Staining with annexin V, which detects the externalization of phosphatidylserine on cells, is a widespread method for the assessment of cell death, and in particular, apoptosis.1 Although additional assays are important for demonstrating apoptosis, many studies use annexin V staining in isolation and directly equate annexin V positivity with apoptosis. Is this a problem? Although cells undergoing apoptosis externalize phosphatidylserine, describing annexin V-positive cells as apoptotic can be misleading. Here we briefly highlight strengths and limitations of assessing apoptosis using annexin V staining.
Apoptosis is a defined cell death mechanism, the central features of which include: activation of caspases, changes in the plasma membrane, nuclear condensation, DNA fragmentation and cell shrinkage. Importantly these changes occur before the loss of plasma membrane integrity.2 Other forms of cell death have been defined such as necrosis and ‘autophagic' cell death whereas numerous others linger on the edge of formal definition.3 These alternate forms of cell death can have overlapping biochemical features, some of which have also been ascribed to apoptosis.
The biochemical features of apoptosis have been used to develop a range of assays, with the cleavage of caspases, DNA fragmentation, mitochondrial depolarization and membrane changes being popular. Perhaps the most commonly used assay is annexin V staining, which is based on the changes in plasma membrane lipid asymmetry that occur early in apoptosis. The plasma membrane is highly organized consisting of two leaflets of distinct composition. The outer leaflet is electrostatically neutral being made up of phosphatidylcholine and sphingomyelin, whereas the inner leaflet contains the aminophospholipids phosphatidylethanolamine and phosphatidylserine (PS), and the negatively charged phosphatidic acid. This asymmetry is maintained in an energy dependent manner by specific lipid transporters. On caspase activation, the membrane protein Xkr8 is cleaved and ‘scrambles' the plasma membrane lipids, such that the asymmetry is lost.4 Cell surface phosphatidylserine provides an ‘eat me' signal which results in the rapid removal of apoptotic cells by phagocytes. Annexin V preferentially binds to negatively charged phospholipids such as PS, and when fluorescently labeled, it provides a rapid, simple and sensitive assay to quantitate the proportion of cells with exposed PS. It requires relatively few cells and can be added to multi-parameter approaches such as flow cytometry to simultaneously detect a number of other cellular features.5, 6 Because lipid asymmetry is also lost upon disruption of plasma membrane integrity, this is often combined with membrane-impermeable stains to detect cells that undergo apoptosis. The simplicity of the assay along with its active promotion by industry has resulted in its extensive use and the terms annexin V-positive and apoptosis have for many become synonymous. Unfortunately despite the elegant simplicity of the assay, its interpretation is not that simple.
How specific is annexin V staining for apoptosis? Loss of plasma membrane asymmetry occurs in most cells undergoing apoptosis, although cells lacking Xpr8, which occurs in some cancers4 fail to do so, and defective autophagy appears to also disrupt this process, resulting in false negative results.7 Another reason for not detecting or underestimating apoptosis by annexin V staining is the rapid removal of cells in early apoptosis by phagocytic cells. This commonly occurs in vivo, but can also occur in in vitro cultures where phagocytes are present, such as blood or bone marrow cultures. However, externalization of phosphatidylserine can occur independently of apoptosis. In particular, a calcium dependent lipid scramblase, TMEM16, causes loss of lipid asymmetry upon elevation of intracellular calcium in the absence of cell death.8 Healthy monocytes and macrophages can stain positive following the phagocytosis, stressed tumor cells and tumor vascular endothelium as well as activated platelets can also bind annexin V. In addition, viral entry into host cells and fertilization of ova cause transient externalization of phosphatidylserine.
