies their respective cell in the lymph gland and, therefore, will be extremely useful for assessing phenotypes in various experimental contexts, such as genetic screening. This triple recombinant marker line, as well as the new, individual fluorescent dome-MESO transgenic lines, will be made freely available to the research community.
Figure 1. A new triple-fluorescence reporter line identifies mature, progenitor, and niche cells within the hematopoietic lymph gland.
A) Differentiated blood cells in the cortical zone of a third-instar larval lymph gland are marked by DsRed protein (red) expression driven by the Hemolectin enhancer (Hml-DsRed). B) Blood progenitor cells in the medulary zone express EBFP2 protein (cyan) under the control of the domeless MESO enhancer (dome-MESO-EBFP2). C) PSC cells express EGFP protein (green) under the control of the hedgehog PSC enhancer (hh-EGFP). D) A combined image (Merge) showing differential marking of blood cell populations juxtaposed in the lymph gland primary lobe. DNA (blue) is labeled with TO-PRO-3 (Molecular Probes) in each panel. E) Overview of the generation of the dome-MESO-EGFP and dome-MESO-EBFP2 constructs. The 2.8 kb dome-MESO enhancer (1) was amplified by PCR from Drosophila genomic DNA, along with an added 5’ CACC nucleotide sequence for Gateway® Directional TOPO® cloning into the pENTR/D-TOPO® entry vector (2; Life Technologies). Upon directional cloning of the dome-MESO enhancer into the Gateway entry vector (3), the enhancer was recombined with the Ganesh-G2 Gateway destination vector (4), thereby creating the dome-MESO-EGFP Drosophila transformation vector (5). The DNA coding sequence for nuclear-localized Enhanced Blue Fluorescent Protein 2 (EBFP2-NLS) was PCR amplified from the expression vector pLV-EBFP2-nuc (Addgene) as an AgeI/MfeI fragment (6). The dome-MESO-EBFP2 expression vector (7) was subsequently created by removing the EGFP-NLS DNA sequence from the dome-MESO-EGFP vector by digesting with AgeI and MfeI restriction endonucleases and then ligating in the EBFP2-NLS DNA sequence into the vector using the same restriction sites.
In contrast to other organs such as the imaginal discs, brain, gut, and fat body, the lymph gland is likely to be unfamiliar to most Drosophilists. Because of its relatively small size and delicate nature, the lymph gland presents a significant challenge to anyone interested in analyzing it by dissection and microscopy. To provide guidance in this area, a protocol for lymph gland dissection is described, and is followed by a standard immunostaining procedure and a summary of how to mount high quality, intact lymph glands on glass slides for microscopic analysis (see Boxes 2, 3, and 4).
Analysis of adult hemocytes
As mentioned, relatively little is known about adult hemocytes or hematopoiesis, owing primarily to a lack of useful tools and methods. In contrast to larvae, which are akin to fluid-filled balloons, adult bodies are rigid and have a relatively lower hemolymph volume, making analysis of circulating cells by “bleeding” more challenging. This becomes even more difficult in light of the fact that many, if not most, adult hemocytes are sessile, associated with the adult body wall and internal organs and structures. Many such adult hemocytes can be visualized through the cuticle in whole-mount preparations using markers such as Hml-gal4 UAS-GFP, however approach this is not very amenable to more detailed analyses of blood cell fate, proliferation, or function. It is clear that more work needs to be done to develop reagents and techniques to make the adult blood system more accessible to molecular genetic analysis. To date, the most commonly applied method for visualizing and analyzing adult hemocytes is to perfuse the adult hemocoel with aqueous buffer, which flushes hemocytes out of the adult and onto a slide for analysis. An adult perfusion protocol is described in Box 7. Recently, such a method was used in the identification of the first adult-specific blood marker, Ad1 [107], and further demonstrated that adult hemocytes maintain NimC1 (P1) expression but lose expression of Hemese (He), the blood-specific pan hemocyte marker found in larval hemocytes.
Summary
As with any developing field of study, continued effort on the part of researchers will enhance the ability to manipulate and analyze the fly hematopoietic system. This advancement will undoubtedly rely in large part on the identification of improved molecular markers offering higher resolution identification of cell types within the hematopoietic lineages, as well as on improved technologies for genetic analysis and imaging. Of particular importance will be approaches that allow for analyses in vivo during the larval, pupal, and adult stages, which may mitigate current difficulties in extracting blood cells from animals for ex vivo analyses. This review has highlighted some of the more useful genetic markers and analytic methods, both by pointing toward expertise in the published literature where appropriate and by providing here a set of basic protocols for dealing with the larval and adult blood system.
