Abstract
The identification of human CD34-negative (CD34−) hematopoietic stem cells (HSCs) provides a new concept for the hierarchy in the human HSC compartment. Previous studies demonstrated that CD34− severe combined immunodeficiency (SCID)-repopulating cells (SRCs) are a distinct class of primitive HSCs in comparison to the well-characterized CD34+CD38− SRCs. However, the purification level of rare CD34− SRCs in 18 lineage marker-negative (Lin−) CD34− cells (1/1000) is still very low compared with that of CD34+CD38− SRCs (1/40). As in the mouse, it will be necessary to identify useful positive markers for a high degree of purification of rare human CD34− SRCs. Using 18Lin−CD34− cells, we analyzed the expression of candidate positive markers by flow cytometric analysis. We finally identified CD133 as a reliable positive marker of human CB-derived CD34− SRCs and succeeded in highly purifying primitive human CD34− HSCs. The limiting dilution analysis demonstrated that the incidence of CD34− SRCs in 18Lin−CD34−CD133+ cells was 1/142, which is the highest level of purification of these unique CD34− HSCs to date. Furthermore, CD133 expression clearly segregated the SRC activities of 18Lin−CD34− cells, as well as 18Lin−CD34+ cells, in their positive fractions, indicating its functional significance as a common cell surface maker to isolate effectively both CD34+ and CD34− SRCs.
Keywords: CD133, hematopoietic stem cell, CD34 negative, SCID-repopulating cell, IBMI, cord blood
Introduction
The sialomucin, CD34, has long been believed to be a major positive marker for murine and human hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs).1, 2, 3, 4 Despite the importance of the CD34 antigen as a marker of early HSCs/HPCs in basic research, as well as in clinical HSC transplantation, the biological function of CD34 has not been fully elucidated.1 During the past two decades, the fluorescence-activated cell sorting (FACS) technology has markedly improved, and it is now possible to isolate prospectively murine HSCs using various cell surface markers, including Sca-1, c-kit and CD150.2, 3, 4, 5, 6 In fact, the surface immunophenotype of murine HSCs has been increasingly refined, resulting in the success of the single cell-based transplantation analyses.7, 8, 9 Using such advanced technology, Nakauchi and his co-workers revealed that murine long-term lymphohematopoietic-reconstituting HSCs are lineage marker-negative (Lin−) c-kit+Sca-1+CD34-low/negative (CD34lo/− KSL).10 Collectively, these results established the concept that the most primitive murine HSCs are present in the CD34lo/− KSL cell population.
In contrast, the current understanding of the primitive human HSC compartment is far behind that of the murine HSC system. As in the mouse, the purification of primitive human HSCs requires positive markers. It is well documented that CD34 is expressed on the surfaces of HSCs, as well as the more differentiated HPCs.1, 2, 3, 4 Therefore, it is important to identify additional positive markers for the purification of primitive human HSCs at the single-cell level. The severe combined immunodeficiency (SCID)-repopulating cell (SRC) xenotransplantation assay provides a gold standard surrogate assay for human HSCs.11 A number of studies using xenograft assays demonstrated that the bone marrow (BM)- and cord blood (CB)-derived primitive human HSCs are enriched in the Lin−CD34+CD38− population.2, 3, 4, 11, 12, 13, 14 It was also reported that the Lin−CD34+CD38−CD90+CD45RA− CB fraction contains primitive HSCs, and this activity has been isolated to as few as 10 purified cells.15 Recently, Notta et al.16 published an informative study showing that CB-derived single Lin−CD34+CD38−CD45RA−Thy1+RholoCD49f+ cells were highly efficient in generating long-term multilineage engraftment in NOD-SCID-IL-2Rγc−/− mice,16 providing strong evidence that primitive human CD34+CD38− HSCs express CD49f.
Although the most primitive murine HSCs are defined as CD34− KSL cells,10 primitive human HSCs were believed to be Lin−CD34+CD38− cells.2, 3, 4, 11, 12, 13, 14, 15, 16 However, this long-standing dogma was challenged by several groups.17, 18, 19, 20 Interestingly, these studies suggested that CD34+ HSCs originate from CD34− HSCs. Accordingly, we developed an intra-BM injection (IBMI) method and successfully identified human CB-derived CD34− SRCs using 13 lineage-specific monoclonal antibodies (mAbs) to remove Lin+ cells.21, 22 These CD34− SRCs could generate CD34+CD38− SRCs in vitro as well as in vivo.21, 22, 23 Moreover, our in vivo kinetic analysis clearly demonstrated that only one or two CD34− SRCs had potent human hematopoietic cell reconstitution potentials, which was almost equivalent to that of 5–10 CD34+CD38− SRCs.24, 25 Using this highly efficient SRC assay, we demonstrated that the surface immunophenotype of CD34− SRCs is Lin−CD34−c-kit−flt3−.23, 26 However, the frequency of CD34− SRCs in these 13Lin−CD34− cells is approximately 1/25 000, which is still very low in comparison to the frequency (1/40) of CD34+CD38− SRCs in 13Lin−CD34− cells.21, 22, 24 To more effectively purify CD34− SRCs, we then developed a high-resolution purification method using 18Lin mAbs, which can enrich CD34− SRCs to 1/1000.25
As our goal is to purify the CD34− HSCs to the single-cell level, it was necessary to identify specific positive markers for CD34− HSCs. Using these 18Lin−CD34− cell populations, we extensively analyzed the expression of candidate positive markers, including known HSC markers and various adhesion molecules, by FACS. Finally, we identified CD133, a five-transmembrane glycoprotein,27 as a positive marker of human CB-derived CD34− SRCs (HSCs) and succeeded in highly purifying primitive human CD34− SRCs (HSCs) to the level of 1/142 cells. Moreover, CD133 expression clearly segregated the SRC activities of 18Lin−CD34− cells, as well as 18Lin−CD34+ cells, in their positive fractions. These results indicate that CD133 is a common cell surface maker that can be used to isolate effectively both CD34+ and CD34− SRCs.
Materials and methods
Collection of CB samples and processing of Lin− cells
CB samples were obtained from normal full-term deliveries with signed informed consent. This study was approved by the Institutional Review Board of Kansai Medical University. The CB-derived Lin− mononuclear cells were separated using an EasySep Human Progenitor Cell Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada) and manipulated by using the RoboSep (StemCell Technologies) according to the manufacturer's instructions.
