Abstract
QRS duration (QRSd) is used to diagnose left bundle branch block (LBBB) and is important for determining cardiac resynchronization therapy eligibility. The same QRSd thresholds established decades ago are used for all patients. However, significant inter-individual variability of normal QRSd exists and individualized QRSd thresholds may improve diagnosis and intervention strategies. Prior work reported left ventricular (LV) mass and papillary muscle location predicted QRSd in healthy subjects, but the relationship in diseased ventricles is unknown. We aimed to determine the association between LV anatomy and QRSd in cardiomyopathy patients. Patients referred for primary prevention implantable defibrillators (n=166) received cardiac magnetic resonance imaging and those with normal conduction (without bundle branch or fascicular block) and LBBB were studied. LV mass, length, internal diameter, end diastolic volume (LVEDV), septal and lateral wall thickness, and papillary muscle location were measured. In normal conduction patients, LV length (r=0.35, p<0.001), mass (r=0.32, p<0.001), diameter (r=0.20, p=0.03) and septal wall thickness (r=0.20, p=0.03) had positive correlations with QRSd. In LBBB patients, LV length (r=0.32, p=0.03), mass (r=0.39, p=0.01), diameter (r=0.34, p=0.02), and LVEDV (r=0.32, p=0.04) had positive correlations with QRSd. Contrary to prior studies in healthy subjects, papillary muscle angle (location) was not associated with QRSd in normal conduction or LBBB cardiomyopathy patients. In conclusion, increasing LV anatomical measurements are associated with increasing QRSd in cardiomyopathy patients. Future work should investigate the use of LV anatomical measurements in developing individualized QRSd thresholds for diagnosing conduction abnormalities such as LBBB and identifying candidates for cardiac resynchronization therapy.
Keywords: Left Ventricular Anatomy, QRS Duration, Cardiomyopathy, Left Bundle Branch Block
Introduction
Conventional QRS duration (QRSd) thresholds for diagnosis of cardiac disease were established decades ago and the same thresholds are used for all patient types. For example, QRSd thresholds are used to diagnose left bundle branch block (LBBB) as well as to determine patient eligibility for treatments such as cardiac resynchronization therapy (CRT). Since QRSd inter-individual variability can make diagnosis difficult, determining an individualized baseline QRSd could aid in determining the onset and progression of cardiac conditions. Causes of QRSd variability include differences in cardiac anatomy. Prior work in healthy subjects demonstrated that left ventricular (LV) mass, length, and papillary muscle location predicted QRSd.1 Previous studies also showed that QRSd prolongation is associated with LV structural changes including increases in wall thickness.2,3,4 Other anatomical factors that influence QRSd include fiber orientation, LV geometry, the complexity of the Purkinje network, and the shape of the LV wall.5 However, it is not known if LV anatomical characteristics correlate with QRSd in cardiomyopathy patients referred for primary prevention implantable defibrillators or CRT. The aim of this study was to evaluate the association between LV anatomy and QRSd in cardiomyopathy patients, specifically those with normal conduction and those with LBBB.
Methods
The study was approved by the Johns Hopkins Hospital Institutional Review Board and the FDA Research in Human Subjects Committee. Patients referred to the Johns Hopkins Medical Institutions for implantable cardioverter-defibrillator (ICD) placement for primary prevention of sudden cardiac death and were enrolled between November 2003 and December 2010. This is a retrospective analysis of the cardiovascular magnetic resonance (CMR) arm of the PROSE-ICD study (Prospective Observational Study of Implantable Cardioverter Defibrillators) which has been previously described.6 The inclusion and exclusion criteria have been described previously.7 In brief, patient inclusion required 1) left ventricular ejection fraction (LVEF) ≤35% measured by a clinically indicated non-CMR study (echocardiography, nuclear scintigraphy or ventriculography), 2) coronary angiography, 3) no other indications for ICD placement (e.g. syncope, sustained ventricular arrhythmias, or cardiac arrest), and 4) no contraindications to CMR (e.g. existing cardiac device). Patients were classified as having ischemic cardiomyopathy if they had a history of myocardial infarction or revascularization, evidence of coronary artery stenosis >50% of the left main or proximal left anterior descending coronary arteries, or >50% stenosis of two or more epicardial vessels. Other patients were classified as having non-ischemic cardiomyopathy. Acute myocarditis, congenital heart disease, hypertrophic cardiomyopathy, and infiltrative heart disease were excluded.8 Patients were divided into normal His-Purkinje conduction (normal conduction) and LBBB using the criteria below. Patients not matching the defined normal conduction or LBBB classes and those with right bundle branch block or left anterior fascicular block were excluded from the analysis.