Box 1 | Collection and processing of larval circulating blood cells.
Collect larvae and wash thoroughly. This is important to ensure that the circulating cell preparation is not contaminated with food debris, yeast, and bacteria (which will obscure observation of the cells) from the culture.
Place a 14-well glass slide on the stereo microscope and illuminate with the transmitted light base.
Place a 20 μL drop of 1XPBS on one well spot of a 14-well glass slide. Do not place a drop on each well at the beginning as evaporation will significantly decrease the buffer volume as you proceed. Instead, use one drop at a time.
Place one larva in the drop of buffer.
Using two pairs of forceps, pinch the body wall/cuticle at the posterior of the larva. Keep one forceps in place while tearing the cuticle down the length of the larva toward the anterior. As the hemocoel is opened, hemolymph will begin to stream into the 1XPBS buffer; this should be relatively easy to observe because of differential fluid densities. To obtain a clean preparation of isolated blood cells, it is essential to grab and tear only the cuticle. Avoid damaging internal organs particularly the gut, the contents of which will significantly contaminate the sample and will be difficult to wash away from the slide surface.
Once the larval hemocoel is open, use forceps to gently move the larva through the buffer to evenly distribute the hemolymph and hemocytes throughout.
Using single tines from two different forceps, gently remove the carcass from the drop of buffer containing the blood cells. Do this slowly so that minimal buffer is removed. Do NOT use one pair of forceps to pick up the carcass as too much buffer will also be removed due to capillary action between the tines.
Repeat for remaining samples.
Carefully place slide into humidified chamber. Incubate for 30 minutes to let blood cells settle onto the glass surface.
Remove slide from the humidified chamber.
Under the microscope, carefully remove the 1XPBS buffer from each drop using a pipette.
Add 20 μL of freshly prepared fixative to each well and place into humidified chamber for 10 minutes at room temperature.
Under the microscope, remove fixative and replace with 20 μL of 1×PBS for short term storage at 4C or proceed with subsequent assay of choice, such as immunostaining. For steps using buffers with detergents, volumes of 5-10 μL are recommended to avoid mixing between wells due to loss of surface tension. All steps should be carried out in a humidified chamber to avoid evaporation.
After processing, place a small drop of VECTASHIELD on each well, followed by a glass cover slip.
Seal with nail polish around the edges and proceed to imaging.
Box 2 | Lymph gland dissection.
Using a large transfer pipette, place several large drops of 1XPBS on the silicone dissection plate.
Place one larva in each drop. As technique improves, several larvae can be placed in each drop without interference.
Place the plate on a standard stereo dissecting microscope with a transmitted light base.
Illuminate the sample. If lighting makes use of standard light bulbs (instead of a cold LED source), take care to work quickly so that the larvae/samples do not overheat. Dissect for a maximum of 30 minutes, with samples stored on ice, before fixation.
Using forceps, orient larva dorsal-side up, anterior to the right (for a right-handed person).
Using the left hand, gently hold larvae at 75% length (A=0%, P=100%).
Using the right hand, pinch the dorsal cuticle (only, no internal organs) at 70-75% length, and slowly pull toward the anterior. Pull the entire length, stopping at the mouth hooks. This will open the body cavity without damaging internal structures. Sometimes the dorsal cuticle will completely detach or the ventral cuticle also; either is ok as long as the body cavity is open without disrupting internal organs. Alternative method: Using both pairs of forceps, rip the larva in half, then invert the anterior part by pushing the mouth hooks into the body cavity longitudinally (imagine inverting a sock by pushing your hand inward at the toes). Once inverted, proceed with step 10 below. Note: this method opens the gut, which can make subsequent steps more difficult because food debris, yeast, and bacteria cloud the buffer.
Use both forceps to sever the larvae (internal organs and ventral cuticle) completely at 75% length (near where the larva was being held by the left hand).
Stabilize the anterior portion of the larva with the left hand by grabbing the ventral cuticle. Using the right hand forceps, reach inside the body cavity and directly grasp the mouth hooks (do not grab them externally as this will include cuticle).
Gently pull the mouth hooks out, separating them from the body wall and cuticle. Removing the mouth hooks in this manner will bring along a complex of structures that includes the eye imaginal discs, the brain, the dorsal vessel, the prothoracic (ring) gland, and the lymph gland; salivary glands are also commonly included.
Gently separate these lymph gland-containing complexes from any residual tissues such as fat body.