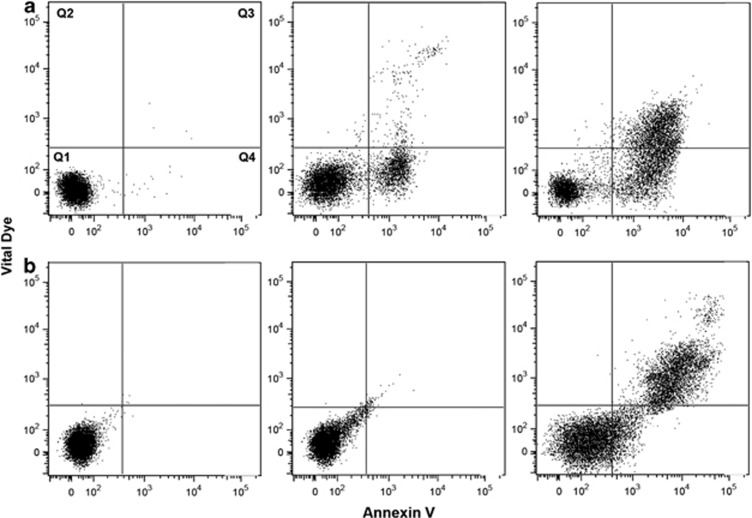
The failure to ensure that the cells retain an intact plasma membrane is a common error in the assessment and reporting of annexin V data. If the plasma membrane integrity is lost, even transiently, annexin V will bind to cells regardless of whether or how they have died.5, 6, 7 This can be particularly problematic when cells have been isolated by mechanical means, have undergone electroporation, transfection or transduction, all of which can cause at least transient leakiness of the plasma membrane and loss of membrane asymmetry. A vital dye must be included to determine that phosphatidylserine has been externalized (annexin V-positive/vital dye negative, Figure 1a Q4). Nonetheless, cells are regularly reported as being annexin V-positive and apoptotic without data confirming the integrity of the plasma membrane. In these situations it is impossible to know whether cells had externalized phosphatidylserine or a permeabilized plasma membrane. The initial papers describing annexin V staining in apoptosis carefully referred to annexin V-positive/vital dye negative cells (Figure 1a Q4) as apoptotic and those that were dual positive or positive for the vital dye alone as necrotic or simply as dead (Figure 1a Q2).6 Later, this dual positive population was referred to as late apoptotic9 and although this definition is correct if apoptosis is known to be the mechanism of cell death, the term has led to considerable confusion. This is because some scientists concluded that dual staining with annexin V and a vital dye is diagnostic of late apoptosis, whether or not apoptosis has occurred. If a clear annexin V-positive/vital dye negative population is not apparent (Figure 1b), then the evidence for apoptosis is clearly insufficient, but unfortunately plots like those shown in Figure 1b are not uncommonly presented to attest that cells have died by apoptosis. Even when an annexin V-positive/vital dye negative population is present, additional evidence should be obtained before concluding that apoptosis is the mechanism of cell death.
Figure 1.
Different staining patterns for annexin V/PI staining resulting from treatment of the same cells with different agents (a and b) over time. The quadrants are named on the first dot plot.
Perhaps the most common supporting evidence for apoptosis is the cleavage of caspases. Many assays are available to quantitate caspase cleavage including western blotting, flow cytometric and ELISA-based assays, but some only permit the user to determine a fold increase in caspase cleavage or activity following treatment. This can be deceptive and inclusion of a positive control where apoptosis is the known death mechanism is important. Although the specific cleavage of caspase-3 and caspase-7 is indicative of their activation (and can be detected with cleaved product-specific antibodies), the cleavage of other caspases is not.10 An alternative, such as the use of fluorescent caspase substrates for staining, is problematic in that these agents often stain non-specifically.11
So how does one determine whether apoptosis is the death mechanism identified? There are several answers to this question and largely it depends on the system being investigated and resources available. Reduced DNA content as assessed by flow cytometry using DNA intercalating dyes such as propidium iodide to detect a sub-G1 peak is another common method. Although this is a very useful assay for quantitation once apoptosis has been confirmed, it is not evidence for apoptosis per se, as the DNA of dead cells can also be degraded by extracellular DNases that gain access to the nucleus.12 We would suggest that at a minimum at least one functional assay be undertaken in addition to the descriptive methods. The most simple is pan-caspase inhibition. Although caspase inhibitors are not particularly specific and cells can die via another mechanism if caspases are blocked, the application of a pan-caspase inhibitor should reduce the number of dead cells or at least delay the process when death is due to apoptosis. If the cells are susceptible to genetic manipulation then overexpression of proteins that inhibit apoptosis or knockdown of those required provides stronger evidence. Apoptosis that proceeds via the mitochondrial pathway is blocked by anti-apoptotic Bcl-2 proteins such as Bcl-2 and Bcl-xL, and sensitized by specific antagonists of these proteins such as ABT-737.13 Use of enforced expression of these proteins and/or specific antagonists can greatly aid in drawing mechanistic conclusions.