Clinically indicated 12-lead ECGs were acquired with a GE-Marquette system before ICD implantation as previously described.7 ECGs were analyzed by two observers and classified in consensus by the following criteria.7 1) Normal Conduction: QRSd < 120 ms and 2) LBBB: QRS duration ≥ 140 ms (men) or 130 ms (women), QS or rS in V1 with mid-QRS notching/slowing in ≥ 2 of the leads I, aVL, V1, V2, V5 or V6. The new strict LBBB criteria, with different thresholds for men and women, were proposed previously because men have larger ventricles and longer QRS duration than women.7,9,10 Multiple independent studies have shown that they improve prediction of which patients benefit from CRT,11,12,13 likely identifying which patients have a true LBBB.14,15
Of the 235 primary prevention ICD patients available for analysis, 167 met the inclusion criteria for this study and had analyzable data. One LBBB patient was excluded because his LV mass was >8 standard deviations and QRSd was >5 standard deviations from the mean of other LBBB patients (subject LV mass = 525.30 g, QRSd = 244 ms).
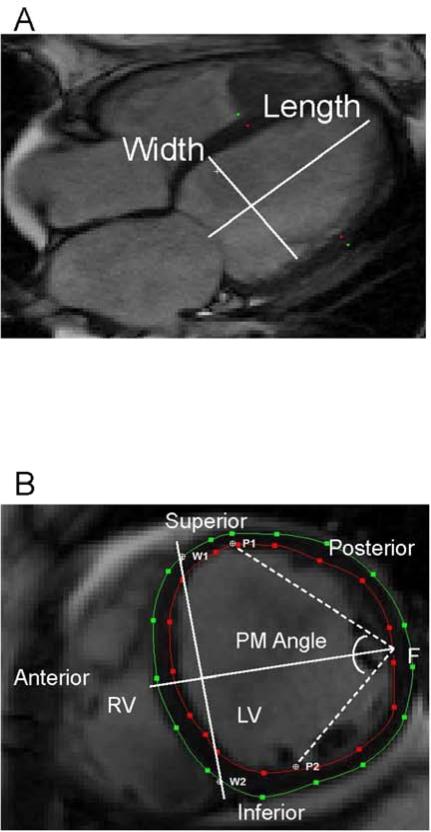
The cine and CMR protocol have been described previously.7,16,8 In summary, patients were imaged with a 1.5T scanner (Signa CV/I, GE Healthcare Technologies, WI, USA or Avanto, Siemens Medical Systems, Erlangen, Germany). LV mass and end diastolic volume measurements (LVEDV) were performed with CINEtool (GE Healthcare, WI, USA), as previously described. All images were analyzed subsequently using the image analysis program Segment (Segment 1.9, Medviso, AB, Lund, Sweden) to determine LV papillary muscle angle, LV length, LV diameter, ventricular septal wall thickness, and LV lateral wall thickness (Figure 1). Papillary muscle angle was measured as previously described, in the short axis view in the plane where the papillary muscle inserts into the LV wall (Figure 1B).5,1 LV diameter and wall thicknesses were measured at this level at end-diastole. LV length was measured from the four-chamber long axis view (Figure 1A). In brief, papillary muscle angle was determined by defining anterior and posterior papillary muscle insertion points (P1 and P2) along with ventricular septal wall border points (W1 and W2) where the right ventricle meets the left ventricle. A perpendicular bisector line was then drawn between the W1W2 segment and is used to determine the free wall midpoint (F). Two segments (P1F and P2F) are drawn between the midpoint (F) and the papillary muscle insertion points (P1 and P2). The papillary muscle angle is defined as the angle between P1F and P2F segments.