Carefully remove the complex in a small drop of 1XPBS retained between the tines of a single forceps) to a microfuge tube containing cold 1mL 1XPBS + 1 drop 1XPBST on ice. The PBST break the surface tension of the buffer, allowing samples to sink to the bottom of the tube.
Collect as many lymph gland complexes as needed.
Replace buffer with 1 mL of freshly prepared fixative.
Place microfuge tube on mixer (Nutator, orbital shaker, or similar) for 30 minutes.
Proceed to immunostaining (described below) or other analytical method.
For short term storage, wash fixed tissue with 1 mL 1XPBS for 5 minutes; repeat; store at 4°C.
Box 3 | Lymph gland immunostaining.
For this procedure, lymph glands are left attached to the mouth hook/eye disc/brain complex, 1) to provide a “handle” so that lymph glands are not damaged because of physical manipulation and 2) because these tissues are often convenient immunostaining controls
With lymph glands either in fix or 1XPBS in a 1.5 mL microfuge tube, discard buffer.
Add 1mL 1XPBST (1XPBS + 0.4% Triton), place on mixer (Nutator or similar) for 15 minutes; repeat twice.
During the washing stage, prepare 10% normal goat serum (NGS)/1xPBST blocking solution.
Discard wash buffer from lymph glands and replace with 1 mL blocking solution; block in for 30 minutes.
Dilute primary antibody in blocking solution at desired concentration.
Discard blocking solution from lymph glands and replace with 100 μL or greater volume of primary antibody and mix well. For smaller volumes and/or limited primary antibody, 0.6 mL microfuge tubes, PCR tubes, or Terasaki microtiter plates (with 10 μL wells) can be used.
Place tubes in a rack. For short term incubations at room temperature, intermittent mixing or placement on an orbital shaker is helpful.
Incubate for 3 hours at room temperature or overnight at 4C.
Remove primary antibody.
Add 1mL 1XPBST, place on mixer for 15 minutes; repeat twice.
Discard wash buffer from lymph glands and replace with 1 mL blocking solution; block 10-30 minutes.
Dilute secondary antibody (usually 1:250 for lymph glands) in blocking solution at desired concentration.
Discard blocking solution from lymph glands and replace with 100 μL or greater volume of primary Incubate in secondary antibody (diluted usually 1:250) in block for 3 hours at room temperature or overnight at 4C.
Remove secondary antibody.
Add 1mL 1XPBST, place on mixer for 15 minutes, discard buffer; repeat once.
Add 1mL 1XPBST + DNA stain (DAPI, etc.), place on mixer for 15 minutes, discard buffer.
Add 1mL 1XPBS, place on mixer for 15 minutes, discard buffer; repeat once (these wash steps remove detergent and excess DNA stain).
Use pipette and dissection microscope to remove as much buffer as possible.
Add a drop of VECTASHIELD to the lymph glands in the 1.5 mL microfuge tube.
Store in refrigerator or begin mounting procedure.
Box 4 | Mounting fixed lymph glands for microscopy.
Dissection of lymph glands itself can be difficult even for people with lots of practice with other tissues such as imaginal discs, however mounting them successfully after processing is just as difficult. Fewer things are more frustrating that dissecting high quality lymph gland samples, only to destroy them during the mounting process. As with dissection, many approaches will work depending on the skill level of the investigator. Below is the primary method taught to individuals in our laboratory.
Transfer lymph gland complexes (in VECTASHIELD) from the microfuge tube to a glass slide using a small transfer pipette or a pipette with a 200 μL tip cut by a razor blade to enlarge the opening.
Place a drop of VECTASHIELD on a new slide.
Using forceps, move lymph gland complexes from the first slide to the second, taking care to minimize the transfer of mounting medium.
Once transferred, use forceps to form the VECTASHIELD into a square or rectangle shape, leaving 5-8 mm of dry area before the side edges.
Carefully separate the lymph gland complexes and distribute them in the rectangle of medium. Use of single tines of the forceps is recommended because surface tension of the medium will cause it to flow readily between both tines of a single forceps, which will disrupt your tissues on the slide.
Place one tine of one forceps underneath the posterior end of the dorsal vessel, lift slightly, and gently pull the lymph glands (and anterior structures) toward the straight, perpendicular edge of the medium where it meets the glass slide. The brain and discs create drag that straightens out the lymph glands (dorsal vessel) as you move through the medium.
