Abstract
During social interactions an individual’s behavior is largely governed by the subset of signals emitted by others. Discrimination of ‘self’ from ‘other’ regulates the territorial urine countermarking behavior of mice. To identify the cues for this social discrimination and understand how they are interpreted, we designed an olfactory-dependent countermarking assay. We find Major Urinary Proteins (MUPs) sufficient to elicit countermarking, and unlike other vomeronasal ligands that are detected by specifically tuned sensory neurons, MUPs are detected by a combinatorial strategy. A chemosensory signature of ‘self’ that modulates behavior is developed via experience through exposure to a repertoire of MUPs. In contrast, aggression can be elicited by MUPs in an experience-independent but context dependent manner. These findings reveal that individual-emitted chemical cues can be interpreted based on their combinatorial permutation and relative ratios, and they can transmit both fixed and learned information to promote multiple behaviors.
Introduction
During social behavior each participant emits a variety of sensory cues. The receiver likely uses multiple neural strategies in order to identify those cues that are sent by others within the milieu of all detected cues. How self-emitted cues are detected and filtered to allow receivers to respond specifically to non-self cues is largely unknown. In addition to direct interaction with conspecifics, male mice also communicate by proxy; they deposit urine odor cues in the environment to advertize their presence to females and rival males (Desjardins et al., 1973; Rich and Hurst, 1999). If another male’s mark is encountered by a dominant male, he will reply with a ‘countermark’ to indicate command of the territory (Rich and Hurst, 1999). This behavior is metabolically costly, therefore contact with a self-deposited mark does not initiate marking behavior (Nevison et al., 2000). Identification of the behavior-promoting ligands, the olfactory strategy that enables the discrimination between self and other, and the responding sensory neurons will provide a tractable system to begin to address the neural mechanisms that distinguish self from other.
Instead of being tuned to a specific ligand, main olfactory neurons detect molecular features of odorants (Malnic et al., 1999). Therefore, depending on the diversity of its molecular features each ligand activates multiple sensory neurons and each neuron detects multiple ligands; termed ‘combinatorial coding’. This strategy enables a limited number of receptors to capture a large amount of information. The main olfactory system functions to recognize the identity of the odor blend through the composition of its repertoire and does not easily discriminate individual odorants. In contrast, stimulation of the vomeronasal organ (VNO) has been shown to mediate identical behavioral responses whether the ligand is purified or in the context of a native odor blend (Kimoto et al., 2005). This difference may enable the VNO to initiate fixed responses to specialized ligands. The bioactivity of very few VNO ligands has been solved. Purifying additional ligands and solving their function is necessary to study how this sensory system evaluates the environment.
Mouse urine is composed of a large number of volatile odors as well as peptides and proteins that function as chemosignals to promote social behavior. A subset of proteins, Major Urinary Proteins (MUPs), are produced in a testosterone- and growth hormone-dependent manner primarily by adult males (Finlayson et al., 1965; Hastie et al., 1979; Knopf et al., 1983; Szoka and Paigen, 1978). MUPs have been shown to be detected by vomeronasal sensory neurons (VSNs) (Chamero et al., 2011; Chamero et al., 2007; Papes et al., 2010). In contrast to main olfactory neurons, VSNs have been found to be tuned to specific cognate ligands (Haga et al., 2010; Leinders-Zufall et al., 2000; Nodari et al., 2008). This requires evolution of a unique receptor for each ligand. The mouse reference genome encodes 21 MUPs, all species-specific, 15 of which are extremely similar, with some proteins varying by only a single amino acid (Logan et al., 2008; Mudge et al., 2008). These observations are consistent with a rapidly-evolving gene family. It is not known whether such ligands can be uniquely distinguished by co-evolving sensory neurons or if they are detected by a limited number of VSNs which would render the individual gene products functionally redundant. As evidence against redundancy, an individual does not express all of the 21 MUPs, rather individual males stably express discrete subsets of 4–12 of the MUPs throughout their lifetime (Robertson et al., 1997). While wild-caught brothers each emit a unique MUP profile, all inbred males of the same strain emit identical MUPs and males of other strains may express a different MUP subset (Cheetham et al., 2009). Why individuals express varying repertoires of these specialized ligands is not known.
Recombinant MUP proteins (rMUPs) have been shown to promote male-male territorial aggression (Chamero et al., 2007), female attraction, and conditioned place preference (Roberts et al., 2012; Roberts et al., 2010). MUPs have additionally been proposed to play a role in signaling individual identity for countermarking behavior based on three observations. 1) MUPs are lipocalins, which fold into degradation resistant β-barrel structures that effectively persist in the environment (Flower, 1996; Hurst et al., 1998). 2) Male mice emit an extraordinarily high MUP concentration (20 mg/ml) in their urine (Szoka and Paigen, 1978). Protein excretion in urine is unusual in mammals due to high metabolic cost, suggesting their function is likely to be a species-specific evolved trait. 3) The unique MUP repertoire of each individual is stable throughout his lifetime and has been proposed to be a potential protein ‘bar-code’ of individuality (Hurst et al., 2001). Indeed, male mice increase their marking when they encounter MUP-containing urine fractions (Humphries et al., 1999). Males can discriminate between native urine and the same urine spiked with rMUPs (Hurst et al., 2001). However, the role of MUPs in countermarking may be indirect because the β-barrel structure of MUPs binds volatile urine molecules (Bacchini et al., 1992; Novotny et al., 1999), retaining them in the environment, extending their potency as volatile odor cues (Hurst et al., 1998), and transporting them into the mucus-filled VNO lumen that is otherwise not readily accessible to volatiles (Meredith and O’Connell, 1979). These MUP-associated ligands are sufficient to activate VNO neurons and promote social behavior (Leinders-Zufall et al., 2000; Novotny, 2003). Whether mice detect MUP type to promote countermarking through differences in volatile ligands, through simultaneous detection of MUPs and their ligands, or through the MUPs themselves has not been determined (Hurst and Beynon, 2004; Hurst et al., 2001). Further, how MUPs can be necessary to regulate a variety of disparate social behaviors is not known.
The large MUP repertoire provides an experimental toolkit to investigate the sensory logic underlying social behavior. Here, we fractionate urine to identify the underlying bioactive cues and confirm that the MUP fraction elicits countermarking. We further assay recombinant proteins to determine that the MUPs alone, not the bound odor molecules, are each relevant to promote countermarking behavior. We use both calcium imaging and electrophysiology and find that VSNs employ a combinatorial coding strategy to sense and interpret the identity and concentration of MUPs in the environment. Surprisingly, we find that MUP bioactivity to instruct countermarking behavior depends not on individual MUP ligands, but on the blend of the entire detected MUP repertoire as a whole. Through behavioral manipulations we demonstrate that the ability of the encountered MUPs to signal ‘self’ or ‘other’ varies with previous MUP sensory experience. In contrast, we find that two particular MUPs are predetermined to innately elicit male-male aggression, a stereotyped output that is not modulated by concentration, experience, or the entire detected MUP repertoire. Through behavioral analysis we show that the decision to respond to detected MUPs with either aggression or countermarking depends upon the extended sensory context. Overall, we find that males use MUP ligands to regulate two different behaviors, each with a different sensory coding strategy. Aggression is highly tuned and is promoted by dedicated ligands. In contrast, countermarking utilizes combinatorial sensory coding and the propensity of each ligand to promote behavior varies based on the experience of the receiving animal.