Figure 1.
LV anatomical and papillary muscle angle measurements. (A) The long axis view was used to measure LV length. (B) Short axis view, at the level of endocardial insertion of the papillary muscle, was used for the measurement of LV wall thicknesses, LV diameter, and papillary muscle angle. PM-angle papillary muscle angle; W1, W2-ventricular septal wall border points; P1-Anterior papillary muscle; P2- Posterior papillary muscle; F-Free wall midpoint.
Baseline patient characteristics were compared between ECG conduction types with oneway ANOVA (Kruskal-Wallis). Associations between LV anatomical measurements and QRSd were assessed by linear regression. Categorical variables were evaluated by Chi-square. All statistical analyses were performed using SigmaPlot (version 11.0, CA, USA). P-values <0.05 were considered statistically significant.
Results
The 166 patients had a mean age of 56 years, were 23% female, had a mean LVEF of 28%, had 49% ischemic cardiomyopathy etiology, and had a distribution of NYHA heart failure classes of: 23% Class I, 48% Class II and 29% Class III. LBBB patients, n=44 (compared to non-bundle/fascicular block patients, n=122) were older, more commonly female and Caucasian, had smaller LV scar size, higher QRSd, less ischemic and had higher NYHA heart failure class (Table 1). LBBB patients also had larger LV mass, LV length and LVEDV, but smaller LV wall thicknesses. There was no significant difference in LV diameter or papillary muscle angle between groups (Table 1).
Table 1.
Baseline Characteristics by Conduction Type
| Characteristics | Normal Conduction (n=122) | LBBB (n=44) | P-value |
|---|---|---|---|
| Age (years) | 54.4 ± 12.1 | 59.6 ± 11.5 | 0.01 |
| Female | 22 (18%) | 16 (36%) | 0.01 |
| Left Ventricular Ejection Fraction (%) | 28.4 ± 9.0 | 25.4 ± 8.6 | 0.06 |
| Scar size (%LV) | 13.9 ± 13.4 | 6.6 ± 9.5 | 0.001 |
| QRS duration (ms) | 98.2 ± 10.8 | 160.4 ± 16.2 | <0.001 |
| Ischemic etiology | 68 (55.7%) | 13 (30%) | 0.003 |
| New York Heart Association | <0.001 | ||
| Class I | 37 (30%) | 2 (5%) | |
| Class II | 64 (52%) | 15 (34%) | |
| Class III | 21 (17%) | 27 (61%) | |
| Body Surface Area (m2) | 2.07 ± 0.3 | 2.04 ± 0.3 | 0.47 |
| Caucasian | 78 (64%) | 34 (77%) | |
| African American | 39 (32%) | 9 (20%) | 0.13 |
| Other | 5 (4%) | 0 (0%) | |
| LV length (mm) | 95.7 ± 9.8 | 101.0 ± 11.9 | 0.004 |
| LV diameter (mm) | 65.4 ± 8.8 | 67.7 ± 10.3 | 0.18 |
| LV mass (g) | 144.5 ± 45.2 | 159.3 ± 42.3 | 0.04 |
| Ventricular septal wall thickness (mm) | 7.1 ± 2.6 | 5.5 ± 2.3 | <0.001 |
| LV lateral wall thickness (mm) | 5.8 ± 2.2 | 4.7 ± 1.4 | 0.002 |
| Papillary Muscle Angle (°) | 108.8 ± 16.0 | 107.9 ± 14.0 | 0.73 |
| LV End Diastolic Volume (mL) | 236.7 ± 75.6 | 271.0 ± 95.2 | 0.04 |
LBBB=Left Bundle Branch Block, LV=Left Ventricle
Continuous variables are reported as mean ± standard deviation and categorical variables are reported as n (% of population).