Draw the dorsal vessel/lymph glands out past the perpendicular edge of the medium and onto the dry part of the slide. As this occurs, the volume of medium around the lymph gland decreases, causing the lymph gland and other structures to “sit down”onto the glass surface, instead of just floating around. It is critical that the tissues make contact with the slide, otherwise the samples will dislodge and become contorted upon coverslipping.
Repeat this process for each lymph gland around the perimeter of the medium, such that each one is well spaced and straight.
Separate the brain and imaginal discs from the lymph glands of each complex. This is most easily achieved by using a scissoring motion with single tines of two different forceps (avoid placing both tines of a pair of forceps into the medium). Place the tines in a “Figure X” over the dorsal vessel anterior to the primary lobes, usually at or near the location of the ring gland. Hold one tine still while drawing the other tine across the glass surface; as the tines pass each other, the dorsal vessel is severed. Push the brain complex away from the lymph gland, toward the center of the medium pool.
Once the lymph glands and brain complexes have been separated, discard or move most of the brain complexes (and any other tissue) to a new slide. Place one brain near each corner of the medium rectangle and one or two in the center of the medium; these will serve as spacers to protect the lymph glands from severe compression by the cover slip.
Place one short edge of an appropriately sized cover slip down onto the glass slide near, but not in, the medium. Take care to center it longitudinally. Place the tines of a forceps under the opposite end and slowly lower the cover slip onto the medium. To avoid air bubbles and misalignment, do NOT drop it onto the medium. Wait for the medium to distribute between the cover slip and the slide. Slight pressure on the cover slip with the forceps can help distribute the medium, however be careful not to compress the tissues. If more mounting medium is needed, it can be added using a pipette tip at the edge of the cover slip. Excess medium should be removed at the edge with a pipette or wicked away using a Kimwipe.
Once the cover slip is down, seal the cover slip around the edges using nail polish. Allow to air dry for a few minutes, then store slides (horizontal is best) at 4 °C until they can be imaged.
Box 5 | Staining for Reactive Oxygen Species (ROS) in the lymph glands.
Special Materials
1X Schneider's Drosophila Medium + L-Glutamine (GIBCO, cat. no. 11720)
Anhydrous Dimethyl Sulfoxide (DMSO) > 99.9% (Sigma-Aldrich, cat. no. 276855)
Dihydroethidium – special packaging (Molecular Probes, cat no. D11347)
VECTASHIELD Mounting Medium (Vector laboratories, cat. no. H-1000)
Micro Spot Plate – 3 well (Electron Microscopy Sciences, cat. no. 71561- 01)
12-well microscope slides with hydrophobic barrier (Erie Scientific).
Dissect lymph glands in room temperature Schneider's medium. Do not use cold 1XPBS, which may inhibit respiration, thereby interfering ROS production. Because these samples are not kept on ice or immediately fixed, dissection times less than 30 minutes are optimal for limiting tissue degradation.
Quickly reconstitute DHE in anhydrous DMSO (see reagent set up). Reconstituted dye solution should appear slightly pink in color; a more intense color such as purple may be indicative of oxidation of the dye.
Make DHE staining solution by adding 1 μL of the reconstituted DHE/DMSO to 1mL of Schneider's medium (in a microfuge tube) to give a final concentration of approximately 30μM. Vortex sample 15-30 seconds, but not more.
Remove Schneider's medium from lymph glands and replace with the DHE staining solution. Incubate 5 minutes in the dark, on an orbital shaker at room temperature.
Wash three times, 5-minutes each, with Schneider's medium in the dark, on an orbital shaker at room temperature.
Lightly fix for 5 minutes in 7% formaldehyde/1XPBS.
Wash once in 1XPBS.
Immediately mount lymph glands on a glass slide in VECTASHIELD medium.
Image immediately using fluorescence microcopy.
Box 6 | Staining for Reactive Oxygen Species (ROS) in circulating cells.
Bleed larvae into 20 μL Schneider's medium. Mix thoroughly and take care to remove as little liquid volume as possible when removing the carcass.
Let hemocytes settle 20-30 minutes in a humidified chamber at room temperature.
Toward the end of the settling period, reconstitute DHE and prepare DHE staining solution as described above for lymph glands.
Wash settled hemocytes by gently removing Schneider's medium from the edge of each well using a pipette, and replace with 20 μL Schneider's medium.
Remove Schneider's medium from each well and replace with 20 μL DHE staining solution. Incubate 5 minutes in a dark, humidified chamber.
Remove DHE staining solution and wash each well of cells twice, 5 minutes each, with 20 μL Schneiders medium.
Lightly fix cells with 20 μL of 4% formaldehyde/1XPBS for 5 minutes.