Immunostaining of Lin− cells and purification of Lin−CD34+/−CD133+/− cells
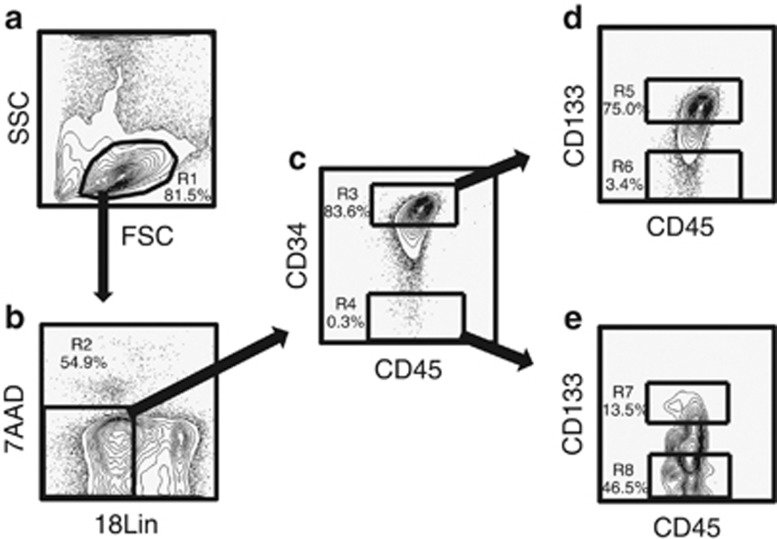
The pooled Lin− cells from multiple donors were stained with various mAbs (see Supplementary Table S1) for 30 min on ice in Ca2+− and Mg2+-free phosphate-buffered saline (PBS−) (Nakalai Tesque, Kyoto, Japan) containing 2% fetal calf serum (FCS) (Biofill, Elsternwick, VIC, Australia) (PBS−/FCS). We used fluorescein isothiocyanate-conjugated 18Lin mAbs against CD2, CD16, CD24 and CD235a (Dako, Kyoto, Japan); CD3, CD7, CD10, CD11b, CD20, CD41 and CD66c (Beckman Coulter, Fullerton, CA, USA); CD19 and CD56 (BD Biosciences, San Jose, CA, USA); CD4, CD14, CD33 and CD127 (eBioscience, San Diego, CA, USA); CD45RA (Southern Biotech, Birmingham, AL, USA); a Pacific Blue-conjugated anti-CD45 mAb (BioLegend, San Diego, CA, USA); an apophycocyanin (APC)-conjugated anti-CD34 mAb (BD Biosciences) and a phycoerythrin (PE)-conjugated anti-CD133 mAb (Miltenyi Biotec, Bergish Gladbach, Germany). The cells were then washed once thoroughly with PBS−/FCS, and resuspended in a 7-amino-actinomycin D (7-AAD) (Beckman Coulter)-containing PBS−/FCS solution before the flow cytometric (FCM) analyses or FACS. For all multicolor analyses, Fluorescence Minus One controls, wherein all other specific mAbs were present in the same tube minus the one of the interest, which is replaced with an appropriate isotype-matched control, were included for determining the fluorescent threshold. The stained cells were then sorted into four fractions, including 18Lin−CD34+/−CD133+/− cells, using a FACS Aria III (BD Biosciences) (Figure 1).
Figure 1.
Representative FACS profiles of CB-derived 18Lin−CD45+CD34+/−CD133+/− cells. (a) The FSC/SSC profile of immunomagnetically separated Lin− cells. The R1 gate was set on the blast–lymphocyte window. (b) The R2 gate was set on the 18Lin− living cells. (c) The 18Lin− living cells (R2) were subdivided into two fractions: 18Lin−CD45+CD34+ (R3) and CD34− (R4) cells, according to their expression levels of CD34. The definitions of CD34+/− cells are as follows: the CD34+ fraction contains cells expressing maximum APC fluorescence intensity (FI) to 5% the level of FI. The CD34− level of FI was determined based on the Fluorescence Minus One controls. (d) The 18Lin−CD34+ cells residing in the R3 gate were further subdivided into two fractions: 18Lin−CD45+CD133+ (R5) and CD133− (R6) cells, according to their expression levels of CD133. The definitions of CD133+/− cells are as follows: the CD133+ fraction contains cells expressing maximum PE FI to 15% the level of FI and the CD133− level of FI was determined based on the Fluorescence Minus One controls. (e) The R4-gated cells were further subdivided into two fractions: 18Lin−CD45+CD34−CD133+ (R7) and CD133− (R8) cells. The definitions of CD133+/− cells are the same as described above.
Clonal cell culture
Colony-forming cells (CFCs) were assayed using our standard methylcellulose cultures as reported.21, 23, 25
Coculture with human BM-derived mesenchymal stromal cells and the analysis of culture-generated cells by FCM
First, we established human BM-derived mesenchymal stromal cells (MSCs) using Lin−CD45− cells as reported.28 In this study, we used the MSCs established from human BM-derived Lin−CD45−CD271+SSEA-4+ cells designated as DP MSCs, which expressed a high level of the nestin gene and had supporting potential for human CB-derived CD34+/− SRCs.
A total of 1 × 103 purified 18Lin−CD34+/−CD133+/− cells per well of a 24-well plate (BD Falcon, San Jose, CA, USA) were plated onto pre-established irradiated (1200 cGy using a 137Cs-γ irradiator) DP MSC layers in StemPro-34 medium (Gibco Laboratories, Grand Island, NY, USA); a cocktail of cytokines including 50 ng/ml stem cell factor (ATgen, Gyeonggi, South Korea), 50 ng/ml flt3 ligand (FL) (R&D Systems, Minneapolis, MN, USA), 100 ng/ml thrombopoietin, 10 ng/ml interleukin (IL)-3 (R&D Systems), 100 U/ml intereukin-6 (IL-6) (kindly provided by Ajinomoto Co. Inc., Tokyo, Japan) and 10 ng/ml granulocyte colony-stimulating factor and 5% FCS (HyClone Laboratories, Logan, UT, USA). The granulocyte colony-stimulating factor and thrombopoietin were generous gifts from Kyowa Kirin Company (Tokyo, Japan). After 1 week, all of the cells were collected by vigorous pipetting, and a portion was used for the cell counting using a counting chamber. The cells were then stained with 7-AAD, an APC-conjugated anti-CD34 mAb (BD Biosciences) and a Pacific Blue-conjugated anti-CD45 mAb (BioLegend). The number of CD34+ cells and the expression of various lineage markers, including CD33, CD14, CD11b and CD41, was analyzed by five-color FCM (FACS CantoII; BD Biosciences), as described below.
NOG mice
Six-week-old female NOD/Shi-SCID/IL-2Rγcnull (NOG) mice29 were purchased from the Central Institute of Experimental Animals (Kawasaki, Japan). All mice were handled under sterile conditions and were maintained in germ-free isolators located in the Central Laboratory Animal Facilities of Kansai Medical University. The animal experiments were approved by the Animal Care Committees of Kansai Medical University.