Results
MUPs are sufficient to promote countermarking behavior
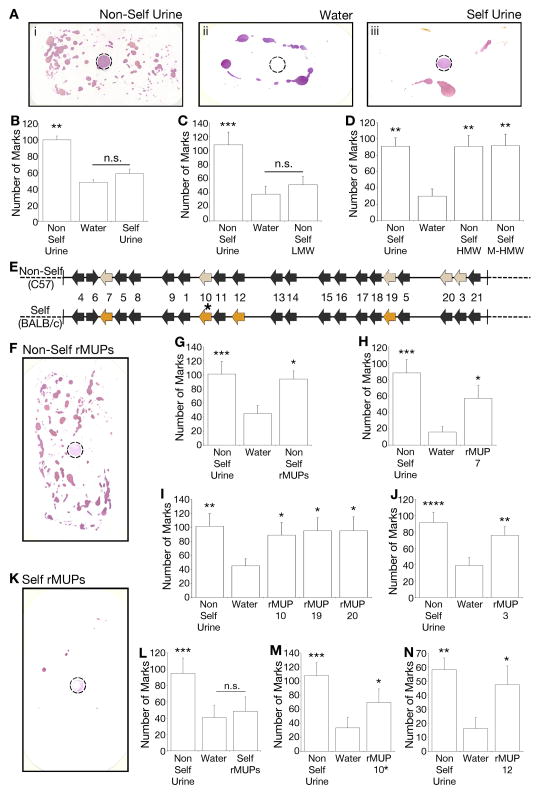
To isolate the urinary cues that promote and regulate countermarking, we devised an olfactory-mediated behavioral assay. BALB/cByJ male mice were placed in an empty cage lined with Whatman paper spotted with 50 μl of an olfactory stimulus. After 5 minutes, the animal was removed and urine marks revealed by ninhydrin treatment were quantified (Figure 1A–B). The cues that signal self are likely to contain a genetic component because it has previously been shown that the marking response to urine from any male of the same inbred strain is identical to that elicited by self-emitted urine (Nevison et al., 2000). Our assay corroborates this known characteristic of countermarking behavior, since a spot of non-self urine (from C57BL/6J males) is able to promote robust countermarking from stimulus naïve test males of the BALB/c strain, while a spot of self-emitted (BALB/c) urine generates a response similar to that evoked by water (Figure 1A–B, S1A–B). This behavior is dependent on social status, as only dominant males mark in response to non-self male cues (Figure S1A). Males also mark to female urine, though this behavior is evoked irrespective of social status of the receiving male (Figure S1A). Marking behavior was not simply the result of environmental novelty, because marking was not enhanced by the presence of the attractive odorant eugenol or the repulsive odor of ethanol (Figure S1C) (Logan et al., 2012). Females and castrated males did not show marking behavior in our assay (Figure S1D–E) (Desjardins et al., 1973; Kimura and Hagiwara, 1985). These controls confirm the robustness and reliability of our olfactory-mediated assay to investigate the role of olfactory cues in the release of countermarking behavior.
Figure 1. MUPs are sufficient to elicit marking behavior.
(A) Representative blots of urine marks deposited by BALB/c males in response to olfactory stimuli (dotted circle): non-self (C57BL/6J) urine (i), water (ii), or self (BALB/cByJ) urine (iii). (B–D) Quantification of urine marks to total urine (B), low molecular weight (LMW) fraction of non-self urine (C), or high molecular weight fraction with (HMW) or without (M-HMW) bound small molecules (D). (E) Genomic representation of Mup gene cluster. Colored arrows indicate genes expressed by non-self (C57BL/6J – top panel) or self (BALB/cByJ - bottom) strains. * indicates the Q159K MUP10 allelic variant present in BALB/cByJ, all other MUPs have the same amino acid sequences between the two strains. (F–G) Behavioral response to a mixture of non-self recombinant MUPs (rMUP3+rMUP7+rMUP10+rMUP19+rMUP20). (H–J) Quantification of behavioral responses to individual non-self rMUPs. (K–L) Behavioral response to a mixture of self-emitted rMUPs (rMUP7+rMUP10*+rMUP12+rMUP19); and (M–N) to individual self-emitted rMUPs (also see panels H & I). n=8–16. Mean + standard error of the mean (SEM). p-values determined by a repeated measures one-way ANOVA, with a Greenhouse-Geisser correction, followed by Bonferroni multiple comparisons test or by Friedman’s non-parametric test followed by Dunn’s multiple comparison test. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. n.s. = non-significant. p-values determined by comparison to water. See also Figure S1.
To isolate the male chemosignal(s) that promotes countermarking in our assay, we size-fractionated the bioactive non-self (C57BL/6J) urine and assayed countermarking from BALB/c males. While distinctive volatile odors that vary between individuals compose the small molecule-containing low molecular weight (LMW) fraction, we found this fraction lacked significant bioactivity. Instead, consistent with previous reports, males displayed a robust increase in marking behavior specifically towards the MUP-containing high molecular weight (HMW) fraction (Figure 1C–D) (Hurst et al., 2001; Nevison et al., 2003). MUPs are a large family of environmentally stable proteins that fold into a β-barrel structure which binds small hydrophobic molecules (Flower, 1996). While MUPs have been previously implicated in promoting countermarking behavior their precise role has not been determined (Hurst and Beynon, 2004). It has been postulated that they may act indirectly, perhaps serving to stabilize small molecules in the environment or transporting them into the mucus filled VNO (Beynon and Hurst, 2003, 2004; Humphries et al., 1999; Hurst and Beynon, 2004; Hurst et al., 2001; Hurst et al., 1998; Nevison et al., 2003; Novotny, 2003). However, when MUP-bound small molecules were competitively displaced by incubation of the HMW fraction with behaviorally inert menadione (Figure S1F) (Chamero et al., 2007; Xia et al., 2006) prior to assaying for behavior, we found that the countermarking-promoting bioactivity was retained indicating that these small molecules do not instruct countermarking behavior (M-HMW; Figure 1D).