Remove fixative and wash once with 1XPBS.
Remove 1XPBS and add a small volume (~2 μL) of VECTASHIELD to each well. Coverslip the slide.
Image immediately using fluorescence microscopy.
Box 7 | Collection of adult hemocytes by perfusion.
Anesthetize adults of interest on a standard carbon dioxide pad under the dissecting microscope
Using forceps and microscissors, cut a small slit or hole in the posterior abdomen; this should be slightly lateral so that internal organs, genitalia, and the gut are not disrupted.
Place a 10 μL drop of 1XPBS onto a well of a 12-well hydrophobic slide.
Using a mouth pipette, draw 1XPBS into a drawn glass capillary needle.
Grasp the adult fly by the wing using forceps and gently push the tip of the needle into the lateral thorax.
Place cut posterior end of the adult fly in or near the drop of 1XPBS and gently perfuse buffer through the adult.
Monitor the drop of buffer on the slide for hemolymph streaming into it; when the drop approximately doubles in size, halt perfusion and remove the adult carcass from the drop.
Place slide in a humidified chamber for 30 minutes.
Carefully remove buffer using a pipette and replace with 20 μL of freshly prepared fixative.
Return to humidified chamber for 10 minutes.
Carefully remove fixative and replace with 20 μL of 1XPBS, or continue with processing as described previously.
Acknowledgments
Because of space limitations, the review could not be exhaustive in its description of the hematopoietic system. We apologize to those whose important work in the field is not cited here. This work was supported by NIH grants R01 HL067395 and R21 AI094048 to U.B, and a China Scholarship Council award to T.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizki TM. Blood cells of Drosophila as related to metamorphosis. In: Campbell FL, editor. Physiology of Insect Development. Chicago University Press; Chicago, IL.: 1956. pp. 91–94. [Google Scholar]
- 2.Rizki TM, Rizki RM. Properties of the larval hemocytes of Drosophila melanogaster. Experientia. 1980;36:1223–1226. [Google Scholar]
- 3.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14:213–222. [PubMed] [Google Scholar]
- 4.Rizki TM. The circulatory system and associated cells and tissues. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. Academic Press; New York, London: 1978. [Google Scholar]
- 5.Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230(2):243–57. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 6.Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16(2-3):103–10. doi: 10.1016/0145-305x(92)90011-z. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243(1):65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- 8.Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5(5):673–90. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AP. Hemocyte types: their structures, synonymies, interrelationships, and taxonomic significance. In: Gupta AP, editor. Insect Hemocytes: Development, forms, functions, and techniques. Cambridge University Press; 1979. pp. 85–127. [Google Scholar]
- 10.Shrestha R, Gateff E. Ultrastructure and cytochemistry of the cell types of the larval hematopoietic organs and hemolymph of Drosophila melanogaster. Dev. Growth Differ. 1982;24:65–82. doi: 10.1111/j.1440-169X.1982.00065.x. [DOI] [PubMed] [Google Scholar]
- 11.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5(3):441–50. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 12.Hetru C, Troxler L, Hoffmann JA. Drosophila melanogaster antimicrobial defense. J Infect Dis. 2003;187(Suppl 2):S327–34. doi: 10.1086/374758. [DOI] [PubMed] [Google Scholar]
- 13.Nappi AJ. Inhibition by parasites of melanotic tumour formation in Drosophila melanogaster. Nature. 1975;255(5507):402–4. doi: 10.1038/255402a0. [DOI] [PubMed] [Google Scholar]
- 14.Samakovlis C, Kimbrell DA, Kylsten P, Engstrom A, Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990;9(9):2969–76. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002;99(4):2152–7. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan CA, Delaney JR, Schneider DS, Anderson KV. Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr Biol. 2007;17(1):67–72. doi: 10.1016/j.cub.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol. 2000;10(13):781–4. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- 18.Matova N, Anderson KV. Rel/NF-kappaB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc Natl Acad Sci U S A. 2006;103(44):16424–9. doi: 10.1073/pnas.0605721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3(3):e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fessler LI, Nelson RE, Fessler JH. Drosophila extracellular matrix. Methods Enzymol. 1994;245:271–94. doi: 10.1016/0076-6879(94)45016-1. [DOI] [PubMed] [Google Scholar]
- 21.Franc NC. Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences. Front Biosci. 2002;7:d1298–313. doi: 10.2741/A841. [DOI] [PubMed] [Google Scholar]
- 22.Murray MA, Fessler LI, Palka J. Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev Biol. 1995;168(1):150–65. doi: 10.1006/dbio.1995.1068. [DOI] [PubMed] [Google Scholar]