IBMI of purified cells
IBMI was carried out as reported previously.21, 22, 23, 24, 25
The SRC assay and the serial analysis of human CD45+ cell engraftment in NOG mice by FCM
The SRC assays were performed using a previously reported method.21, 22, 23, 24, 25 In this study, 5 × 103 purified CB-derived 18Lin−CD34+/−CD133+/− cells were transplanted by IBMI into sublethally irradiated (250 cGy using a 137Cs-γ irradiator) 8-week-old NOG mice. The repopulation of human CD45+ hematopoietic cells in the NOG mouse BM was serially analyzed by our aspiration method, as reported.21, 24, 25 In brief, 1–2 μl of BM fluids were serially aspirated from the contralateral sites (right tibia) of each mouse 6, 12 and 18 weeks after transplantation, and the BM fluid was then diluted with PBS−/FCS plus 1% heparin. The mice were finally killed 18–20 weeks after transplantation and the BM cells were collected by crushing the pairs of femurs and tibiae of each mouse in a mortar. The cells were suspended in PBS−/FCS and the remaining bone tissues were removed using a cell strainer (BD Falcon). The repopulation of human hematopoietic cells in the murine BM was determined by detecting the number of 7-AAD− cells positively stained with Pacific Blue-conjugated anti-human CD45 mAb by six-color FCM (FACS CantoII; BD Biosciences). The cells were also stained with a PE-Cy7-conjugated anti-mouse CD45.1 mAb (Beckman Coulter); fluorescein isothiocyanate-conjugated anti-human CD19 (eBioscience), CD11b (Beckman Coulter) and CD235a (DAKO) mAbs; PE-conjugated anti-human CD33, CD14 and CD41 (Beckman Coulter); and an APC-conjugated anti-human CD34 mAb (BD Biosciences) for the detection of human stem/progenitor, B-lymphoid and myeloid hematopoietic cells. For the precise analysis of the T/NK-cell development in the thymus and spleen, the cells obtained from these organs were stained with fluorescein isothiocyanate-conjugated anti-human CD3 (Beckman Coulter), PE-conjugated anti-human CD4 and an APC-conjugated anti-human CD8 mAbs (eBioscience) and a fluorescein isothiocyanate-conjugated anti-human CD56 mAb (BD Biosciences).
The mice were scored as positive if more than 0.01% of the total murine BM cells were human CD45+ cells. In separate experiments, we confirmed the detection limit of human CD45+ cells in mouse BMs was 0.005% (Supplementary Figure S1).
Secondary transplantation
For secondary transplantations, murine BM cells were obtained 18–20 weeks after transplantation from the pairs of femurs and tibiae of engrafted primary recipient NOG mice that received 5 × 103 18Lin−CD34+/−CD133+ cells. One out of the five portions of the whole BM cells were transplanted by IBMI into sublethally (250 cGy) irradiated secondary recipient NOG mice. Eighteen weeks after transplantation, the presence of human CD45+ cells in the secondary recipients' BM was analyzed by FCM, as described for primary transplantation.
In vivo limiting dilution analysis
To assess the frequency of SRCs in the CB-derived 18Lin−CD34−CD133+ cells, various numbers of cells (200, 400 and 800; n=15) were transplanted into NOG mice by IBMI, as reported.21, 22, 23, 24, 25, 26 The mice were killed 12 weeks after transplantation, and the human cell repopulation in the mouse BM was analyzed by FCM as described above.
Statistical analyses
In the limiting dilution analysis (LDA) (Figure 6), the frequencies of SRCs were calculated using a software application for the LDA as reported.30 In Figures 2 and 3 and Supplementary Figure S3, the differences in the mean colony numbers or expression levels of each pair were examined by two-tailed Student's t-test.
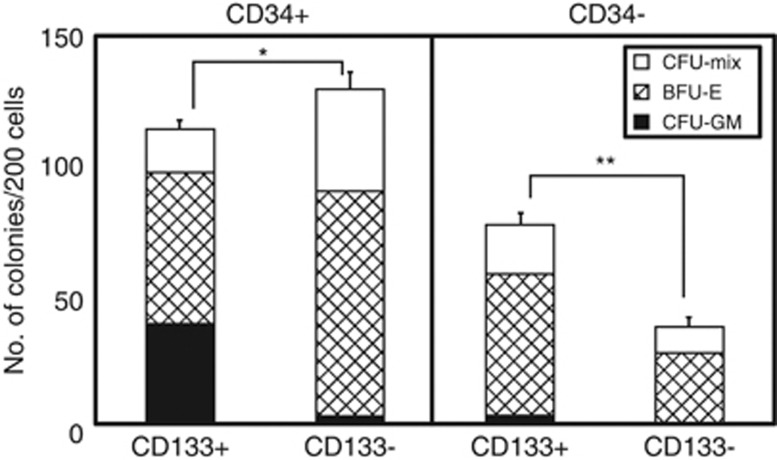
Figure 2.
Colony-forming capacities of sorted 18Lin−CD45+CD34+/−CD133+/− cells. Two hundred sorted 18Lin−CD45+CD34+/−CD133+/− cells were cultured in methylcellulose at 37 °C with 5% CO2, 5% O2 and 90% N2 in the presence of stem cell factor, thrombopoietin, IL-3, granulocyte/macrophage colony-stimulating factor, granulocyte colony-stimulating factor and erythropoietin for 12 to 14 days. The number and composition of various colonies derived from the 18Lin−CD45+CD34+/−CD133+/− cells are shown. The types of colonies identified in situ were granulocytes (CFU-G), macrophages (CFU-M), granulocyte/macrophage (CFU-GM), erythroid burst (BFU-E) and erythrocyte-containing mixed (CFU-Mix). Open, shaded and closed bars represent the number of CFU-Mix, BFU-E and CFU-GM (including CFU-G, CFU-M and CFU-GM) colonies, respectively. The numbers of all the types of hematopoietic colonies were determined as the mean of quadruple cultures. The data represent the means±s.d. *P<0.05; **P<0.01.
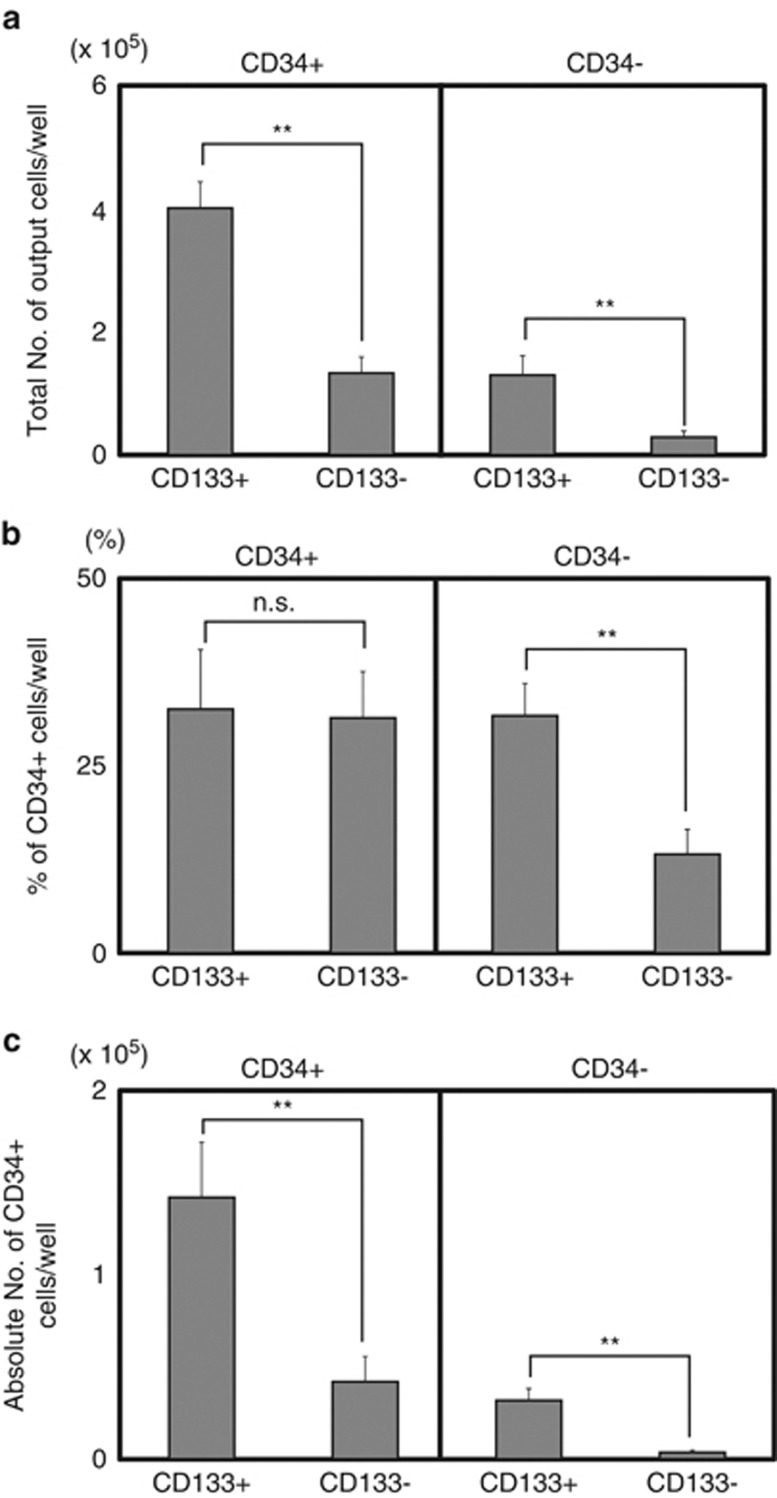
Figure 3.