Of the 21 Mups encoded in the genome (Logan et al., 2008; Mudge et al., 2008) wild-caught individuals stably express variable subsets of 4–12 MUP proteins in their urine (Hurst and Beynon, 2004). The MUP expression of lab strains is genetically fixed so that all members of the same strain express identical MUP proteins and some strains express a different, but fixed, MUP repertoire (Cheetham et al., 2009; Robertson et al., 1997) Though the overall amount of excreted protein can vary with social experience the ratios of MUPs expressed is thought to be stable (Janotova and Stopka, 2011). Like MHC peptides, MUPs display the hallmarks of individuality cues, however, such a function has only been tested in the presence of urinary small molecules, therefore, the significance of such customized protein excretion remains unknown (Hurst and Beynon, 2004; Hurst et al., 2001; Leinders-Zufall et al., 2004). We have previously isolated and cloned the MUP repertoires from both the C57BL/6J and BALB/cByJ lab strains (Figure 1E, S1G) (Chamero et al., 2007; Logan et al., 2008; Mudge et al., 2008; Robertson et al., 1997). A spot containing a mixture of bacterially-expressed recombinant MUPs (rMUPs) corresponding to the five native MUPs excreted in non-self C57BL/6J urine resulted in increased marking behavior from BALB/c males, indistinguishable from the response elicited by native urine (Figure 1F–G, S1H). MUPs are detected by TRPC2-expressing VSNs (Chamero et al., 2007) therefore, we tested the marking response of TRPC2 deficient BALB/c males in our behavioral assay. We found that while these mutants marked in response to female cues, they did not display marking behavior to non-self male cues (Figure S1I). This indicates that there are at least two different ligands that promote marking behavior; a male-emitted cue that is detected by TRPC2 expressing neurons and a female emitted cue that is TRPC2 independent. Together, these experiments indicate that the MUPs, devoid of their small molecule urinary ligands, serve as the male-emitted sensory cue that is sufficient to initiate countermarking behavior.
Previously, MUP20 also known as Darcin, has been shown to be attractive to females and generate a conditioned place preference, while other MUPs do not share this bioactivity (Roberts et al., 2012; Roberts et al., 2010). To determine if one particular MUP from the non-self mixture is inherently dedicated to generate countermarking, individual rMUPs were singly assayed. We found no rMUP to be uniquely specialized to promote countermarking. Instead, each rMUP from the non-self repertoire is equally sufficient to elicit a robust marking response compared to water (Figure 1H–J). However, a blend of the four rMUPs corresponding to those excreted in self-urine was devoid of countermarking bioactivity, which is consistent with the response to self-emitted urine (Figure 1K–L). Surprisingly, such lack of activity is not due to an intrinsic difference between self and non-self MUPs, because individual self rMUPs assayed alone were each sufficient to generate increased countermarking compared to the control (Figure 1M–N, also HI). Moreover, the ability of individual rMUPs that compose the self-emitted MUP repertoire to elicit countermarking indicates that, despite constant exposure to their own urine, the failure of mice to display countermarking behavior to self-emitted cues is not due to sensory habituation. To evaluate whether orthologous MUPs similarly initiate countermarking, we tested a recombinant-expressed cat MUP, FelD4, which promotes fear behavior in mice (Papes et al., 2010). We found that mice did not countermark to this rMUP (Figure S1J). Together, our data reveal that individual mouse MUPs are each equally sufficient to promote countermarking, however the repertoire of self-emitted MUPs fails to generate behavior.
Vomeronasal sensory neurons detect MUPs combinatorially
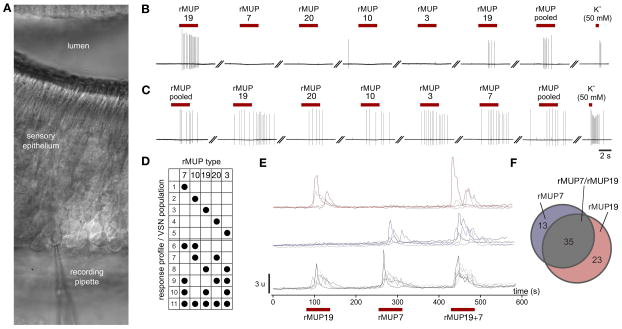
To function as an effective social cue, the signals that indicate self and other should be readily discernible by the receiver. The protein sequence of individual mouse MUPs can be up to 99% identical (Logan et al., 2008; Mudge et al., 2008). To determine the extent to which MUPs are differentially sensed, we recorded spike activity from basal VSNs in VNO slice preparations in response to repetitive, randomized stimulation with individual rMUPs (Hagendorf et al., 2009) (Figure 2A). We found 25 out of 1,006 basal neurons (2.5%) that responded to at least one single rMUP. 52% of these neurons (13/25) were selectively activated by one single rMUP ligand (Figure 2B, S2A), a response pattern consistent with the highly tuned pheromone detection properties previously observed in VSNs (Haga et al., 2010; Leinders-Zufall et al., 2000; Nodari et al., 2008). Unexpectedly, each rMUP activates at least 3 – 4 additional VSN populations (2C–D, S2B–D). Some VSNs respond to specific combinations of few MUPs (5/25 neurons; 20%) (Figure 2D, S2B, D) while others are broadly tuned ‘generalists’ that detect every rMUP tested (7/25 neurons; 28%) (Figure 2C, S2C, D). Together, the five rMUP ligands activate at least 11 VSN populations and the variation in VSN tuning enables individual rMUPs to be recognized by a unique combination of VSNs (Figure 2D, S2D). We additionally used a higher-throughput calcium imaging approach to analyze single dissociated VSNs (Chamero et al., 2007) and similarly found individual rMUPs to stimulate both broadly and narrowly tuned neurons (Figure 2E–F, S2E). Both methods of analysis indicate that each MUP activates multiple VSN types and one VSN can detect multiple MUPs. This sensory strategy differs from the concept of highly tuned neurons that have been found to detect known mouse pheromones (Haga et al., 2010; Leinders-Zufall et al., 2000; Nodari et al., 2008) and, at least in part, appears to mirror the combinatorial coding strategy employed by canonical olfactory neurons in the main olfactory epithelium (Malnic et al., 1999).
Figure 2. VSNs utilize a combinatorial code to detect MUPs.
(A) VNO slice preparation to analyze individual sensory neurons showing the location of the recording pipette in the basal layer. (B–C) Original ‘loose-seal’ traces from two representative VSNs repetitively responding to a single (B) or all (C) rMUPs. Red bars = stimulation. Stimuli were applied in random order, ISI = 60″. Recordings are representative of VSN population #3 and #11 (panel D), respectively. (D) Summary of 11 distinct populations of neurons observed during extracellular recordings. (E) Representative calcium transients imaged from dissociated VSNs sequentially stimulated with rMUP7 and rMUP19, which only differ by two amino acids (F56V & E140K), followed by a mixture of both MUPs. Colors indicate three distinct populations of VSNs based on response profile. Black bars indicate stimulus application. 3,767 cells imaged, sequentially exposed to all stimuli. (F) Venn diagram quantifying the 3 distinct populations of neurons observed to respond to the two MUP stimuli by calcium imaging, colors correspond to VSN population in panel (E). See also Figure S2.