- 23.Yasothornsrikul S, Davis WJ, Cramer G, Kimbrell DA, Dearolf CR. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198(1-2):17–25. doi: 10.1016/s0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- 24.Sears HC, Kennedy CJ, Garrity PA. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130(15):3557–65. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- 25.Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4(5):431–43. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- 26.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 27.Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38(4):567–80. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 28.Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, et al. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J Biol Chem. 2004;279(46):48466–76. doi: 10.1074/jbc.M408597200. [DOI] [PubMed] [Google Scholar]
- 29.Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416(6881):644–8. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- 30.Ramet M, Pearson A, Manfruelli P, Li X, Koziel H, et al. Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity. 2001;15(6):1027–38. doi: 10.1016/s1074-7613(01)00249-7. [DOI] [PubMed] [Google Scholar]
- 31.Royet J, Reichhart JM, Hoffmann JA. Sensing and signaling during infection in Drosophila. Curr Opin Immunol. 2005;17(1):11–7. doi: 10.1016/j.coi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123(2):335–46. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17(7):649–54. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 34.Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309(5742):1874–8. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 35.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101(6):671–84. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 36.Castillejo-Lopez C, Hacker U. The serine protease Sp7 is expressed in blood cells and regulates the melanization reaction in Drosophila. Biochem Biophys Res Commun. 2005;338(2):1075–82. doi: 10.1016/j.bbrc.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 37.Chosa N, Fukumitsu T, Fujimoto K, Ohnishi E. Activation of prophenoloxidase A1 by an activating enzyme in Drosophila melanogaster. Insect Biochem Mol Biol. 1997;27(1):61–8. doi: 10.1016/s0965-1748(96)00070-7. [DOI] [PubMed] [Google Scholar]
- 38.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21(11):2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Kambris Z, Lemaitre B, Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem. 2006;281(38):28097–104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- 40.Rizki TM, Rizki RM. Paracrystalline inclusions of D. melanogaster hemocytes have prophenoloxidases. Genetics. 1985;110(S98) [Google Scholar]
- 41.Bidla G, Dushay MS, Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci. 2007;120(Pt 7):1209–15. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 42.Peeples EE, Geisler A, Whitcraft CJ, Oliver CP. Comparative studies of phenol oxidase activity during pupal development of three lozenge mutants (lz8,lz,lzk) of Drosophila melanogaster. Genetics. 1969;62(1):161–70. doi: 10.1093/genetics/62.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizki TM, Rizki RM. Alleles of lz as suppressors of the Bc-phene in Drosophila melanogaster. Genetics. 1981;97(S90) [Google Scholar]
- 44.Bidla G, Lindgren M, Theopold U, Dushay MS. Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev Comp Immunol. 2005;29(8):669–79. doi: 10.1016/j.dci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Russo J, Dupas S, Frey F, Carton Y, Brehelin M. Insect immunity: early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology. 1996;112(Pt 1):135–42. doi: 10.1017/s0031182000065173. [DOI] [PubMed] [Google Scholar]
- 46.Luo H, Rose PE, Roberts TM, Dearolf CR. The Hopscotch Jak kinase requires the Raf pathway to promote blood cell activation and differentiation in Drosophila. Mol Genet Genomics. 2002;267(1):57–63. doi: 10.1007/s00438-001-0632-7. [DOI] [PubMed] [Google Scholar]
- 47.Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101(1):108–11. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Lemaitre B. The road to Toll. Nat Rev Immunol. 2004;4(7):521–7. doi: 10.1038/nri1390. [DOI] [PubMed] [Google Scholar]
- 49.Romeo Y, Lemaitre B. Drosophila immunity: methods for monitoring the activity of Toll and Imd signaling pathways. Methods Mol Biol. 2008;415:379–94. doi: 10.1007/978-1-59745-570-1_22. [DOI] [PubMed] [Google Scholar]
- 50.Small C, Paddibhatla I, Rajwani R, Govind S. An introduction to parasitic wasps of Drosophila and the antiparasite immune response. J Vis Exp. 2012;(63):e3347. doi: 10.3791/3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartenstein V. Blood cells and blood cell development in the animal kingdom. Annu Rev Cell Dev Biol. 2006;22:677–712. doi: 10.1146/annurev.cellbio.22.010605.093317. [DOI] [PubMed] [Google Scholar]
- 52.Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288(5463):146–9. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 53.Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120(7):1829–37. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- 54.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132(11):2521–33. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 55.Rugendorff AE, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Rouxs Arch. Dev. Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- 56.Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130(20):4955–62. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- 57.Robertson CW. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 1936;59:351–399. [Google Scholar]