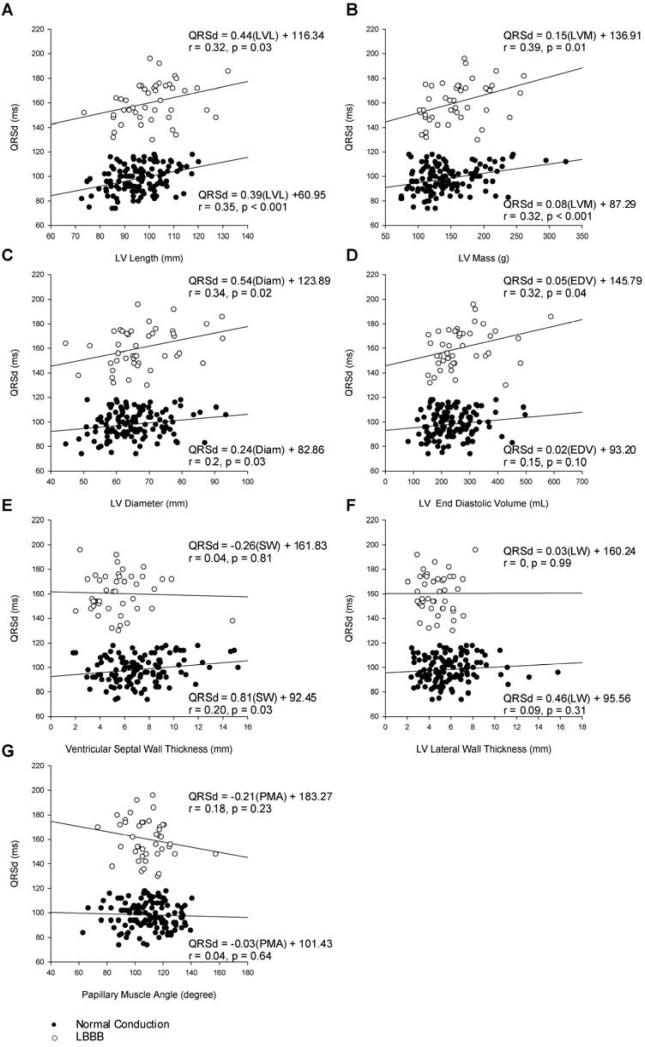
Figure 2 shows scatterplots and linear regression results for the correlation between each anatomical measurement and QRSd. LV length, LV diameter, and LV mass all had positive correlations with QRSd in both the normal and LBBB conduction groups. In the normal conduction patients, there was a 10 ms QRSd increase per 3.9 mm increase in LV length (r=0.35, p<0.001), 10 ms QRSd increase per 2.4 mm LV diameter increase (r=0.20, p=0.02), and 10 ms QRSd increase per 0.8 g increase in LV mass (r=0.32, p<0.001). In the LBBB patients, the increase in QRSd per change in length (10 ms per 4.4 mm; r=0.32, p=0.03) was similar to that in the normal conduction group, while the increase in QRSd per change in LV diameter (10 ms per 5.4 mm; r=0.34, p=0.02) and LV mass (10 ms per 1.5 g; r=0.39, p=0.01) was approximately double that in the normal conduction group.
Figure 2.
Correlation between QRSd and left ventricle A) Length (LVL), B) Mass (LVM), C) LV Diameter (Diam), D) LV End Diastolic Volume (EDV), E) Ventricular Septal Wall (SW), F) LV Lateral Wall (LW), and G) Papillary Muscle Angle (PMA).
QRSd was also associated with ventricular septal wall thickness in the normal conduction (10 ms per 8.1 mm; r=0.20, p=0.03), but not in the LBBB group; while QRSd was associated with LVEDV in the LBBB (10 ms per 0.5 mL; r=0.32, p=0.04), but not in the normal conduction group. We found no correlation between QRSd and LV lateral wall thickness or papillary muscle angle in either conduction type group. Sub analyses of the normal conduction group stratified by scar, ischemic etiology, gender, and mass did not increase the correlation of LV anatomical measurements with QRSd.