Vomeronasal sensory neurons detect relative ratios of MUP ligands
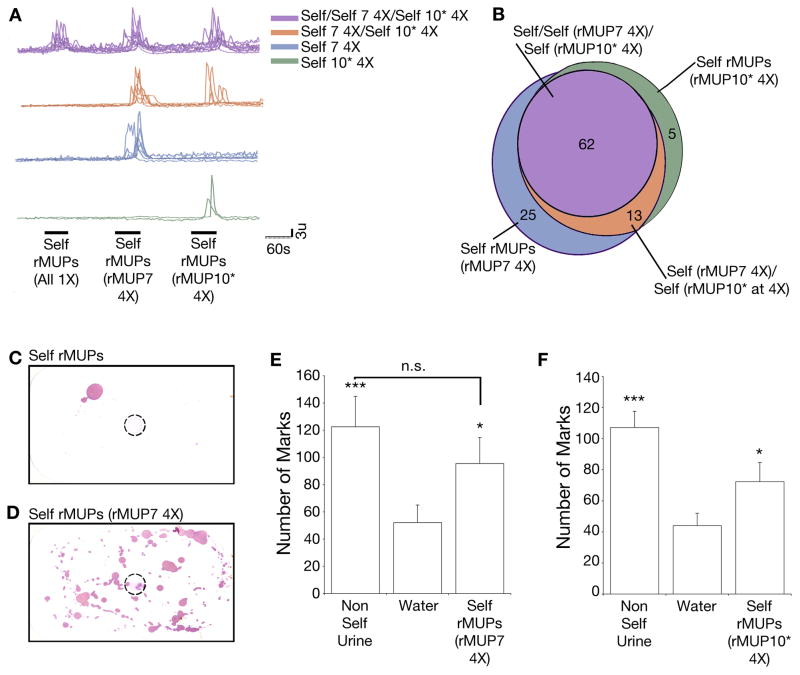
Another hallmark of MUP ligand expression is that individual MUPs are emitted at different ratios between different inbred strains, or among wild-caught individuals (although the ratio appears to be fixed in an individual). Therefore, the same MUP may be expressed at different concentrations between wild individuals (Cheetham et al., 2009; Robertson et al., 1997). Whether differences in concentration are detected by males and used to expand the complexity of sensory information coding for behavior is not known. We used calcium imaging to assay the dose response of VSNs to three different rMUPs and found each to elicit a unique effective concentration and inflection point (Figure S3A). To determine if vomeronasal response profiles could allow discrimination of relative MUP ratios, we compared the response of single neurons to the blend of self-rMUPs with all rMUPs at the same concentration, to an identical blend except the ratio of one rMUP component was increased four-fold. We found that this change in rMUP ratio resulted in the stimulation of new VSN populations (Figure 3A–B). An increased ratio of rMUP7 activated an additional 38 neurons (1.5%), while altering the ratio of rMUP10* activated an additional 18 VSNs (0.7%). To determine if this concentration-dependent change in VSN response is functionally meaningful to the receiving male, we spotted the countermarking arena with the self-rMUP mixture in which the relative ratio of one rMUP was increased four-fold. This change indeed endowed the previously inactive self-MUP repertoire with countermarking bioactivity (Figure 3C–F). Our experiments indicate that the population of MUP-detecting VSNs can signal both the identity and the concentration of MUP ligands. Unlike the highly tuned response of other pheromone detecting VSNs (Haga et al., 2010; Leinders-Zufall et al., 2000; Nodari et al., 2008), this alternate combinatorial coding strategy can utilize fewer sensory receptors to transmit a large quantity of information about rapidly-evolving and extremely similar ligands such as MUPs.
Figure 3. Combinatorial code enables VSNs to detect relative ratios of MUPs.
(A–B) Representative calcium transients from VSNs (A), quantified in (B). 2,515 cells imaged, sequentially exposed to self-rMUPs, self-rMUPs with rMUP7 at 4X, and self-rMUPs with rMUP10* at 4X. Colors indicate four distinct populations of VSNs based on response profile. Black bars indicate stimulus application. (C–D) Countermarking behavior in response to a spot (dotted circle) of self-rMUPs (C), or self-rMUPs where the ratio of an individual MUP (rMUP7) was increased 4-fold (4X) (D). (E–F) Quantification of behavioral response to self-rMUPs where the ratio of an individual MUP, rMUP7 (E) or rMUP10* (F), was increased 4-fold. n = 12. Mean + SEM. p-values determined by a repeated measures one-way ANOVA, with a Greenhouse-Geisser correction, followed by Bonferroni multiple comparisons test or by Friedman’s Test followed by Dunn’s multiple comparison test. See also Figure S3.
Marking behavior depends on the composition of the detected MUP repertoire
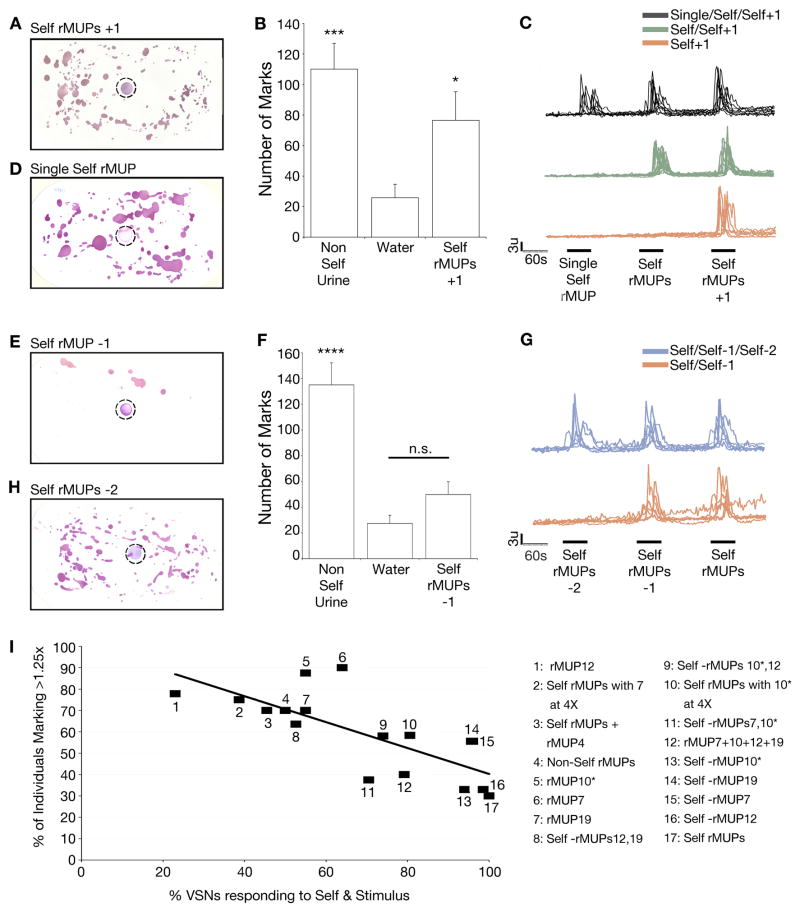
To investigate how self-emitted cues regulate behavior, we examined whether the blend of self-MUPs is sufficient to prevent countermarking. We assayed marking to the self-emitted rMUP repertoire that had been spiked with an additional unfamiliar rMUP (self-rMUPs +1) and found robust countermarking, indicating that the receiver is not solely utilizing the mere presence of self-MUPs to regulate marking behavior (Figure 4A–B). Imaging analysis of the VSN response to the self-MUPs +1 blend revealed the stimulation of additional VSNs that were not activated by the self-MUP blend (Figures 4C, S4A). In comparison, a single rMUP (equally capable of generating behavior; Figure 1H, 4D) only activated a subset of the neuron types stimulated by the full self-rMUP blend (Figure 4C). We further investigated the nature of the MUP code by removing just a single MUP from the self-rMUP blend (self rMUPs −1) and found no countermarking behavior, similar to the response to self rMUPs (Figure 4E–F). Correspondingly, the VSN activity evoked by both behaviorally inactive mixtures (self rMUPs −1, and self-rMUPs) was indistinguishable (Figure 4G). However, when we produced a more dramatic alteration of the self-MUP blend by removing any combination of two of the four rMUPs (self rMUPs −2), only a subset of the neurons activated by the self MUPs were stimulated (Figure 4G, S4B) and detection of the self rMUPs −2 mixtures again resulted in an increase in countermarking behavior compared to water (Figure 4H, S4C). These findings suggest that VSN activity is capable of resolving the identity of MUPs comprising a blend.