- 58.Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138(24):5379–91. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106(12):4805–9. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stofanko M, Kwon SY, Badenhorst P. A misexpression screen to identify regulators of Drosophila larval hemocyte development. Genetics. 2008;180(1):253–67. doi: 10.1534/genetics.108.089094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waltzer L, Ferjoux G, Bataille L, Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 2003;22(24):6516–25. doi: 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milchanowski AB, Henkenius AL, Narayanan M, Hartenstein V, Banerjee U. Identification and characterization of genes involved in embryonic crystal cell formation during Drosophila hematopoiesis. Genetics. 2004;168(1):325–39. doi: 10.1534/genetics.104.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2(8):E196. doi: 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446(7133):320–4. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans IR, Hu N, Skaer H, Wood W. Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development. 2010;137(10):1625–33. doi: 10.1242/dev.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tucker PK, Evans IR, Wood W. Ena drives invasive macrophage migration in Drosophila embryos. Dis Model Mech. 2011;4(1):126–34. doi: 10.1242/dmm.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173(3):405–16. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168(4):567–73. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans IR, Zanet J, Wood W, Stramer BM. Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J Vis Exp. 2010;(36) doi: 10.3791/1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreira CG, Regan JC, Zaidman-Remy A, Jacinto A, Prag S. Drosophila hemocyte migration: an in vivo assay for directional cell migration. Methods Mol Biol. 2011;769:249–60. doi: 10.1007/978-1-61779-207-6_17. [DOI] [PubMed] [Google Scholar]
- 71.Sampson CJ, Williams MJ. Protocol for ex vivo incubation of Drosophila primary post-embryonic haemocytes for real-time analyses. Methods Mol Biol. 2012;827:359–67. doi: 10.1007/978-1-61779-442-1_23. [DOI] [PubMed] [Google Scholar]
- 72.Sinenko SA, Hung T, Moroz T, Tran QM, Sidhu S, et al. Genetic manipulation of AML1-ETO-induced expansion of hematopoietic precursors in a Drosophila model. Blood. 2010;116(22):4612–20. doi: 10.1182/blood-2010-03-276998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreira CG, Jacinto A, Prag S. Drosophila integrin adhesion complexes are essential for hemocyte migration in vivo. Biol Open. 2013;2(8):795–801. doi: 10.1242/bio.20134564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tirouvanziam R, Davidson CJ, Lipsick JS, Herzenberg LA. Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc Natl Acad Sci U S A. 2004;101(9):2912–7. doi: 10.1073/pnas.0308734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vilmos P, Nagy I, Kurucz E, Hultmark D, Gateff E, et al. A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev Comp Immunol. 2004;28(6):555–63. doi: 10.1016/j.dci.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101(39):14192–7. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crozatier M, Vincent A. Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis Model Mech. 2011;4(4):439–45. doi: 10.1242/dmm.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krzemien J, Dubois L, Makki R, Meister M, Vincent A, et al. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446(7133):325–8. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- 79.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17(3):348–53. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pennetier D, Oyallon J, Morin-Poulard I, Dejean S, Vincent A, et al. Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc Natl Acad Sci U S A. 2012;109(9):3389–94. doi: 10.1073/pnas.1109407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tokusumi T, Shoue DA, Tokusumi Y, Stoller JR, Schulz RA. New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis. 2009;47(11):771–4. doi: 10.1002/dvg.20561. [DOI] [PubMed] [Google Scholar]
- 82.Bourbon HM, Gonzy-Treboul G, Peronnet F, Alin MF, Ardourel C, et al. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev. 2002;110(1-2):71–83. doi: 10.1016/s0925-4773(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 83.Hombria JC, Brown S, Hader S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288(2):420–33. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 84.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147(7):1589–600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16(5):756–63. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avet-Rochex A, Boyer K, Polesello C, Gobert V, Osman D, et al. An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev Biol. 2010;10:65. doi: 10.1186/1471-213X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14(4):394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tokusumi T, Tokusumi Y, Hopkins DW, Shoue DA, Corona L, et al. Germ line differentiation factor Bag of Marbles is a regulator of hematopoietic progenitor maintenance during Drosophila hematopoiesis. Development. 2011;138(18):3879–84. doi: 10.1242/dev.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461(7263):537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103(41):15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owusu-Ansah E, Yavari A, Banerjee U. A protocol for _in vivo_ detection of reactive oxygen species. 2008.