Discussion
This retrospective analysis of PROSE-ICD trial data determined that LV anatomical measurements correlate with QRSd in cardiomyopathy patients with normal conduction and with LBBB. Specifically, LV length, LV diameter, and LV mass increase with prolonged QRSd in all cardiomyopathy patients. Additionally, LVEDV correlates with QRSd in LBBB patients, while the ventricular septal wall thickness correlates with QRSd in normal conduction patients. Overall, considering both groups, LV dilation is more closely associated with QRSd than is LV wall thickness in cardiomyopathy patients. Larger LVEDV prolonged QRSd more than increases in LV wall thickness did. This agrees with electrocardiographic simulation studies where ECGs were produced from anatomical models of dilated and hypertrophied hearts.17 In addition, the LV walls in LBBB patients were significantly thinner compared to those of normal conduction patients, which is consistent with a prior study in which LV walls in LBBB patients were thinner than in healthy subjects.18
The degree of correlation between QRSd and significant anatomical measurements in cardiomyopathy patients is consistent with that found in healthy patients. Previous studies in healthy subjects with normal conduction demonstrated that LV mass,2,1 LV length1, LV diameter,2 and LV wall thickness2 all correlated with QRSd. Our findings are also consistent with results of previous studies in patients with known cardiovascular disease, where QRS duration was associated with LV mass and LVEDV.19 QRSd also correlated also LV mass, LV diameter, and ventricular septal and LV lateral walls in Framingham Heart study participants without heart failure or myocardial infarction.2
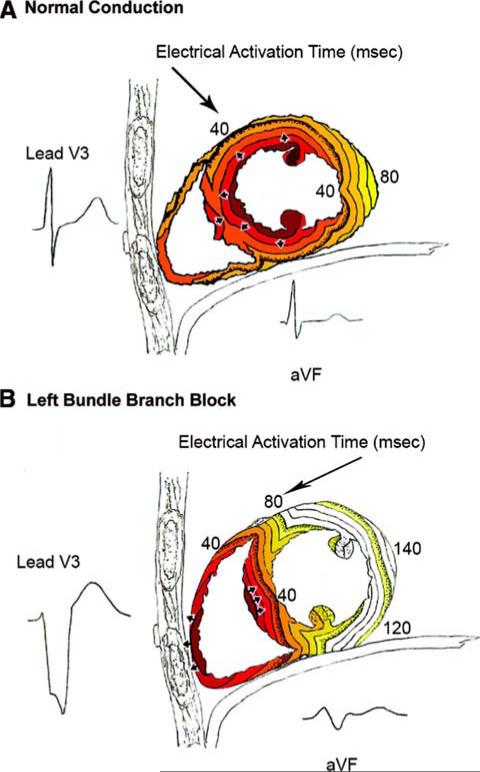
The correlation between papillary muscle angle and QRSd in healthy patients1 was not present in cardiomyopathy patients. Figure 3A shows electrical activation in normal conduction patients starting in the endocardium and papillary muscles. Figure 3B depicts electrical activation in LBBB where activation begins in the RV and must proceed through the septum before reaching the LV endocardium. It was expected that there would be no correlation between papillary muscle angle and QRSd in LBBB patients; however, in normal conduction patients, it can be postulated that as papillary muscle angle increases, the insertion point of the papillary muscle moves towards the lateral wall. Therefore QRSd would be shorter because there is less distance for the electrical wavefront to travel before reaching the LV lateral wall. In our study, dilation of the LV in cardiomyopathy patients may have confounded the relation between the papillary muscle angle and QRSd.
Figure 3.
(Reprint from Circ Arrhythmia Electrophysiol 2008;1:327-336.) Timing of electrical activation (depolarization) wavefronts in normal conduction (A) and LBBB (B). For reference, 2 QRS-T waveforms are shown in their anatomic locations (V3 on the chest and aVF inferiorly). Electrical activation starts at the small arrows and spreads in a wavefront with each colored line representing successive 10 ms. In normal conduction, activation begins within both the LV and RV endocardium. In LBBB, activation only begins in the RV and must proceed through the septum before reaching the LV endocardium (i.e., this pattern in the septum is opposite to that seen in normal conduction).