Coculture of 18Lin−CD45+CD34+/−CD133+/− cells with DP MSCs. A total of 1 × 103 purified 18Lin−CD45+CD34+CD133+/− cells (left panel) and 18Lin−CD45+CD34−CD133+/− cells (right panel) were cocultured with DP MSCs in the presence of six cytokines (SCF+FL+TPO+IL-3+IL-6+G-CSF) for 1 week. Each coculture contained 22 wells. (a) The total numbers of output cells per well. (b) The percentages of CD34 expression on the culture-generated CD45+ cells are shown. (c) The absolute numbers of CD34+ cells maintained/produced in cocultures with DP MSCs are shown. The data represent the means±s.d. **P<0.01; NS, not significant; G-CSF, granulocyte colony-stimulating factor; SCF, stem cell factor; TPO, thrombopoietin.
Results
Identification of candidate positive markers of CD34− SRCs (HSCs)
First, we extensively explored candidate positive markers that could be useful for the efficient purification of rare CB-derived CD34− SRCs (HSCs), including known HSC markers and various adhesion molecules, using highly purified CB-derived 18Lin−CD34+/− cells25 by multicolor FCM. Representative data are shown in Supplementary Figure S2. Known HSC markers, including Tie2, KDR, ABCG2 and CD150, were not expressed on either 18Lin−CD34+ or 18Lin−CD34− cells. On the other hand, CD133, c-kit, CD90, CXCR4, CD49f and CD93 were expressed on both types of cells. The 18Lin−CD34+/− cells could be subdivided into positive and negative fractions. In order to select a good positive marker from these candidate molecules, we back-gated the positive fractions of these markers in 18Lin−CD34− cells into forward scatter/side scatter (FSC/SSC) scattergrams. As clearly seen in Supplementary Figure S2, the distribution of c-kit+, CD90+, CXCR4+, CD49f+ and CD93+ cells in the 18Lin−CD34− cells was scattered inside and around the blast window. In contrast, the 18Lin−CD34−CD133+ cells were concentrated in the low FSC blast window. On the basis of these data, we selected CD133 as a candidate positive marker for human CB-derived CD34− SRCs (HSCs).
Purification of human cord blood-derived 18Lin−CD34+/−CD133+/− cells
First, the R1 gate was set on the blast–lymphocyte window (Figure 1a). Next, the 7-AAD−18Lin− cells were gated as R2, as shown in Figure 1b. The 18Lin−CD45+ CD34+ and 18Lin−CD45+CD34− cells were then gated as R3 and R4 (Figure 1c), respectively. The 18Lin−CD45+CD34+/− cells were further subdivided into four distinct populations gated as R5 (18Lin−CD45+CD34+CD133+), R6 (18Lin−CD45+CD34+CD133−), R7 (18Lin−CD45+CD34−CD133+) and R8 (18Lin−CD45+CD34−CD133−), based on their surface CD133 expression (Figures 1d and e). Then, the 18Lin−CD34+CD133+/− (R5 and R6) and 18Lin−CD34−CD133+/− (R7 and R8) fractions were sorted for the subsequent in vitro cultures and in vivo SRC assays. As shown in Figures 1d and e, 75% of the 18Lin−CD45+CD34+ fraction (R3) and 13.5% of the 18Lin−CD45+CD34− fraction (R4) expressed CD133.
The hematopoietic colony-forming capacity of CB-derived 18Lin−CD34+/−CD133+/− cells
The CFC capacities of the CB-derived 18Lin−CD34+ and CD34− cells were unique (Figure 2). The plating efficiencies of 18Lin−CD34+CD133+, CD34+CD133−, CD34−CD133+ and CD34−CD133− cells were 57%, 65%, 39% and 19%, respectively. Interestingly, the 18Lin−CD34−CD133+/− cells mainly formed erythroid burst (BFU-E) (71 and 73%) and colony-forming unit (CFU)-Mix (mostly erythro/megakaryocytes containing mixed colonies) colonies (25 and 27%). On the contrary, they formed few CFU-granulocyte/macrophage (CFU-GM) colonies (4 and 0%). On the other hand, the 18Lin−CD34+CD133+ cells formed all types of CFCs, including CFU-GM, BFU-E and CFU-Mix. It is of interest that the 18Lin−CD34+CD133− cells formed mainly BFU-E (68%) and CFU-Mix (30%) colonies, and few CFU-GM (2%) colonies, which was a CFC pattern similar to that of the 18Lin−CD34− cells. These results are consistent with our previous report,25 and again confirmed that the CFC capacities can be detected in the CB-derived 18Lin−CD45+CD34− cell fraction. These results indicated that the CB-derived 18Lin−CD45+CD34− cell fraction contains HPCs.