- 92.Sinenko SA, Mathey-Prevot B. Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 2004;23(56):9120–8. doi: 10.1038/sj.onc.1208156. [DOI] [PubMed] [Google Scholar]
- 93.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2(8):E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, et al. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13(15):3438–47. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asha H, Nagy I, Kovacs G, Stetson D, Ando I, et al. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163(1):203–15. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurucz E, Vaczi B, Markus R, Laurinyecz B, Vilmos P, et al. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 2007;58(Suppl):95–111. doi: 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- 97.Honti V, Cinege G, Csordas G, Kurucz E, Zsamboki J, et al. Variation of NimC1 expression in Drosophila stocks and transgenic strains. Fly (Austin) 2013;7(4) doi: 10.4161/fly.25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun A, Lemaitre B, Lanot R, Zachary D, Meister M. Drosophila immunity: analysis of larval hemocytes by P-element-mediated enhancer trap. Genetics. 1997;147(2):623–34. doi: 10.1093/genetics/147.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tokusumi T, Sorrentino RP, Russell M, Ferrarese R, Govind S, et al. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS One. 2009;4(7):e6429. doi: 10.1371/journal.pone.0006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rus F, Kurucz E, Markus R, Sinenko SA, Laurinyecz B, et al. Expression pattern of Filamin-240 in Drosophila blood cells. Gene Expr Patterns. 2006;6(8):928–34. doi: 10.1016/j.modgep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, et al. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 2005;7(3):335–50. doi: 10.1111/j.1462-5822.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 102.Honti V, Kurucz E, Csordas G, Laurinyecz B, Markus R, et al. In vivo detection of lamellocytes in Drosophila melanogaster. Immunol Lett. 2009;126(1-2):83–4. doi: 10.1016/j.imlet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 103.del Valle Rodriguez A, Didiano D, Desplan C. Power tools for gene expression and clonal analysis in Drosophila. Nat Methods. 2012;9(1):47–55. doi: 10.1038/nmeth.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9(5):703–9. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 105.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141(3):536–48. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007;46(20):5904–10. doi: 10.1021/bi700199g. [DOI] [PubMed] [Google Scholar]
- 107.Honti V, Csordas G, Kurucz E, Markus R, Ando I. The cell-mediated immunity of Drosophila melanogaster: hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol. 2014;42(1):47–56. doi: 10.1016/j.dci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, et al. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7(1):73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Bernardoni R, Vivancos V, Giangrande A. glide/gcm is expressed and required in the scavenger cell lineage. Dev. Biol. 1997;191:118–130. doi: 10.1006/dbio.1997.8702. [DOI] [PubMed] [Google Scholar]
- 110.Cho NK, Keyes L, Johnson E, Heller J, Ryner L, et al. Developmental control of blood cell migration by the Drosophila VEGF pathway. 108. Cell. 2002:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 111.Oloffson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279(1):233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 112.Waltzer L, Bataillé L, Peyrefitte S, Haenlin M. Two isoforms of Serpent containing either one or two GATA zinc fingers have different roles in Drosophila haematopoiesis. EMBO J. 2002;21(20):5477–86. doi: 10.1093/emboj/cdf545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127(22):4959–69. doi: 10.1242/dev.127.22.4959. [DOI] [PubMed] [Google Scholar]
- 114.Han Z, Olson EN. Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development. 2005;132(15):3525–36. doi: 10.1242/dev.01899. [DOI] [PubMed] [Google Scholar]
- 115.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6(8):603–5. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kimbrell DA, Hice C, Bolduc C, Kleinhesselink K, Beckingham K. The Dorothy enhancer has Tinman binding sites and drives hopscotch-induced tumor formation. Genesis. 2002;34:23–28. doi: 10.1002/gene.10134. [DOI] [PubMed] [Google Scholar]
- 117.Kurucz E, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, et al. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc Natl Acad Sci U S A. 2003;100(5):2622–7. doi: 10.1073/pnas.0436940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shim J, Mukherjee T, Mondal BC, Liu T, Young GC, et al. Olfactory control of blood progenitor maintenance. Cell. 2013;155(5):1141–53. doi: 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinenko SA, Kim EK, Wynn R, Manfruelli P, Ando I, et al. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev Biol. 2004;273(1):48–62. doi: 10.1016/j.ydbio.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 120.Rizki TM, Rizki RM, Grell EH. A mutant affecting the crystal cells of Drosophila melanogaster. Rouxs Arch. Dev. Biol. 1980;188:91–99. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- 121.Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332(6034):1210–3. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]