For researchers studying death mechanisms, identifying the details involved in the demise of a cell is of key importance. However, for some investigators the mode of cell death is not a major concern as the aim is simply to kill the cell in question. It may not be helpful to state that apoptosis is the mechanism when the only evidence is an annexin V/vital dye stain. It would be better to simply state that the cells are being killed without referring to a specific death mechanism. Rather than reporting the percentage of apoptotic cells or even annexin V-positive cells, a report of the remaining viable fraction is often useful, especially if that fraction can be shown to retain function. Reviewers and editors should insist that the word ‘apoptosis' only be used where apoptosis has been clearly demonstrated. This would not necessarily diminish the overall message of many papers and would help wean the scientific community off the apoptosis addiction.
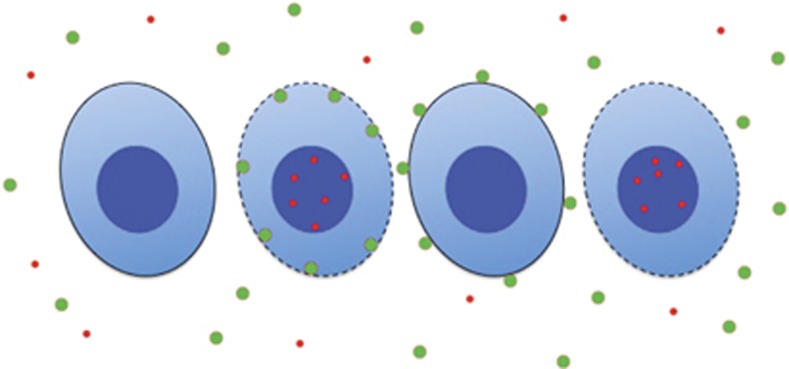
Test Question: Which of the cells in Figure 2 are apoptotic? Answer: You cannot tell. You do not have enough information.
Figure 2.
Cartoon representing cells stained with annexin V (green) and a vital dye (red).
Acknowledgments
LB is supported by an NHMRC Senior Research Fellowship (No. 1042305).
The authors declare no conflict of interest.
References
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Green DR. Means to an End: Apoptosis and other Cell Death Mechanisms. Cold Spring Harbor Laboratory Press: New York, NY, USA; 2011. [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchenko AP. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65:157–172. doi: 10.1007/s10616-012-9481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- Boersma AW, Nooter K, Oostrum RG, Stoter G. Quantification of apoptotic cells with fluorescein isothiocyanate-labeled annexin V in chinese hamster ovary cell cultures treated with cisplatin. Cytometry. 1996;24:123–130. doi: 10.1002/(SICI)1097-0320(19960601)24:2<123::AID-CYTO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McStay GP, Salvesen GS, Green DR. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 2008;15:322–331. doi: 10.1038/sj.cdd.4402260. [DOI] [PubMed] [Google Scholar]
- Smolewski P, Bedner E, Du L, Hsieh TC, Wu JM, Phelps DJ, et al. Detection of caspases activation by fluorochrome-labeled inhibitors: multiparameter analysis by laser scanning cytometry. Cytometry. 2001;44:73–82. doi: 10.1002/1097-0320(20010501)44:1<73::aid-cyto1084>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nagata S. DNA degradation in development and programmed cell death. Annu Rev Immunol. 2005;23:853–875. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]