Coculture with human BM-derived MSCs (DP MSCs)
As reported,21, 23 the CD34− SRCs could produce CD34+ SRCs in vitro. Therefore, 1 × 103 18Lin−CD34+/−CD133+/− cells were cocultured with human BM-derived MSCs (DP MSCs),28 in the presence of six cytokines for 1 week. The 18Lin−CD34+/−CD133+/− cells actively proliferated and maintained/generated CD34+ cells (Figures 3a–c). In the cocultures of 18Lin−CD34+CD133+/− cells, the total number of cells expanded by 130- (1.3 × 105 cells) to 450- (4.5 × 105 cells) fold, which contained approximately 30% of the CD34+ cells, resulting in a significantly higher number of CD34+ cell recovery in the coculture with CD34+CD133+ cells. On the other hand, the total number of cells derived from 18Lin−CD34−CD133+/− cells expanded by 30- (3 × 104 cells) to 130- (1.3 × 105 cells) fold. Both the 18Lin−CD34−CD133+/− cells produced CD34+ cells. However, the percentage and absolute number of CD34+ cells produced from 18Lin−CD34−CD133+ cells (31.7% and 3.2 × 104 cells) were significantly higher than those of 18Lin−CD34−CD133− cells (13.2% and 0.4 × 104 cells). In addition, both the 18Lin−CD34−CD133+/− cells generated higher percentages (13.5 and 11.5%) of CD41+ cells compared with those produced from the 18Lin−CD34+CD133+/− (1.8 and 4.2%) cells (Supplementary Figures S3–D). In contrast, the 18Lin−CD34+/−CD133+ cells generated significantly higher percentages of CD33+, CD11b+ and CD14+ cells than did the 18Lin−CD34+/−CD133− cells (Supplementary Figure S3A–C). Collectively, these results indicated that the 18Lin−CD34−CD133+/− cell fraction contained a substantial number of CD34+ cell-producing HPCs/HSCs, and that the 18Lin−CD34+/−CD133+/− cells showed different in vitro lineage differentiation potential.
SRC activities and multilineage differentiation potentials of CB-derived 18Lin−CD34+/−CD133+/− cells following IBMI
Thereafter, we investigated the SRC activity of the 18Lin−CD34+/−CD133+/− cells using NOG mice. Previously, Lapidot and his co-workers reported elegant studies showing that the chemokine stromal cell-derived factor-1 (CXCL12) and its receptor, CXCR4, has a pivotal role in the homing and repopulation of CD34+ SRCs in NOD/SCID mice.31, 32 Moreover, it was recently reported that the stromal cell-derived factor-1–CXCR4 axis modulates homing, BM retention and the mobilization of HSPCs in a more complex way than was thought previously.33 We previously demonstrated that CB-derived CD34− SRCs expressed lower levels of homing receptors, including CXCR4, and that they could not migrate toward the gradient of stromal cell-derived factor-1 in transwell cultures.21
These data suggested that CB-derived CD34− SRCs cannot home into the BM niche following conventional tail vein injection. We previously demonstrated that human CB-derived CD34− SRCs could only be detected following the IBMI method.21, 22, 23, 24, 25, 26 On the basis of these results, 5 × 103 18Lin−CD34+/−CD133+/− cells were transplanted by IBMI into NOG mice, and the human cell repopulation was analyzed 18–20 weeks after transplantation. Interestingly, all 10 mice that received 18Lin−CD34+/−CD133+ cells (five mice each) were repopulated with human CD45+ cells (Table 1). The level of human cell engraftment was 63.1 to 88.0% (mean, 73.9%) for 18Lin−CD34+CD133+ cells and 11.0 to 52.7% (mean, 30.4%) for 18Lin−CD34−CD133+ cells. In contrast, none of the 12 mice that received 18Lin−CD34+/−CD133− cells (six mice each) were repopulated with human cells. These results demonstrated, for the first time, that the CD133 is a positive marker for human CB-derived CD34− SRCs, and also that CD133 expression clearly segregates the SRC activities of the 18Lin−CD34+/− cells in their positive fraction.
Table 1. The SRC activity of sorted CB-derived 18Lin−CD34+/−CD133+/− cells.
| Type of cells | No. of transplanted cells | % of human CD45+ cellsa (mean) | Incidence of engraftment |
|---|---|---|---|
| 18Lin−CD34+CD133+ | 5000 | 63.1–88.0 (73.9) | 5/5 |
| 18Lin−CD34+CD133− | 5000 | 0 (0) | 0/6 |
| 18Lin−CD34−CD133+ | 5000 | 11.0–52.7 (30.4) | 5/5 |
| 18Lin−CD34−CD133− | 5000 | 0 (0) | 0/6 |
Abbreviations: BM, bone marrow; CB, cord blood; SRC, SCID-repopulating cell.
The human cell repopulation in the right tibia of primary recipient mice was analyzed 18 weeks after transplantation by BM aspiration.
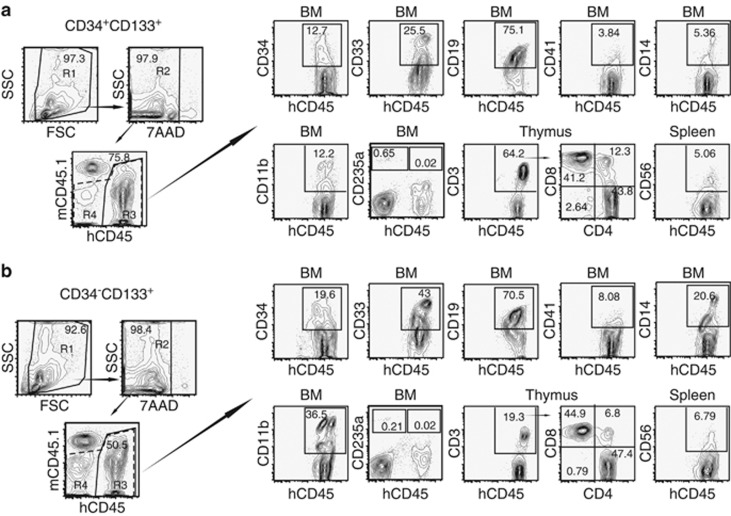
To further evaluate the functional differences between the CD34−CD133+ and CD34+CD133+ SRCs, we studied their multilineage reconstitution abilities in various organs, including the BM, spleen and thymus, in NOG mice that were transplanted with 5 × 103 of 18Lin−CD34+/−CD133+ cells by IBMI. The results of the repopulation patterns are shown in Figure 4. The analyses of the two representative mice that were transplanted with either CD34+CD133+ SRCs (Figure 4a, upper column) or CD34−CD133+ SRCs (Figure 4b, lower column) demonstrated both of the SRCs to have a comparable in vivo differentiation capacity into CD34+ stem/progenitor cells, B-lymphoid, myeloid, monocytic, megakaryocytic and erythroid lineages at 18–20 weeks after the transplantation. In addition, CD4+ and CD8+ single- and double-positive T-lymphoid cells were detected in the CD3+ cell gates of the thymus in both types of NOG mice. The CD56+ natural killer cells were also detected in the spleens in both types of NOG mice. These results confirmed that both the CD34+/−CD133+ SRCs had multilineage differentiation potentials.
Figure 4.
Long-term multilineage reconstitution abilities of CD34+CD133+ and CD34− CD133+ SRCs. First, the R1 gate was set on the total murine BM cells obtained from these two representative NOG mice that received (a) CD34+CD133+ SRCs (upper column) and (b) CD34− CD133+ SRCs (lower column), 20 weeks after transplantation. Thereafter, the human CD45+ cells were gated as R3 (solid line) from the R2-gated 7-AAD− cells. The expression of surface markers, including CD34, CD33, CD19, CD41, CD14 and CD11b, on the R3-gated cells was analyzed by six-color FCM. Only the expression of CD235a was analyzed using the R4 gate (dotted line) containing CD45+/− cells. The percentages of positive cells in each scattergram (CD45, CD34, CD33, CD19, CD41, CD14, CD11b and CD235a) are presented in the indicated squares. The expression of CD3, CD4 and CD8 on the human CD45+ cells in the thymus, and the expression of CD56 on the human CD45+ cells in the spleen was also analyzed and presented. The percentages of positive/negative cells in each scattergram are indicated.