Figure 4. The identity and ratio of detected MUP ensemble modulates behavior.
(A) Countermarking behavior in response to a spot (dotted circle) of self-rMUPs plus the additional novel rMUP4 (self rMUPs +1). (B) Quantification of behavioral response to self-rMUPs plus the additional novel rMUP4 (self rMUPs +1). n = 12. Mean + SEM. (C) Representative calcium transients from VSNs. 2,200 cells imaged, sequentially exposed to rMUP7, self rMUPs, and self rMUP+rMUP4. Colors indicate three distinct populations of VSNs based on response profile. (D) Countermarking behavior in response to a spot (dotted circle) of a single self-rMUP (rMUP7). (E–F) Countermarking behavior in response to a spot of self-rMUPs −1 (self-rMUPs without rMUP 7). (F) Summed marking response to every combination of three of the four self rMUPs (self rMUPs −1). n = 36. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. n.s. = non-significant. p-values determined by comparison to water. (G) Representative calcium transients from VSNs. 3,893 cells imaged, sequentially exposed to self-rMUPs −2, self-rMUPs −1, and self-rMUPs. Colors indicate two distinct populations of VSNs based on response profile. Black bars indicate stimulus application. (H) Countermarking behavior in response to a spot of self-rMUPs −2 (self-rMUPs without rMUP10*and rMUP12). (I) An inverse relationship exists between countermarking behavior and the percentage of VSNs that respond both to the indicated stimulus and to self-rMUPs. Best linear fit, R2=0.56. See also Figure S4.
To analyze the extent to which the population of MUP-responsive VSNs can deviate from that activated by self-emitted MUPs to modulate the display of countermarking we measured the behavioral response of individual males to 16 systematically manipulated rMUP mixtures and quantified the amount of marking behavior each blend induced (Figure S4D). We found that the extent of the behavioral response is increasingly altered as the rMUP mixture deviates from the self-emitted composition (Figure S4D). Next, we analyzed the VSN response to these same 16 MUP mixtures compared to behaviorally inactive self-rMUPs (Figure S4A–B, E–J; 3A–B, 4C, G). We determined the percentage of individual neurons that responded to both manipulated rMUP and the self-rMUP stimuli to quantify the extent to which the identities of the responding VSNs matched the response to self-rMUPs. When we compared the percentage of animals displaying behavioral activity to the percentage of neural activity that was identical to the self MUP-induced neural activity, we found a significant negative correlation (Spearman Rank Correlation Coefficient of −0.79; p<0.01; Figure 4I). A high percentage of overlap in neural activity of any stimulus compared to self-MUPs thus correlates with a low probability of countermarking behavior. In contrast, a low overlap in neural activity correlates with a high probability of countermarking.
Olfactory-mediated innate behavior in the mouse is known to be initiated by the detection of single salient ligands and the surrounding odors in the environment have not been shown to modify their function (Dewan et al., 2013; Haga et al., 2010; Papes et al., 2010; Roberts et al., 2012; Roberts et al., 2010). In contrast, while we do find that each MUP is sufficient to initiate behavior, when confronted with a complex blend of MUPs, the receiver initiates behavior based on the identity and concentration of the entire ensemble of detected MUPs. This signal can be rendered inert if it matches the self-emitted MUP repertoire.
Significance of self-MUP repertoire requires experience
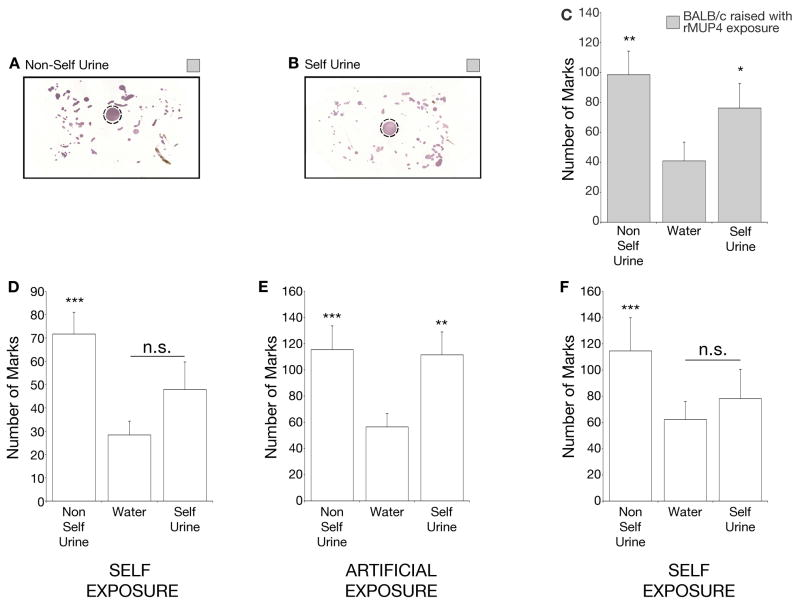
Since wild individuals, or individuals from different lab strains, each express a unique repertoire of MUPs, the ability of one’s emitted MUP repertoire to modulate countermarking may be due to a correspondingly individualized, genetically encoded neural response to MUPs. Alternatively, the ability of one’s own cues to modify behavior could be a result of each individual’s unique sensory experience. To determine if sensory experience with MUPs can modulate countermarking, we aimed to artificially alter the MUP experience of test males with their native MUPs, which we found to be expressed after four weeks of age (Figure S5A). Therefore, we chronically exposed them to an additional MUP that is not part of the BALB/c emitted MUP repertoire (MUP4) starting at three weeks of age. We assayed behavior upon maturity, at 8 weeks of age, and indeed found artificial MUP exposure to cause altered behavior. Subjects that experienced an additional rMUP displayed robust marking behavior to native self urine (Figure 5A–C).
Figure 5. Signature of ‘self’ is based on experience.
(A–B) Countermarking behavior of males raised with rMUP4 following exposure to a spot (dotted circle) of non-self urine (A) or self (BALB/c) urine (B). (C) Behavioral response of males raised with rMUP4 exposure shows marking to self (BALB/c) urine. n = 11. p-values determined by Friedman’s Test followed by Dunn’s multiple comparison test. (D–F) Behavioral response of adult males changes with odor experience. Males were raised among self odors (D), then exposed to non-self male soiled bedding (E), and returned to self odor environment (F). n = 7–15. p-values determined by Friedman’s Test followed by Dunn’s multiple comparison test. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. n.s. = non-significant. p-values determined by comparison to water. See also Figure S5.