While individualized baseline QRSd could aid in the diagnosis of cardiac conditions, the causes of inter-individual variability are numerous. Cardiomyopathy patients can have large variation in QRSd due to several factors including differences in conduction velocity due to fibrosis or scarring.20 QRSd is prolonged with slower conduction velocities in myocardial tissue.19 Conduction velocity can also be slowed in the Purkinje network21 and with LBBB, there may be inter-individual variability with re-entry into the LV Purkinje network.22 Fiber orientation, which can also influence conduction velocity, has been shown to impact electrical excitation both in simulation and in vivo studies.23,24
A limitation of this study is the relatively small number of cardiomyopathy patients with LBBB and p-values should be interpreted with caution due to the multiple comparisons. Larger numbers of patients will be necessary to determine if individual LV anatomic characteristics could have clinical applications for ECG diagnosis. However, to our knowledge, this is the first study to determine the correlation between LV anatomy and QRSd in cardiomyopathy patients referred for primary prevention ICDs with or without CRT.
This study supports that QRSd is associated with LV structural parameters. The importance of individualized QRSd thresholds is already evident by the introduction of new strict LBBB criteria that require a QRSd ≥ 130 ms in women or ≥ 140 ms in men due to men having larger heart sizes than women.9 Further research is necessary to determine if individualized QRSd thresholds can be used to improve the diagnosis of LBBB and select patients for CRT.
Acknowledgments
Financial Support: The study was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (HL103812 to KCW). This project was supported in part by FDA's Critical Path Initiative and appointments to Center for Devices and Radiological Health administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
References
- 1.Hakacova N, Steding K, Engblom H, Sjogren J, Maynard C, Pahlm O. Aspects of Left Ventricular Morphology Outperform Left Ventricular Mass for Prediction of QRS Duration. Ann Noninvasive Electrocardiol. 2010;15:124–129. doi: 10.1111/j.1542-474X.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhingra R, Nam BH, Benjamin EJ, Wang TJ, Larson MG, D'Agostino RB, Levy D, Vasan RS. Cross-sectional relations of electrocardiographic QRS duration to left ventricular dimensions: the Framingham Heart Study. J Am Coll Cardiol. 2005;45:685–689. doi: 10.1016/j.jacc.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Prinzen FW, Cheriex EC, Delhaas T, van Oosterhout MM, Arts T, Wellens HJ, Reneman RS. Asymmetric thickness of the left ventricular wall resulting from asynchronous electric activation: a study in dogs with ventricular pacing and in patients with left bundle branch block. Am Heart J. 1995;130:1045–1053. doi: 10.1016/0002-8703(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 4.Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25:417–423. doi: 10.1016/0735-1097(94)00371-v. [DOI] [PubMed] [Google Scholar]
- 5.Hakacova N, Robinson AC, Maynard C, Wagner GS, Idriss SF. Determination of the mitral papillary muscle positions by the septal-to-free wall arc ratio method. Clin Physiol Funct Imaging. 2009;29:181–186. doi: 10.1111/j.1475-097X.2008.00853.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu KC, Gerstenblith G, Guallar E, Marine JE, Dalal D, Cheng A, Marbán E, Lima JC, Tomaselli GF, Weiss RG. Combined Cardiac Magnetic Resonance Imaging and C-Reactive Protein Levels Identify a Cohort at Low Risk for Defibrillator Firings and Death. Circ Cardiovasc Imaging. 2012;5:178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss DG, Selvester RH, Lima JC, Arheden H, Miller JM, Gerstenblith G, Marbán E, Weiss RG, Tomaselli GF, Wagner GS, Wu KC. ECG Quantification of Myocardial Scar in Cardiomyopathy Patients With or Without Conduction Defects. Circ Arrhythmia Electrophysiol. 2008;1:327–336. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, Tomaselli GF, Lima JC. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;25:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss DG, Selvester RH, Wagner GS. Defining Left Bundle Branch Block in the Era of Cardiac Resynchronization Therapy. Am J Cardiol. 2011;107:927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Strauss DG, Selvester RH. The QRS complex-a biomarker that images the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Mascioli G, Padeletti L, Sassone B, Zecchin M, Lucca E, Sacchi S, Boggian G, Tondo AL, Belvito C, Bakhtadze N, Borrelli A, Sinagra G. Electrocardiographic Criteria of True Left Bundle Branch Block: A Simple Sign to Predict a Better Clinical and Instrumental Response to CRT. PACE. 2012;35:927–934. doi: 10.1111/j.1540-8159.2012.03427.x. [DOI] [PubMed] [Google Scholar]
- 12.van Deursen CM, Blaauw Y, Witjens MI, Debie L, Wecke L, Crijns HM, Prinzen FW, Vernooy K. The value of the 12-lead ECG for evaluation and optimization of cardiac resynchronization therapy in daily clinical practice. J Electrocardiol. doi: 10.1016/j.jelectrocard.2014.01.007. In press. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Zhang P, Li X, Gao Y, Zhu T, Wang L, Li D, Wang J, Yuan C, Guo J. True complete left bundle branch block morphology strongly predicts good response to cardiac resynchronization therapy. Europace. 2013;15:1499–1506. doi: 10.1093/europace/eut049. [DOI] [PubMed] [Google Scholar]
- 14.Risum N, Strauss D, Sogaard P, Loring Z, Hansen TF, Bruun NE, Wagner G, Kisslo J. Left bundle-branch block: The relationship between electrocardiogram electrical activation and echocardiography mechanical contraction. Am Heart J. 2013;166:340–348. doi: 10.1016/j.ahj.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Andersson LG, Wu KC, Wieslander B, Loring Z, Frank TF, Maynard C, Gerstenblith G, Tomaselli GF, Weiss RG, Wagner GS, Ugander M, Strauss DG. Left ventricular mechanical dyssynchrony by cardiac magnetic resonance is greater in patients with strict vs nonstrict electrocardiogram criteria for left bundle-branch block. Am Heart J. 2013;165:956–963. doi: 10.1016/j.ahj.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF, Lima JC, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galeotti L, van Dam PM, Loring Z, Chan D, Strauss DG. Evaluating strict and conventional left bundle branch block criteria using electrocardiographic simulations. Europace. 2013;15:1816–1821. doi: 10.1093/europace/eut132. [DOI] [PubMed] [Google Scholar]
- 18.Kasai T, DePuey EG, Shah AA. Decreased septal wall thickening in patients with left bundle branch block. J Nucl Cardiol. 2004;11:32–37. doi: 10.1016/j.nuclcard.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Stewart RA, Young AA, Anderson C, Teo KK, Jennings G, Cowan BR. Relationship between QRS duration and left ventricular mass and volume in patients at high cardiovascular risk. Heart. 2011;97:1766–1770. doi: 10.1136/heartjnl-2011-300297. [DOI] [PubMed] [Google Scholar]
- 20.Borger van der Burg AE, Bax JJ, Boersma E, Pauwels EJ, Van Der Wall EE, Schalij MJ. Impact of viability, ischemia, scar tissue, and revascularization on outcome after aborted sudden death. Circulation. 2003;108:1954–1959. doi: 10.1161/01.CIR.0000091410.19963.9A. [DOI] [PubMed] [Google Scholar]
- 21.Wit AL, Weiss MB, Berkowitz WD, Rosen KM, Steiner C, Damato AN. Patterns of atrioventricular conduction in the human heart. Circ Res. 1970;27:345–359. doi: 10.1161/01.res.27.3.345. [DOI] [PubMed] [Google Scholar]
- 22.Myerburg RJ, Nilsson K, Gelband H. Physiology of canine intraventricular conduction and endocardial excitation. Circ Res. 1972;30:217–243. doi: 10.1161/01.res.30.2.217. [DOI] [PubMed] [Google Scholar]
- 23.Clayton RH, Bernus O, Cherry EM, Dierckx H, Fenton FH, Mirabella L, Panfilov AV, Sachse FB, Seemann G, Zhang H. Models of cardiac tissue electrophysiology: progress, challenges and open questions. Prog Biophys Mol Biol. 2011;104:22–48. doi: 10.1016/j.pbiomolbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Weiss DL, Keller DJ, Seemann G, Dössel O. The influence of fibre orientation, extracted from different segments of the human left ventricle, on the activation and repolarization sequence: a simulation study. Europace. 2007;9:vi96–vi104. doi: 10.1093/europace/eum213. [DOI] [PubMed] [Google Scholar]