Long-term repopulation patterns of CD34+/−CD133+ SRCs in NOG mice and their secondary repopulating abilities
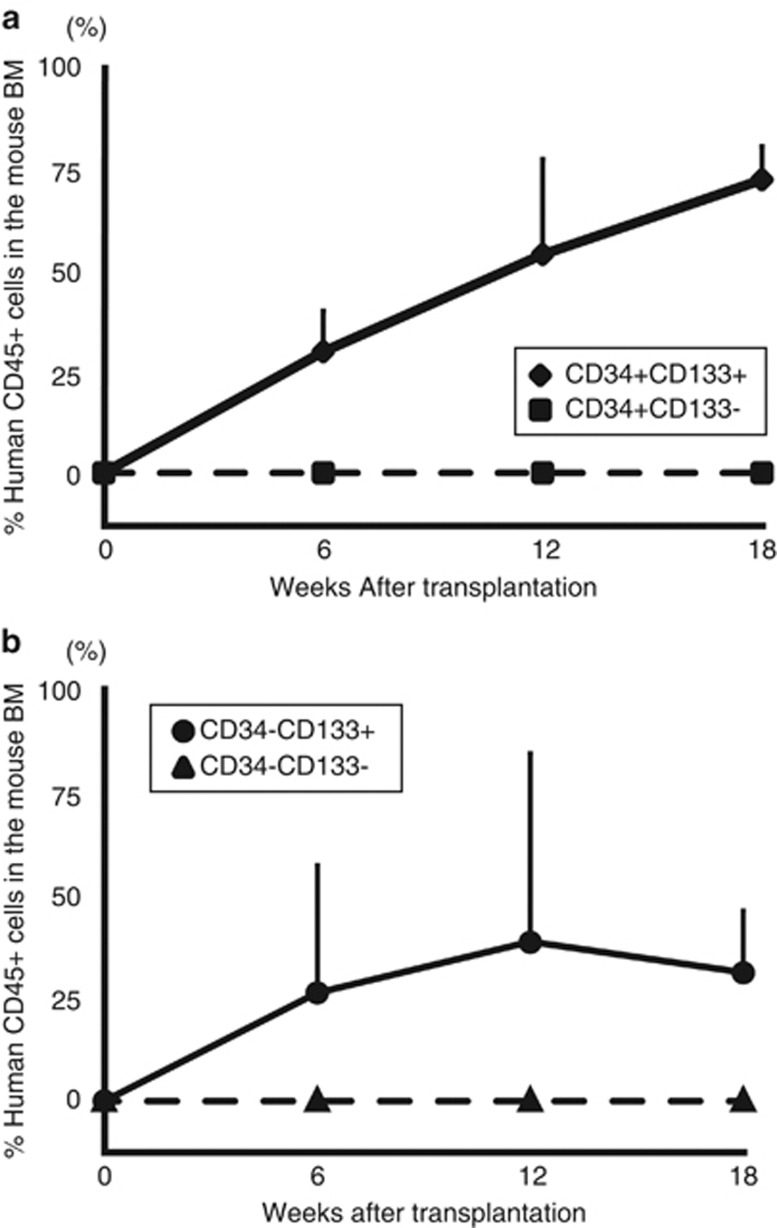
The next approach to characterize the self-renewal potential and the long-term repopulating potential of the CD34− SRCs was to serially analyze the kinetics of BM engraftment for 18 weeks in NOG mice that received transplants of 5 × 103 18Lin−CD34+/−CD133+ cells (Figures 5a and b).
Figure 5.
Long-term human hematopoietic cell reconstitution in NOG mice. A total of 5 × 103 18Lin−CD34+/−CD133+/− cells (a and b) was injected into the left tibiae of NOG mice by IBMI. The human CD45+ cell rates in the contralateral sites of recipient mice were serially analyzed by the BM aspiration method 6, 12 and 18 weeks after transplantation by six-color FCM. The data represent the means±s.d. of the results from five or six mice at each time point.
In these experiments, both the mice that received transplants of CD34+/−CD133+ SRCs showed signs of human cell repopulation at 6 weeks after the transplantation. The repopulation level of all five mice that received both types of SRCs gradually increased, and reached the peak levels at 12–18 weeks after transplantations. As we mentioned above, none of the mice that received 5 × 103 18Lin−CD34+/−CD133− cells were repopulated with human cells (Figures 5a and b).
We then performed the secondary transplantation to analyze their self-renewing capacities of the cells. All of the secondary recipient mice that received the transplants from the primary recipient mice that received 5 × 103 of either of the 18Lin−CD34+/−CD133+ cells by IBMI showed signs of human cell repopulation 18 weeks after the transplantation (Supplementary Table S2). The human CD45+ cell rates in all the secondary recipient mice showed comparable levels of human cell repopulation (0.01–0.03% vs 0.04–0.14%). We confirmed that all of the secondary recipient mice that received transplants of CD34−CD133+, as well as CD34+CD133+ SRCs, showed multilineage human hematopoiesis, including CD19+ and CD33+ cells (Supplementary Figure S4).
These results demonstrated that both the CD34−CD133+ and CD34+CD133+ SRCs had comparable secondary reconstituting abilities, and could sustain long-term (up to 38 weeks) human hematopoiesis in NOG mice, suggesting that both types of SRCs had significant self-renewing potentials.
LDA of CB-derived 18Lin−CD34−CD133+ cells by IBMI
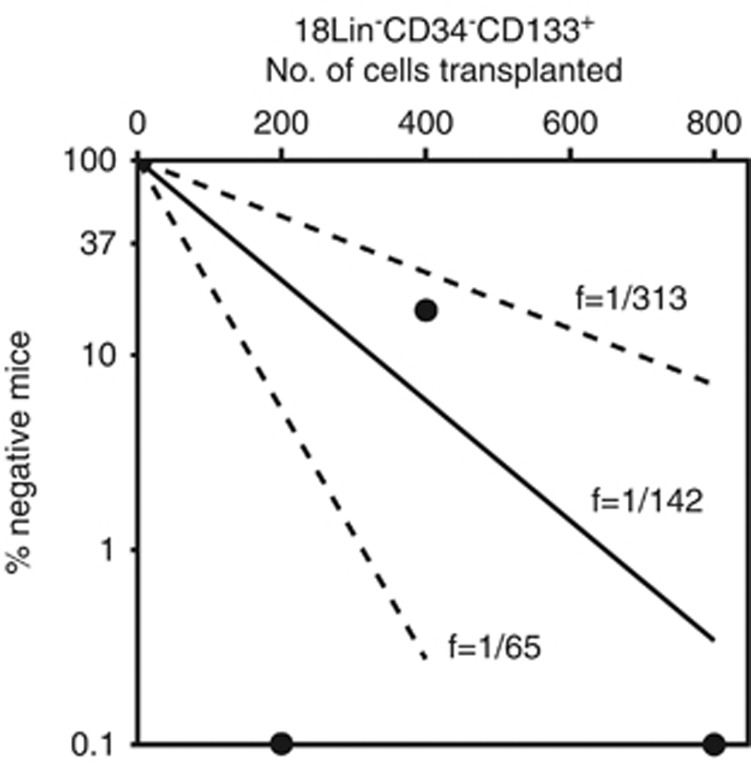
We first reported that the frequency of the CB-derived CD34− SRCs in the 13Lin−CD34− cells was 1/25 000.21 Then, we developed a high-resolution purification method improving our negative selection method, resulting in an incidence of CD34− SRCs of 1/1000.25 In this study, we succeeded in more highly purifying the CD34− SRCs using the identified positive marker, CD133. As expected, a LDA using the IBMI demonstrated the frequency of the CD34− SRCs in the 18Lin−CD34−CD133+ cells to be 1/142, which is the highest level of purification of this unique CD34− HSC population to date. (Figure 6). Therefore, the present method can enrich the CD34− SRCs 180 times more than the previously described negative selection method.21
Figure 6.