We next sought to determine the extent to which odor experience could alter the significance of an established self MUP repertoire in adult males. We first confirmed that self-emitted cues failed to increase marking behavior (Figure 5D). Next, we artificially exposed each subject to an alternate MUP repertoire by housing them in cages that had previously contained adult C57BL/6J males. This ‘artificial exposure’ continuously provided a non-self MUP repertoire experience. Individuals were assayed each week for their marking response to self and non-self urine. After an average of two weeks we observed an increase in marking behavior to native self-urine, compared to water, in the majority of individuals (Figure 5E, S5B). The animals did not correspondingly decrease their response to non-self (C57BL/6J) urine, likely because the artificial exposure contained a mixture of both C57BL/6J as well as self-emitted BALB/c MUPs. Finally, to determine if we could observe further experience-driven plasticity we ensured that males were once again only exposed to self-emitted cues by maintaining them in clean cages. We found the response to self-emitted urine to revert to the original bioactivity; no different from the response to water (Figure 5F, S5B). The ability to repeatedly manipulate the countermarking response to native self-urine indicates that the self-MUP repertoire is not inherently restricted to attenuate countermarking of the emitting male. Rather it is an individual’s experience with the MUP proteins in his immediate environment that defines a unique signature of self which can be used to appropriately direct marking behavior.
A subset of countermarking MUPs additionally promotes male-male aggression
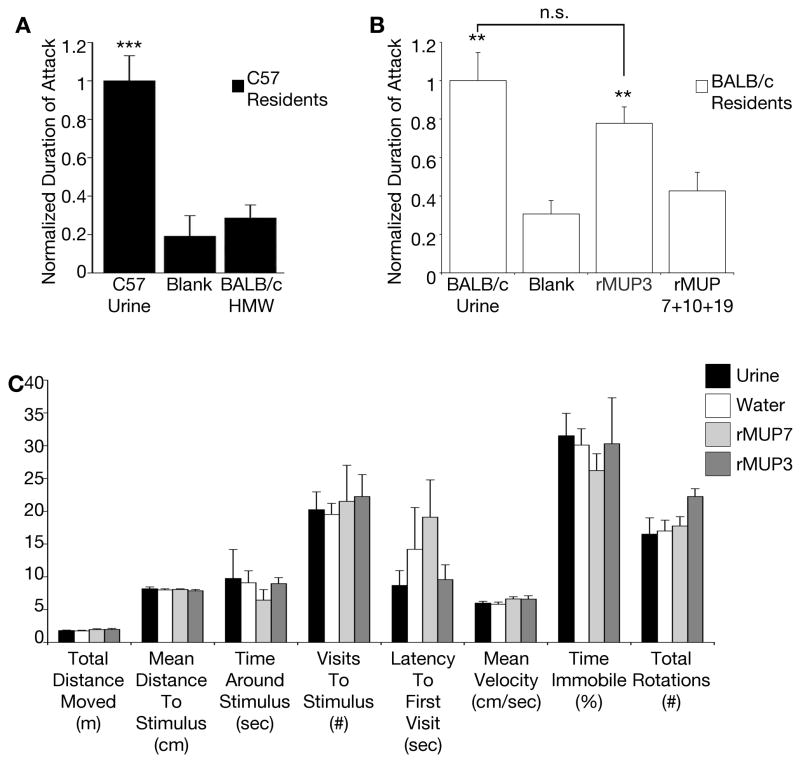
Currently, known mouse pheromones have only been implicated to promote a single function (Haga et al., 2010; Kimoto et al., 2005; Roberts et al., 2010). We have previously shown that the pool of C57BL/6J rMUPs elicits male-male territorial aggression (Chamero et al., 2007). To investigate how MUPs promote multiple behavioral outputs, we assayed rMUPs as stimuli in a resident-intruder assay using C57BL/6J male subjects (Figure 6A) (Chamero et al., 2007). Unlike countermarking, we found that the pool of self rMUPs is not inert; it contains significant aggression-promoting bioactivity (Figure 6A). Moreover, when individually assayed we found that not all rMUPs promote aggression. Three rMUPs were unable to generate any detectible behavior while two proteins, rMUP20 and rMUP3, were each sufficient to release the full fixed action pattern of aggression (Figure 6A). To determine if a change in MUP concentration alters its likelihood to promote aggression, we tested the function of rMUPs at 1X and 4X concentrations. Even when present at high concentrations three MUPs failed to promote the behavior, only rMUP20 and rMUP3 increase the aggressive behavior of males (Figure 6B). Together, these results indicate that, unlike countermarking which utilizes the full repertoire of detected MUPs to initiate behavior, MUP3 and MUP20 promote aggression behavior irrespective of the urine odor milieu, repertoire of MUPs or MUP concentration.
Figure 6. Only a subset of MUPs promotes aggression.
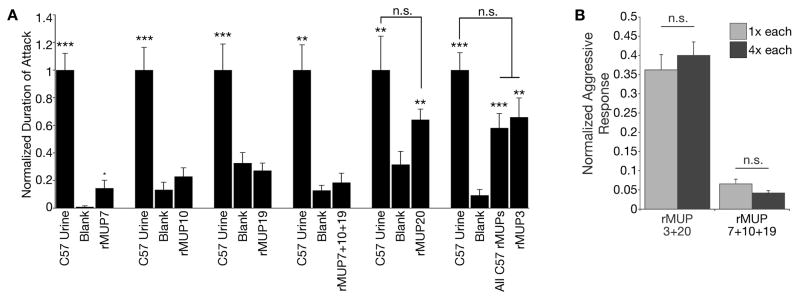
(A) Only a subset of recombinant MUPs swabbed on castrate mice stimulate aggression in the resident-intruder assay. Black bars=C57 male residents, n = 6–51. Mean + SEM. p-values determined by Friedman’s Test followed by Dunn’s multiple comparison test. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. n.s. = non-significant. p-values determined by comparison to water. (B) Aggression-promoting bioactivity of MUPs is not sensitive to ligand concentration. Response to negative control was subtracted from response to positive control and test stimulus. Response to test stimulus was then normalized to the response to positive control in order to compute the values presented. 1X = 5mg/ml of each protein in the mix, 4X = 20mg/ml of each protein in the mix. n = 12.
MUP3 triggers experience-independent innate aggression in a context-dependent manner
Since the countermarking response to self-MUPs requires MUP experience (Figure 5) we aimed to test the extent to which ligand-experience during development influences its capacity to generate aggressive behavior in adults. While all males of the C57BL/6J lab strain express both aggression-promoting MUPs, mass spectrometry analysis shows that BALB/cByJ males do not express either MUP20 or MUP3 (Figure 1E) (Cheetham et al., 2009; Logan et al., 2008). To determine whether BALB/c males express any alternate aggression-promoting MUP(s) we tested the bioactivity of the MUP-containing high molecular weight (HMW) urine fraction from the BALB/c strain in the aggression assay and found no aggression-promoting bioactivity (Figures 7A, S6). This confirms that BALB/c can serve as a natural loss of function strain in which males are experientially naïve to aggression-promoting MUP. When presented with rMUP3 in the resident-intruder assay, we found that a BALB/c male’s innate response is statistically indistinguishable from his aggressive response to the odor of whole urine (Figure 7B). These experiments indicate that rMUP3 is intrinsically specialized with a determined function and its sensory detection activates hardwired neural circuits that generate innate aggression.
Figure 7. MUPs promote innate aggression.
(A) Native BALB/c High Molecular Weight (HMW) fraction does not promote aggression in the resident-intruder assay. Black bars = C57BL/6J male residents. n = 5–20. (B) First detection of rMUP3 stimulates aggression in BALB/c males. White bars = BALB/c male residents. n = 12. Mean + SEM. p-values determined by ANOVA followed by Tukey-HSD post hoc analysis or by Kruskal-Wallis test. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. n.s. = non-significant. (C) Locomotor behavior of test animals during odor-mediated countermarking assay as measured by tracking software. n = 4. All values mean + SEM. No significant differences were found by one-tailed ANOVA. See also Figure S6.
We next began to investigate how MUP3 and MUP20 regulate both aggression and countermarking. 92% of wild-caught males, and most inbred lab strains, have been found to express an aggression-promoting MUP (Armstrong et al., 2005; Roberts et al., 2010). If MUP3 and MUP20 function to elicit aggression, and individuals are continuously expressing these signals, then most males would be constantly aroused to fight. However, we observe that singly housed males do not show arousal and motor patterns consistent with aggressive behavior. We confirmed this by exposing males to an aggression-promoting MUP, rMUP3, in the countermarking assay and quantified six variables of locomotion to determine if it elicits any motor patterns characteristic of aggression. We found the responses to rMUP3 to be indistinguishable from that of the non-aggression-promoting rMUP7, with neither showing any motor patterns of aggression (Figure 7C). This is consistent with observations by us and others that defining motor patterns of aggression (such as lunging, biting, chasing, cornering, and kicking) require a physical target on which to direct attacks (Blanchard et al., 2003; Choi et al., 2011; Stowers et al., 2013) (L.S. unpublished observations), and indicates that the extended sensory environment contributes to MUP significance. Our data provides a framework to begin to investigate the mechanisms by which a single ligand promotes two entirely different motor outputs; with aggression being released only in the presence of a target animal.
Discussion
Very little is known about how the environment is transformed into a meaningful neural code that regulates behavior. Behavioral analysis of mice indicates that a subset of odor cues emitted and detected between members of the same species, termed pheromones, are specialized to generate social behavior (Karlson and Luscher, 1959; Wyatt, 2010). Because few of these specialized odors have been isolated and studied in mammals, there is little understanding of whether they function as originally supposed (Wyatt, 2010). The purification of ligands with a known output enables us to now gain first insight into several basic qualities of the neural strategy that underlies sensory-evoked social behavior. First, we find MUP3 is specialized in that it is intrinsically fixed to promote aggression upon first encounter. This reveals that a subset of the cognate sensory neurons is genetically hardwired to gain access to central neural circuits of aggression (Adams, 2006; Nelson and Trainor, 2007). Second, unlike currently known mouse pheromones (Haga et al., 2010; Roberts et al., 2012; Roberts et al., 2010), we find MUP3 is not sufficient to generate the motor patterns of aggression, instead it requires additional, coincident, sensory information from a target animal (Blanchard et al., 2003; Choi et al., 2011) which may function as a gate to ensure that males do not become aggressive to their own signals. MUP3 and MUP20 can now be used as tools to activate, identify, and begin to study the neural circuits that generate aggression and the mechanisms that regulate the display of the behavior depending on the extended sensory context. Third, we also find aggression-promoting MUPs to be sufficient to evoke a second, entirely different social behavior: countermarking. Surprisingly, this indicates that subsets of VSNs have the capacity to elicit multiple outputs. It will be of great interest to determine how VSNs are organized and regulated to differentially activate multiple downstream circuits. The identification of MUP3 as a bioactive odor cue will provide an essential tool to identify and study the neural circuit mechanisms that underlie the innate response, context dependency, and general circuit structure that generates aggressive behavior.
The MUP sensory code that generates countermarking is strikingly different from that of aggression or other known specialized odor cues (Haga et al., 2010; Papes et al., 2010; Roberts et al., 2010). Our behavioral analysis indicates that MUP-mediated countermarking is dependent on evaluating the entire repertoire of MUPs in the environment. This indicates that there is a neural mechanism to identify and compare all of the sensory activity generated by this ligand family. Second order mitral cell neurons have been shown to monitor multiple glomeruli, of presumably closely related receptors (Wagner et al., 2006). This organization may serve to ‘read’ coincident MUP activity directly in the accessory olfactory bulb, or it may occur through undefined mechanisms in higher-order processing centers. How the detection of multiple MUP ligands informs countermarking, but has no observed effect on aggression remains to be studied.
Previously studied VSNs have been found to be tuned to a cognate ligand. Instead, we find that the subset of MUP-detecting neurons utilize a combinatorial strategy. It has previously been noted that very few neurons in the VNO respond to male mouse odor sources (Isogai et al., 2011). While this may indicate that other sensory neurons, perhaps in the main olfactory epithelium, primarily detect male odors, our data indicate an alternate strategy mediated within the VNO. Combinatorial coding enables a small subset of receptors to identify a large number of structurally similar ligands, provides for the detection of rapidly evolving protein ligands (such as species-specific MUPs), and is able to differentiate relative ratios of individual members thereby increasing their functional coding-capacity. The extent to which MUP sensory neurons are unique among VSNs in utilizing combinatorial coding remains to be determined.
Using simplified laboratory assays, it is possible to control an individual’s experience, internal state, and environment in order to robustly initiate and study social behavior. While this approach is essential for first identification of the underlying general neural characteristics, the neural mechanisms and responses in the wild are likely to be dynamic, more complex, and less predictable. Even in a controlled environment, the probability of pheromones to initiate their cognate behavior depends on the gender, age, social status, and reproductive state of the receiver. Countermarking is an extremely simple, behavior, that has a high probability of occurring in the presence of urine and can serve as a model to study the neural mechanisms that enable and modify the release of behavior. Among mice, and across species, individuals have to decipher the origin of all detected biosignals, whether they are self-generated or emitted by others. While this computation appears effortless, the underlying neural mechanisms remain largely unknown. Our identification of the relevant ligands that signal self and other for countermarking behavior enabled us to determine that the distinction is not due to sensory habituation. Instead, the countermarking action of MUPs can be modulated by previous experience, with the action of self-emitted MUPs rendered inert. This indicates that MUP sensory activity is likely to intersect with a memory of previous MUP experience to inhibit output countermarking behavior. The formation of olfactory memories and the role of experience to modify innate behavior have been difficult to study. The identification of bioactive ligands now provides a relatively simple experimental platform to determine how the olfactory template of the self-emitted ligands is created, where in the brain it is stored, and how it inhibits the release of countermarking.