The frequency of SRCs in the 18Lin−CD34−CD133+ cells. Various numbers of 18Lin−CD34−CD133+ cells (200, 400 and 800) were transplanted to NOG mice (n=15). The human CD45+ cell repopulation in the mouse BM was analyzed by FCM 12 weeks after transplantation. The frequency of SRCs was one per 142 18Lin−CD34−CD133+ cells. For the frequency determination, the middle solid line represents the estimated weighted mean frequency (fWM). The lower and upper dotted lines represent the 95% confidence interval of the fWM.
Discussion
Until recently, all human HSCs/HPCs were believed to be present in the Lin−CD34+ cell fraction.1, 2, 3, 4 However, the existence of the human CD34− HSCs was suggested by several studies.17, 18, 19, 20 Bhatia et al.17 first reported that SRCs were present in human BM- and CB-derived Lin−CD34− cells using tail vein injection. However, the incidence of CD34− SRCs in the Lin−CD34− cell population was 1/125 000, which was very low. In that report, they demonstrated that the frequency of CD34− SRCs increased to 1/38 000 cells after 4 days of short-term culture of these Lin−CD34− cells in the presence of a cocktail of cytokines. This group later reported that ex vivo culture of the AC133+ subset from human CB-derived CD34−CD38−Lin− cells allowed for a detection of SRC activity in NOD/SCID mice by a Southern blot analysis.34 However, the levels of human chimerism in the BM of recipient mice were very low, even after ex vivo culture. Collectively, these results suggested that homing receptors, including CXCR4, may become upregulated on CD34− SRCs during the culture, resulting in the improvement of their engraftment potential by tail vein injection. The existence of long-term repopulating CD34− HSCs in human CB- and BM-derived Lin− cells was also supported by data showing that the CD34− fraction of normal human BM contains cells capable of engraftment and differentiation into CD34+ progenitors, as well as multiple lymphohematopoietic lineages, using the human/sheep competitive engraft model.18 However, studies on human CD34− HSCs have been hindered by the lack of a positive marker comparable to Sca-1 in mice. In this study, we tried to identify useful/reliable positive markers for highly purifying rare CD34− HSCs.
We recently developed a high-resolution purification method using 18Lin mAbs (the previously reported 13Lin mAbs21 plus five Lin mAbs against CD11b, CD33, CD66c, CD45RA and CD127), which can enrich CD34− SRCs to 1/1000.25 Using 18Lin−CD34− cells as a target population, we extensively analyzed the expression of candidate positive markers, including known HSC markers and various adhesion molecules, by multicolor FCM. We finally identified CD133 as a reliable positive marker for human CB-derived CD34− SRCs (HSCs), and succeeded in highly purifying primitive human CD34− HSCs at an incidence of 1/142 cells. This purification level of human CD34− SRCs (HSCs) is the highest reported in the literatures to date. The present data are consistent with earlier studies demonstrating that the AC133+CD34+ population from the CB and BM is enriched for SRC activity.35, 36
It is very important to clarify the molecular mechanisms controlling the maintenance, quiescence, proliferation and differentiation of very primitive CD34− HSCs. For this purpose, it will be necessary to highly purify these CD34− HSCs. This is because if a crude Lin−CD34− cell population is used, then these cells may contain many contaminating lymphocytes and myeloid cells, as shown in Figure 1b and Supplementary Figure S5. Therefore, we cannot obtain accurate information about the gene expression and signal transduction that control the above-mentioned processes in very primitive CD34− HSCs. To truly elucidate the molecular mechanisms controlling the maintenance, quiescence, proliferation and differentiation of very primitive CD34− HSCs, it will be necessary to better purify CD34− HSCs.
Very recently, Bonnet and co-workers37 reported that CD93 is a positive marker of human CB-derived very primitive CD34− HSCs. They transplanted Lin−CD34−CD93+ or Lin−CD34−CD38−CD93+cells, which did not express CD133, by tail vein injection, and demonstrated their SRC activity. In contrast, our identified CB-derived CD34− SRCs could only be detected by IBMI.21, 22, 23, 24, 25, 26 Furthermore, as shown in Supplementary Figure S2, CD93 was only marginally expressed on our highly purified 18Lin−CD34− cells. When we back-gated these cells into the FSC/SSC scattergram, the 18Lin−CD34−CD93+ cells were scattered inside and around the blast window. We further analyzed the expression of CD93 on the CB-derived 18Lin−CD34−CD133+ cells, which contained all of the CD34− SRC activity in the CB demonstrated by the present study. As clearly seen in Supplementary Figure S5, we did not detect any CD93+ cells in our 18Lin−CD34−CD38−/loCD133+ cell population. As reported, their Lin−CD34−CD38−CD93+ cells did not express CD133. However, almost all of the Lin−CD34−CD38−CD93+ cells were included in the 18Lin+ cell fraction (Supplementary Figures S5–R27). This is not surprising because they only depleted mature cells from their target cells by immunomagnetic beads using the StemSep (StemCell Technologies), which only removed strongly Lin+ cells. These immunomagnetically Lin-depleted cells still contained many lymphocytes, monocytes, granulocytes and natural killer cells (see Figure 1b and Supplementary Figure S5), which expressed CD93.38 Human CB-derived CD45RA+-naive T-lymphocytes were previously reported to express CD93.39 Indeed, their incidence of CD34− SRCs in the Lin−CD34−CD38−CD93+ cells was 1/∼6100 cells, which is very low compared with our present data (1/142). A comparison of the results of these two studies is summarized in Supplementary Table S3. Overall, their data are exactly the opposite of our data, thus suggesting the possibility that there may be different classes of CD34− HSCs in the primitive human HSC compartment. Further studies will therefore be required to clarify this important issue.
As our goal is to purify the CD34− HSCs at the single-cell level, it is inevitable that additional specific positive markers for CD34− HSCs will need to be identified besides CD133. Using our newly developed high-resolution purification method, studies to identify additional specific positive markers of CD34− HSCs are now underway in our laboratory. In our model, the CD34− SRCs were more immature than the CD34+CD38− SRCs, as the CD34− SRCs could generate CD34+ SRCs in vitro as well as in vivo,21, 23 and the limited number of CD34− SRCs transplanted into primary recipient mice sustained primary, secondary and tertiary transplantation (over 1 year) in NOG mice, as we recently reported.40 These results may suggest that the hierarchical position of CD34+CD38− SRCs is in the intermediate stage of the HSC compartment, between CD34− SRCs and CD34+CD38+ SRCs.24, 26 However, further studies will be required to fully elucidate the proposed model of the human HSC hierarchy.24, 26
In summary, the present results clearly demonstrated that CD133 is a reliable positive marker of human CB-derived CD34− SRCs (HSCs). Furthermore, CD133 expression clearly segregated the SRC activities of 18Lin−CD34− cells, as well as 18Lin−CD34+ cells, in their positive fractions, indicating that CD133 is a common cell surface marker for both CD34+/− SRCs. More importantly, these findings suggest that the number of CD133+ cells in cord blood units is a more appropriate marker to detect/predict the HSC potential of cord blood stem cell transplantation in comparison to the currently used CD34+ cell numbers.
Acknowledgments
This work was supported by grants-in-aid for Scientific Research C (Grant Nos. 21591251 and 24591432) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a grant from the Science Frontier Program of the MEXT; a grant from the Strategic Research Base Development Program for Private Universities from the MEXT; the MEXT-Supported Program for the Strategic Research Foundation at Private Universities; a grant from the Promotion and Mutual Aid Corporation for Private Schools of Japan; a grant from the Japan Leukemia Research Foundation; a grant from the Mitsubishi Pharma Research Foundation; a grant from the Takeda Science Foundation; a grant from the Terumo Life Science Foundation and a grant from SENSHIN Medical Research Foundation. We are grateful to the Japanese Red Cross Kinki Cord Blood Bank for providing the samples used in this study. Kyowa Kirin Company (Tokyo, Japan) is also acknowledged for providing the various growth factors used in this study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15:293–300. doi: 10.1097/MOH.0b013e328302c7ca. [DOI] [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yimaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Cao Y-A, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowle M, McCaffrey L, Hamilton M, Lyons K, et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Dick JE, Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int J Hematol. 2005;82:389–396. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99:413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JL, Takenaka K, Gan OI, Doedens M, Dick JE. Low rhodamine 123 retention identifies long-term human hematopoietic stem cells within the Lin−CD34+CD38− population. Blood. 2007;109:543–545. doi: 10.1182/blood-2006-06-030270. [DOI] [PubMed] [Google Scholar]
- Yahata T, Muguruma Y, Yumino S, Sheng Y, Uno T, Matsuzawa H, et al. Quiescent human hematopoietic stem cells in the bone marrow niches organize the hierarchical structure of hematopoiesis. Stem Cells. 2008;26:3228–3236. doi: 10.1634/stemcells.2008-0552. [DOI] [PubMed] [Google Scholar]
- Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26:353–360. [PubMed] [Google Scholar]
- Dao MA, Arevalo J, Nolta JA. Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood. 2003;101:112–118. doi: 10.1182/blood-2002-01-0025. [DOI] [PubMed] [Google Scholar]
- Ando K, Nakamura Y, Chargui J, Matsuzawa H, Tsuji T, Kato S, et al. Extensive generation of human cord blood CD34+ stem cells from Lin−CD34− cells in a long-term in vitro system. Exp Hematol. 2000;28:690–699. doi: 10.1016/s0301-472x(00)00154-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, et al. SCID-repopulating cell activity of human cord blood-derived CD34− cells assured by intra-bone marrow injection. Blood. 2003;101:2924–2931. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- Kimura T, Wang J, Matsui K, Imai S, Yokoyama S, Nishikawa M, et al. Proliferative and migratory potentials of human cord blood-derived CD34− severe combined immunodeficiency repopulating cells that retain secondary reconstituting capacity. Int J Hematol. 2004;79:328–333. doi: 10.1532/ijh97.03158. [DOI] [PubMed] [Google Scholar]
- Kimura T, Asada R, Wang J, Kimura T, Morioka M, Matsui K, et al. Identification of long-term repopulating potential of human cord blood-derived CD34−flt3− severe combined immunodeficiency-repopulating cells by intra-bone marrow injection. Stem Cells. 2007;25:1348–1355. doi: 10.1634/stemcells.2006-0727. [DOI] [PubMed] [Google Scholar]
- Kimura T, Matsuoka Y, Murakami M, Kimura T, Takahashi M, Nakamoto T, et al. In vivo dynamics of human cord blood-derived CD34− SCID-repopulating cells using intra-bone marrow injection. Leukemia. 2010;24:162–168. doi: 10.1038/leu.2009.206. [DOI] [PubMed] [Google Scholar]
- Ishii M, Matsuoka Y, Sasaki Y, Nakatsuka R, Takahashi M, Nakamoto T, et al. Development of a high resolution purification method for precise functional characterization of primitive human cord blood-derived CD34-negative SCID-repopulating cells. Exp Hematol. 2011;39:203–213. doi: 10.1016/j.exphem.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Sonoda Y. Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: the significance of the intra-bone marrow injection. J Autoimmun. 2008;30:136–144. doi: 10.1016/j.jaut.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Matsuoka Y, Sasaki Y, Takahashi M, Nakatsuka R, Uemura Y, Inoue M, et al. Prospective isolation and functional characterization of human bone marrow-derived hematopoietic stem cell-supportive mesenchymal stromal cells Blood 20101161575–1576.(abstract 3852). [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Hu Y, Smyth GK. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz E, et al. Rapid and efficient homing of human CD34+CD38−/lowCXCR4+ stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2mnull mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Abdol-Latif A, Schneider G, Kucia M, Morris AJ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34−Lin− and CD34+Lin− hematopoietic stem cells using cell surface marker AC133 and CD7. Blood. 2000;95:2813–1820. [PubMed] [Google Scholar]
- Wynter EA, Buck D, Hart C, Heywood R, Coutinho LH, Clayton A, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–396. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- Drake AC, Khoury M, Leskov I, IIiopoulou BP, Fragoso M, Lodish H, et al. Human CD34+CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID II2rγ−/− (NSG) mice. PLoS One. 2011;6:e18382. doi: 10.1371/journal.pone.0018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjos-Alfonso F, Currie E, Palmer HG, Foster KE, Taussig DC, Bonnet D. CD34− cells at the apex of the human hematopoietic stem cell hierarchy have distinctive cellular and molecular signatures. Cell Stem Cell. 2013;13:161–174. doi: 10.1016/j.stem.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Nepomuceno RR, Tenner AJ. C1qRp, the C1q receptor that enhance phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J Immunol. 1998;160:1929–1935. [PubMed] [Google Scholar]
- Ikewaki N, Yamato H, Kulski JK, Inoko H. Flow cytometric identification of CD93 expression on naïve T lymphocytes (CD4+CD45RA+ cells) in human neonatal umbilical cord blood. J Clin Immunol. 2010;30:723–733. doi: 10.1007/s10875-010-9426-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Matsuoka Y, Iwaki R, Nakatsuka R, Fujioka F, Kohno H, et al. Functional significance of MPL expression in the human primitive hematopoietic stem cell compartment. The 54th ASH Annual Meeting Abstract Available at: https://ash.confex.com/ash/2012/webprogram/paper48699.html (abstract 1195).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.