METHODS SUMMARY
Animals and Behavioral Assays
7–12 week old C57BL/6J and BALB/cByJ males were maintained in groups of 4–5 animals per cage, except those used in behavioral paradigms, which were pair housed. Countermarking behavior was measured by placing test animals into individual clean Whatman-paper lined cages, with 50μl stimulus pipetted onto the center, for 5 minutes. Collected Whatman paper was treated with ninhydrin solution, which binds proteins and turns purple upon baking, allowing visualization of urine marks. The blots were then digitally scanned to quantify the number of spots per sheet. All animal procedures were in compliance with institutional guidelines, Institutional Animal Care and Use Committee, European Union legislation (Directive 86/609/EEC) and FELASA recommendations.
Stimuli
Urine was freshly gathered from wild-type mice for use in all experiments. rMUPs were prepared using the pMAL Protein Fusion and Purification system (Chamero et al., 2007). Each rMUP was presented at 5mg/ml (1X) for behavioral assays and diluted 1:300 for calcium imaging. Wherever proteins were presented in a blend, each protein was present at 5 mg/ml (for a total of 10–25 mg/ml protein in the blend). Control stimuli were used at the following concentrations: 4 mg/ml menadione in ethanol, 20 mg/ml BSA, 70% ethanol in water, and 100nM eugenol in water.
Calcium Imaging
Transient increase in free Ca2+ concentration in dissociated VSNs was determined by ratiometric fura-2 fluorescence (Chamero et al., 2007). Experiments were limited to three stimuli to maintain viability of the neurons. Dissociated VSNs were sequentially exposed to all listed stimuli and response profiles were scored. Neural responses were scored if they met all of the following criteria: (a) ≥1.5x increase in fluorescence ratio (over baseline signal) during stimulus presentation, and (b) ≥1.5x increase in fluorescence ratio (over baseline signal) response to the positive control stimulus, c) less than 1.5x (over baseline signal) increase in fluorescence ratio outside the stimulus presentation window.
Supplementary Material
Highlights.
Detected MUP repertoire signals ‘self’ and ‘other’ to promote countermarking behavior
VNO neurons use combinatorial coding to resolve MUP identity and concentration
A single MUP promotes innate aggression irrespective of MUP repertoire
MUP-repertoire, -context, and -experience promotes different behavioral outputs
Acknowledgments
We thank K. Spencer, C.H. Engelhardt, and S. Lipartowski for excellent technical assistance. C. Dulac, L.B. Vosshall, and C.I. Bargmann provided critical advice on the manuscript. This work was supported by the Skaggs Foundation (T-H. K. and L.S.), the Ellison Medical Foundation (L.S.), the NIH-NIDCD (L.S. R01 DC006885, R01 DC009413), the Deutsche Forschungsgemeinschaft (M.S. SP724/7-1) and the Volkswagen Foundation (M.S. 83533). MS is a Lichtenberg Professor of the Volkswagen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DB. Brain mechanisms of aggressive behavior: an updated review. Neuroscience and biobehavioral reviews. 2006;30:304–318. doi: 10.1016/j.neubiorev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ. Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem J. 2005;391:343–350. doi: 10.1042/BJ20050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchini A, Gaetani E, Cavaggioni A. Pheromone binding proteins of the mouse, Mus musculus. Experientia. 1992;48:419–421. doi: 10.1007/BF01923448. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Hurst JL. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochemical Society transactions. 2003;31:142–146. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Hormones and behavior. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, Abramowitz J, Birnbaumer L, Zufall F, Leinders-Zufall T. G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12898–12903. doi: 10.1073/pnas.1107770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL. Limited variation in the major urinary proteins of laboratory mice. Physiol Behav. 2009;96:253–261. doi: 10.1016/j.physbeh.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dewan A, Pacifico R, Zhan R, Rinberg D, Bozza T. Non-redundant coding of aversive odours in the main olfactory pathway. Nature. 2013 doi: 10.1038/nature12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson JS, Asofsky R, Potter M, Runner CC. Major urinary protein complex of normal mice: origin. Science. 1965;149:981–982. doi: 10.1126/science.149.3687.981. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Hagendorf S, Fluegge D, Engelhardt C, Spehr M. Homeostatic control of sensory output in basal vomeronasal neurons: activity-dependent expression of ether-a-go-go-related gene potassium channels. J Neurosci. 2009;29:206–221. doi: 10.1523/JNEUROSCI.3656-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie ND, Held WA, Toole JJ. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979;17:449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Humphries RE, Robertson DH, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mice. Animal behaviour. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. BioEssays: news and reviews in molecular, cellular and developmental biology. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Animal behaviour. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janotova K, Stopka P. The level of major urinary proteins is socially regulated in wild Mus musculus musculus. J Chem Ecol. 2011;37:647–656. doi: 10.1007/s10886-011-9966-8. [DOI] [PubMed] [Google Scholar]
- Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Hormones and behavior. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Knopf JL, Gallagher JF, Held WA. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Molecular and cellular biology. 1983;3:2232–2240. doi: 10.1128/mcb.3.12.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, SPC, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current biology: CB. 2012;22:1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DW, Marton TF, Stowers L. Species specificity in major urinary proteins by parallel evolution. PloS one. 2008;3:e3280. doi: 10.1371/journal.pone.0003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Meredith M, O’Connell RJ. Efferent control of stimulus access to the hamster vomeronasal organ. The Journal of physiology. 1979;286:301–316. doi: 10.1113/jphysiol.1979.sp012620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge JM, Armstrong SD, McLaren K, Beynon RJ, Hurst JL, Nicholson C, Robertson DH, Wilming LG, Harrow JL. Dynamic instability of the major urinary protein gene family revealed by genomic and phenotypic comparisons between C57 and 129 strain mice. Genome Biol. 2008;9:R91. doi: 10.1186/gb-2008-9-5-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature reviews Neuroscience. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in mouse scent marks is involatile. Proceedings Biological sciences/The Royal Society. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. The consequences of inbreeding for recognizing competitors. Proceedings Biological sciences/The Royal Society. 2000;267:687–694. doi: 10.1098/rspb.2000.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochemical Society transactions. 2003;31:117–122. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Ma W, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc R Soc Lond B Biol Sci. 1999;266:2017–2022. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Animal behaviour. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC biology. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DH, Hurst JL, Bolgar MS, Gaskell SJ, Beynon RJ. Molecular heterogeneity of urinary proteins in wild house mouse populations. Rapid Commun Mass Spectrom. 1997;11:786–790. doi: 10.1002/(SICI)1097-0231(19970422)11:7<786::AID-RCM876>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stowers L, Cameron P, Keller JA. Ominous odors: olfactory control of instinctive fear and aggression in mice. Current opinion in neurobiology. 2013;23:339–345. doi: 10.1016/j.conb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka PR, Paigen K. Regulation of mouse major urinary protein production by the Mup-A gene. Genetics. 1978;90:597–612. doi: 10.1093/genetics/90.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2010;196:685–700. doi: 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- Xia J, Sellers LA, Oxley D, Smith T, Emson P, Keverne EB. Urinary pheromones promote ERK/Akt phosphorylation, regeneration and survival of vomeronasal (V2R) neurons. The European journal of neuroscience. 2006;24:3333–3342. doi: 10.1111/j.1460-9568.2006.05244.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.